Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea
Abstract
1. Introduction
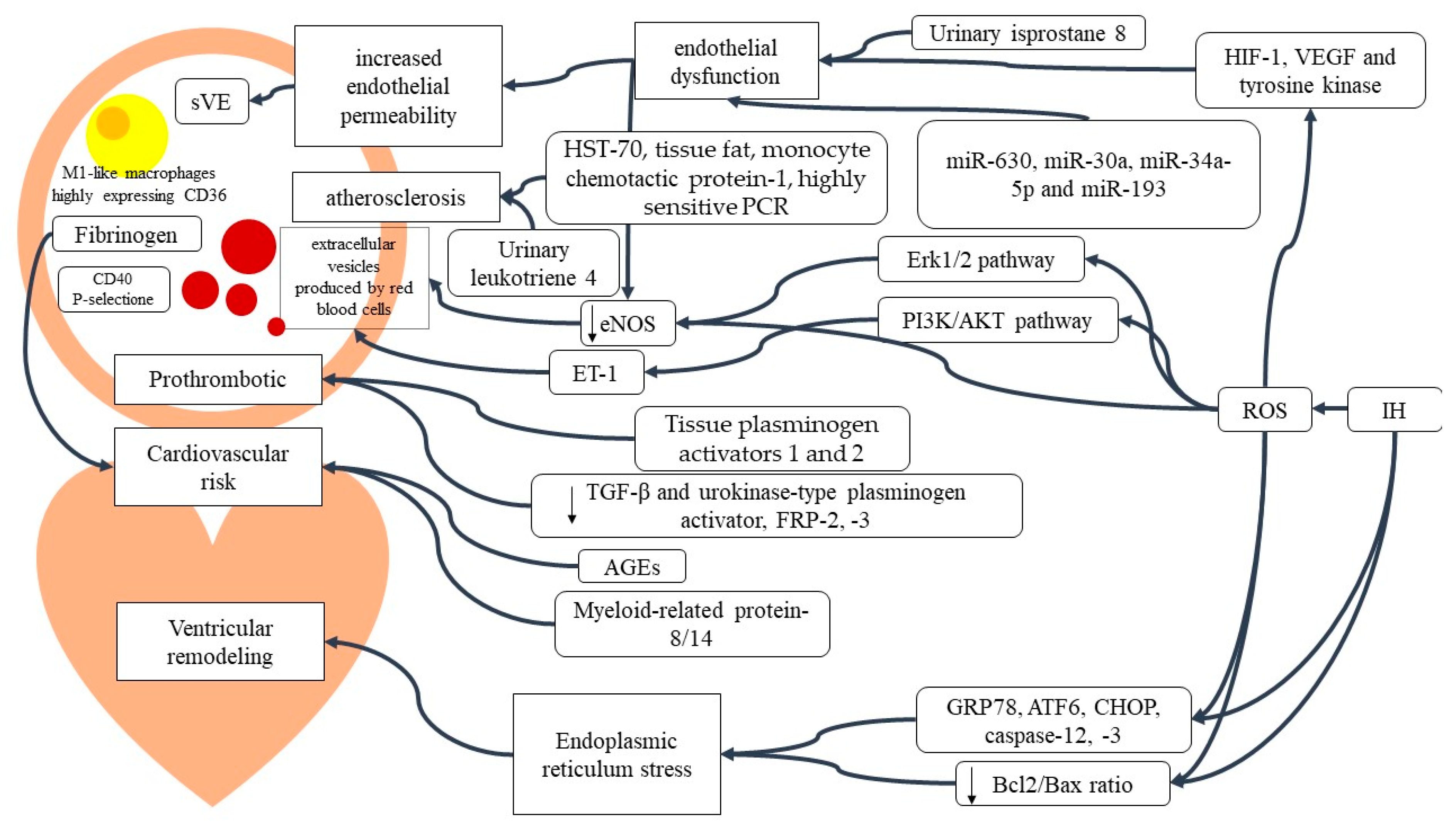
2. Results
2.1. Oxidative Stress (OS)
2.1.1. Mitochondrial Involvement
2.1.2. Inflammatory Signaling
2.1.3. Peripheral Blood Cells
2.1.4. Cardiovascular Implication of OSAS Inflammation
2.2. Circulating Metabolites
2.3. Urine Molecules
2.4. Neurotransmitters
OSAS, Neurocognition and Neurofilament
2.5. Potential Therapies
2.5.1. Antioxidants
2.5.2. Non-Antioxidant-Based Therapy
2.6. Diagnostic Biomarkers
3. Discussion
4. Materials and Methods
4.1. Literature Research
4.2. Inclusion and Exclusion Criteria
4.3. Data Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epstein, L.J.; Kristo, D.; Strollo, P.J.J.r.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Iannella, G.; Vicini, C.; Colizza, A.; Meccariello, G.; Polimeni, A.; Greco, A.; de Vincentiis, M.; de Vito, A.; Cammaroto, G.; Gobbi, R.; et al. Aging effect on sleepiness and apneas severity in patients with obstructive sleep apnea syndrome: A meta-analysis study. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 3549–3556. [Google Scholar] [CrossRef]
- Flemons, W.W.; Buysse, D.; Redline, S. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Lira, A.B.; de Sousa Rodrigues, C.F. Evaluation of oxidative stress markers in obstructive sleep apnea syndrome and additional antioxidant therapy: A review article. Sleep Breath. 2016, 20, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Mohit; Tomar, M.S.; Sharma, D.; Nandan, S.; Pateriya, A.; Shrivastava, A.; Chand, P. Emerging role of metabolomics for biomarker discovery in obstructive sleep apnea. Sleep Breath. 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Young, T.B.; Lind, B.K.; Shahar, E.; Samet, J.M.; Redline, S.; D’Agostino, R.B.; Newman, A.B.; Lebowitz, M.D.; Pickering, T.G. Association of sleep-disordered breathing sleep apnea, and hypertension in a large community-based study. J. Am. Med. Assoc. 2000, 283, 1829–1836. [Google Scholar] [CrossRef]
- Li, J.; Thorne, L.N.; Punjabi, N.M.; Sun, C.K.; Schwartz, A.R.; Smith, P.L.; Marino, R.L.; Rodriguez, A.; Hubbard, W.C.; O’Donnell, C.P.; et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005, 97, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L. Cardiovascular morbidity in obstructive sleep apnea: Oxidative stress, inflammation, and much more. Am. J. Respir. Crit. Care Med. 2008, 177, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Sorkin, J.D.; Katzel, L.I.; Goldberg, A.P.; Schwartz, A.R.; Smith, P.L. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am. J. Respir. Crit. Care Med. 2002, 165, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Mignot, E. The genetics of sleep disorders. Lancet Neurol. 2002, 1, 242–250. [Google Scholar] [CrossRef]
- Hukins, C.A. Obstructive sleep apnea—Management update. Neuropsychiatr. Dis. Treat. 2006, 2, 309–326. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, H.; Lee, H.; Ahn, S.; Noh, G. Biomechanical effect of mandibular advancement device with different protrusion positions for treatment of obstructive sleep apnoea on tooth and facial bone: A finite element study. J. Oral Rehabil. 2018, 45, 948–958. [Google Scholar] [CrossRef]
- Ferguson, A.; Ono, T.; Lowe, A.A.; Al-Majed, S.; Love, L.L.; Fleetham, J.A. A short-term controlled trial of ant adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax 1997, 52, 362–368. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004, 126, 313–323. [Google Scholar] [CrossRef]
- Celec, P.; Jurkovičová, I.; Buchta, R.; Bartík, I.; Gardlík, R.; Pálffy, R.; Mucska, I.; Hodosy, J. Antioxidant vitamins prevent oxidative and carbonyl stress in an animal model of obstructive sleep apnea. Sleep Breath. 2013, 17, 867–871. [Google Scholar] [CrossRef]
- Pilkauskaite, G.; Miliauskas, S.; Sakalauskas, R. Reactive oxygen species production in peripheral blood neutrophils of obstructive sleep apnea patients. Sci. World J. 2013, 2013, 421763. [Google Scholar] [CrossRef]
- Olszewska, E.; Rogalska, J.; Brzóska, M.M. The association of oxidative stress in the uvular mucosa with obstructive sleep apnea syndrome: A clinical study. J. Clin. Med. 2021, 10, 1132. [Google Scholar] [CrossRef]
- Gabryelska, A.; Stawski, R.; Sochal, M.; Szmyd, B.; Białasiewicz, P. Influence of one-night CPAP therapy on the changes of HIF-1α protein in OSA patients: A pilot study. J. Sleep Res. 2020, 29, e12995. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Li, N.; Yao, X.; Zhou, L. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Biomol. Biomed. 2017, 17, 47–53 . [Google Scholar] [CrossRef]
- Gabryelska, A.; Szmyd, B.; Szemraj, J.; Stawski, R.; Sochal, M.; Białasiewicz, P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J. Clin. Sleep Med. 2020, 16, 1761–1768. [Google Scholar] [CrossRef]
- Schulz, R.; Hummel, C.; Heinemann, S.; Seeger, W.; Grimminger, F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am. J. Respir. Crit. Care Med. 2002, 165, 67–70. [Google Scholar] [CrossRef] [PubMed]
- El-Solh, A.A.; Mador, M.J.; Sikka, P.; Dhillon, R.S.; Amsterdam, D.; Grant, B.J.B. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest 2002, 121, 1541–1547. [Google Scholar] [CrossRef]
- Aydin, Ş.; Özdemir, C.; Küçükali, C.I.; Sökücü, S.N.; Giriş, M.; Akcan, U.; Tüzün, E. Reduced peripheral blood mononuclear cell ROCK1 and ROCK2 levels in obstructive sleep apnea syndrome. In Vivo 2018, 32, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jelic, S.; Lederer, D.J.; Adams, T.; Padeletti, M.; Colombo, P.C.; Factor, P.H.; Le Jemtel, T.H. Vascular inflammation in obesity and sleep apnea. Circulation 2010, 121, 1014–1021. [Google Scholar] [CrossRef]
- Harki, O.; Tamisier, R.; Pépin, J.L.; Bailly, S.; Mahmani, A.; Gonthier, B.; Salomon, A.; Vilgrain, I.; Faury, G.; Briançon-Marjollet, A. VE-cadherin cleavage in sleep apnoea: New insights into intermittent hypoxia-related endothelial permeability. Eur. Respir. J. 2021, 58, 2004518. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Li, X.; Du, Y.; Li, L.; Hu, C.; Zhang, J.; Qin, Y.; Wei, Y.; Zhang, H. Extracellular Vesicles Derived from Intermittent Hypoxia–Treated Red Blood Cells Impair Endothelial Function Through Regulating eNOS Phosphorylation and ET-1 Expression. Cardiovasc. Drugs Ther. 2020, 35, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; Chen, G.P.; Lin, P.L.; Huang, J.C.; Lin, X.; Qi, J.C.; Lin, Q.C. Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci. 2018, 193, 194–199. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Álvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Terán-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating plasma extracellular microvesicle MicroRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Dai, Y.; Ma, Z.; Zhang, S.; Wang, L.; Lin, Q. Endothelial cell autophagy in chronic intermittent hypoxia is impaired by miRNA-30a-mediated translational control of Beclin-1. J. Cell. Biochem. 2019, 120, 4214–4224. [Google Scholar] [CrossRef]
- Lv, X.; Wang, K.; Tang, W.; Yu, L.; Cao, H.; Chi, W.; Wang, B. miR-34a-5p was involved in chronic intermittent hypoxia-induced autophagy of human coronary artery endothelial cells via Bcl-2/beclin 1 signal transduction pathway. J. Cell. Biochem. 2019, 120, 18871–18882. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Huang, J.; Chen, Q.; Chen, L.; Feng, D.; Zhang, S.; Huang, X.; Huang, Y.; Lin, Q. MiR-146a-5p mediates intermittent hypoxia-induced injury in H9c2 cells by targeting XIAP. Oxid. Med. Cell. Longev. 2019, 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwak, J.W.; Lee, K.E.; Cho, H.S.; Lim, S.J.; Kim, K.S.; Yang, H.S.; Kim, H.J. Can mitochondrial dysfunction be a predictive factor for oxidative stress in patients with obstructive sleep apnea? Antioxid. Redox Signal. 2014, 21, 1285–1288. [Google Scholar] [CrossRef]
- Seo, Y.J.; Ju, H.M.; Lee, S.H.; Kwak, S.H.; Kang, M.J.; Yoon, J.H.; Kim, C.H.; Cho, H.J. Damage of inner ear sensory hair cells via mitochondrial loss in a murine model of sleep apnea with chronic intermittent hypoxia. Sleep 2017, 40, 9. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Dong, Y.; Zhang, X.; Ding, N.; Liu, J.; Hutchinson, S.Z.; Lu, G.; Zhang, X. Adiponectin alleviates genioglossal mitochondrial dysfunction in rats exposed to intermittent hypoxia. PLoS ONE 2014, 9, 10. [Google Scholar] [CrossRef]
- Stl, P.S.; Johansson, B. Abnormal mitochondria organization and oxidative activity in the palate muscles of long-term snorers with obstructive sleep apnea. Respiration 2012, 83, 407–417. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Olszewska, E.; Pietrewicz, T.M.; Świderska, M.; Jamiołkowski, J.; Chabowski, A. A Case-Control Study on the Changes in High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-Alpha Levels with Surgical Treatment of OSAS. Int. J. Mol. Sci. 2022, 23, 14116. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-κB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Toujani, S.; Kaabachi, W.; Mjid, M.; Hamzaoui, K.; Cherif, J.; Beji, M. Vitamin D deficiency and interleukin-17 relationship in severe obstructive sleep apnea-hypopnea syndrome. Ann. Thorac. Med. 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Olszewska, E.; Rogalska, J.; Brzóska, M.M. Osteoprotegerin, Chitinase 3-like Protein 1, and Cardiotrophin-1 as Potential Biomarkers of Obstructive Sleep Apnea in Adults—A Case-Control Study. Int. J. Mol. Sci. 2023, 24, 2607. [Google Scholar] [CrossRef] [PubMed]
- Akinnusi, M.; Jaoude, P.; Kufel, T.; El-Solh, A.A. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 2013, 17, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Chen, Y.C.; Tseng, C.C.; Chang, H.C.; Su, M.C.; Wang, T.Y.; Lin, Y.Y.; Zheng, Y.X.; Chang, J.C.; Chin, C.H.; et al. Aberrant DNA methylation of the toll-like receptors 2 and 6 genes in patients with obstructive sleep apnea. PLoS ONE 2020, 15, 2. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.W.; Su, M.C.; Chen, C.J.; Chen, K.D.; Liou, C.W.; Tang, P.; Wang, T.Y.; Chang, J.C.; Wang, C.C.; et al. Whole genome DNA methylation analysis of obstructive sleep apnea: IL1R2, NPR2, AR, SP140 methylation and clinical phenotype. Sleep 2016, 39, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.; Amin, R.S.; Calvin, A.D.; Davison, D.; Somers, V.K. Severity of obstructive sleep apnea is associated with elevated plasma fibrinogen in otherwise healthy patients. Sleep Breath. 2014, 18, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Takamizawa, A.; Kinoshita, O.; Kubo, K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology 2006, 11, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Steffanina, A.; Proietti, L.; Antonaglia, C.; Palange, P.; Angelici, E.; Canipari, R. The plasminogen system and transforming growth factor-β in subjects with obstructive sleep apnea syndrome: Effects of CPAP treatment. Respir. Care 2015, 60, 1643–1651. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Stróżyński, A.; Olszewska, E. Is the oxidative stress in obstructive sleep apnea associated with cardiovascular complications? Systematic review. J. Clin. Med. 2020, 9, 3734. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Sochal, M. Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea-A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1808. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Blüher, M.; Jumpertz, R.; Wiesner, T.; Wirtz, H.; Bosse-Henck, A.; Stumvoll, M.; Batkai, S.; Pacher, P.; Harvey-White, J.; et al. Circulating anandamide and blood pressure in patients with obstructive sleep apnea. J. Hypertens. 2012, 30, 2345–2351. [Google Scholar] [CrossRef]
- Findley, L.J.; Boykin, M.; Fallon, T.; Belardinelli, L. Plasma adenosine and hypoxemia in patients with sleep apnea. J. Appl. Physiol. 1988, 64, 556–561. [Google Scholar] [CrossRef]
- Gonzaga, C.C.; Gaddam, K.K.; Ahmed, M.I.; Pimenta, E.; Thomas, S.J.; Harding, S.M.; Oparil, S.; Cofield, S.S. Calhoun DA Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J. Clin. Sleep Med. 2010, 6, 363–368. [Google Scholar] [CrossRef]
- Day, R.M.; Matus, I.A.; Suzuki, Y.J.; Yeum, K.J.; Qin, J.; Park, A.M.; Jain, V.; Kuru, T.; Tang, G. Plasma levels of retinoids, carotenoids and tocopherols in patients with mild obstructive sleep apnoea. Respirology 2009, 14, 1134–1142. [Google Scholar] [CrossRef]
- Papandreou, C. Independent associations between fatty acids and sleep quality among obese patients with obstructive sleep apnoea syndrome. J. Sleep Res. 2013, 22, 569–572. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Qian, Y.; Guan, J.; Yi, H.; Zou, J.; Wang, Y.; Meng, L.; Zhao, A.; Yin, S.; et al. Metabolomics Profiling for Obstructive Sleep Apnea and Simple Snorers. Sci. Rep. 2016, 6, srep30958. [Google Scholar] [CrossRef]
- Lebkuchen, A.; Carvalho, V.M.; Venturini, G.; Salgueiro, J.S.; Freitas, L.S.; Dellavance, A.; Martins, F.C.; Lorenzi-Filho, G.; Cardozo, K.H.M.; Drager, L.F. Metabolomic and lipidomic profile in men with obstructive sleep apnoea: Implications for diagnosis and biomarkers of cardiovascular risk. Sci. Rep. 2018, 8, 11270. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Arnaud, C.; Fitzpatrick, S.F.; Gaucher, J.; Tamisier, R.; Pépin, J.L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190006. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.K.; Tosun, M.; Alcelik, A.; Yilmaz, B.; Talay, F. Serum omentin levels in patients with obstructive sleep apnea. Sleep Breath. 2014, 18, 391–395. [Google Scholar] [CrossRef]
- Imayama, I.; Prasad, B. Role of leptin in obstructive sleep apnea. Ann. Am. Thorac. Soc. 2017, 14, 1607–1621. [Google Scholar] [CrossRef]
- Schiza, S.E.; Mermigkis, C.; Bouloukaki, I. Leptin and leptin receptor gene polymorphisms and obstructive sleep apnea syndrome: Is there an association? Sleep Breath. 2015, 19, 1079–1080. [Google Scholar] [CrossRef]
- Gharib, S.A.; Hayes, A.L.; Rosen, M.J.; Patel, S.R. A pathway-based analysis on the effects of obstructive sleep apnea in modulating visceral fat transcriptome. Sleep 2013, 36, 23–30. [Google Scholar] [CrossRef]
- Badran, M.; Gozal, D. PAI-1: A Major Player in the Vascular Dysfunction in Obstructive Sleep Apnea? Int. J. Mol. Sci. 2022, 23, 5516. [Google Scholar] [CrossRef]
- Jordan, W.; Berger, C.; Cohrs, S.; Rodenbeck, A.; Mayer, G.; Niedmann, P.D.; von Ahsen, N.; Rüther, E.; Kornhuber, J.; Bleich, S. CPAP-therapy effectively lowers serum homocysteine in obstructive sleep apnea syndrome. J. Neural Transm. 2004, 111, 683–689. [Google Scholar] [CrossRef]
- Ozkan, Y.; Fırat, H.; Şimşek, B.; Torun, M.; Yardim-Akaydin, S. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath. 2008, 12, 149–154. [Google Scholar] [CrossRef]
- Paik, M.J.; Kim, D.K.; Nguyen, D.T.; Lee, G.; Rhee, C.S.; Yoon, I.Y.; Kim, J.W. Correlation of daytime sleepiness with urine metabolites in patients with obstructive sleep apnea. Sleep Breath. 2014, 18, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Wu, X.; Luo, Y.J.; Wang, L.; Qu, W.M.; Li, S.Q.; Huang, Z.L. Signaling mechanism underlying the histamine-modulated action of hypoglossal motoneurons. J. Neurochem. 2016, 137, 277–286. [Google Scholar] [CrossRef]
- Kubin, L. Sleep-wake control of the upper airway by noradrenergic neurons, with and without intermittent hypoxia. Prog. Brain Res. 2014, 209, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Grace, K.P.; Hughes, S.W.; Shahabi, S.; Horner, R.L. K+ Channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir. Physiol. Neurobiol. 2013, 188, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Sochal, M. Evaluation of HIF-1 Involvement in the BDNF and ProBDNF Signaling Pathways among Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2022, 23, 14876. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Strzelecki, D.; Białasiewicz, P.; Sochal, M. BDNF and proBDNF Serum Protein Levels in Obstructive Sleep Apnea Patients and Their Involvement in Insomnia and Depression Symptoms. J. Clin. Med. 2022, 11, 7135. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; He, G.P.; Xiao, X.P.; Gu, C.; Chen, H.Y. Relationship between brain-derived neurotrophic factor and cognitive function of obstructive sleep apnea/hypopnea syndrome patients. Asian Pac. J. Trop. Med. 2012, 5, 906–910. [Google Scholar] [CrossRef]
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S255–S270. [Google Scholar] [CrossRef]
- Flores, K.R.; Viccaro, F.; Aquilini, M.; Scarpino, S.; Ronchetti, F.; Mancini, R.; Di Napoli, A.; Scozzi, D.; Ricci, A. Protective role of brain derived neurotrophic factor (BDNF) in obstructive sleep apnea syndrome (OSAS) patients. PLoS ONE. 2020 15, e0227834. [CrossRef]
- Miao, F.; Shuang, L.; Yin, Y.; Huo, H.; Kui, Z.; Hui, L. 7,8-Dihydroxyflavone protects retinal ganglion cells against chronic intermittent hypoxia—Induced oxidative stress damage via activation of the BDNF/TrkB signaling pathway. Sleep Breath. 2022, 26, 287–295. [Google Scholar] [CrossRef]
- Gabryelska, A.; Sochal, M.; Turkiewicz, S.; Białasiewicz, P. Relationship between HIF-1 and circadian clock proteins in obstructive sleep apnea patients—Preliminary study. J. Clin. Med. 2020, 9, 1599. [Google Scholar] [CrossRef]
- Kaminska, M.; O’Sullivan, M.; Mery, V.P.; Lafontaine, A.L.; Robinson, A.; Gros, P.; Martin, J.G.; Benedetti, A.; Kimoff, R.J. Inflammatory markers and BDNF in obstructive sleep apnea (OSA) in Parkinson’s disease (PD). Sleep Med. 2022, 90, 258–261. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, Y.; Liu, H.; Xu, P. The Role of Inflammation in Cognitive Impairment of Obstructive Sleep Apnea Syndrome. Brain Sci. 2022, 12, 1303. [Google Scholar] [CrossRef]
- Arslan, B.; Şemsi, R.; İriz, A.; Dinçel, A.S. The evaluation of serum brain-derived neurotrophic factor and neurofilament light chain levels in patients with obstructive sleep apnea syndrome. Laryngoscope Investig. Otolaryngol. 2021, 6, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Chi, L.; Ke, Y.; Luo, C.; Qian, S.; Gozal, D.; Liu, R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol. Dis. 2007, 28, 206–215. [Google Scholar] [CrossRef]
- Ling, J.; Yu, Q.; Li, Y.; Yuan, X.; Wang, X.; Liu, W.; Guo, T.; Duan, Y.; Li, L. Edaravone improves intermittent hypoxia-induced cognitive impairment and hippocampal damage in rats. Biol. Pharm. Bull. 2020, 43, 1196–1201. [Google Scholar] [CrossRef]
- Sadasivam, K.; Patial, K.; Vijayan, V.K.; Ravi, K. Antioxidant treatment in obstructive sleep apnoea syndrome. Indian, J. Chest Dis. Allied Sci. 2011, 53, 153–162. [Google Scholar]
- Grebe, M.; Eisele, H.J.; Weissmann, N.; Schaefer, C.; Tillmanns, H.; Seeger, W.; Schulz, R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2006, 173, 897–901. [Google Scholar] [CrossRef]
- MacRea, M.; Martin, T.; Zagrean, L.; Jia, Z.; Misra, H. Role of leptin as antioxidant in obstructive sleep apnea: An in vitro study using electron paramagnetic resonance method. Sleep Breath. 2013, 17, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lu, J.; Guan, Y.F.; Li, S.J.; Hu, T.T.; Xie, Z.S.; Wang, F.; Peng, X.H.; Liu, X.; Xu, X.; et al. Estrogen/ERR-α signaling axis is associated with fiber-type conversion of upper airway muscles in patients with obstructive sleep apnea hypopnea syndrome. Sci. Rep. 2016, 6, 27088. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y. Effects of genistein and estrogen on the genioglossus in rats exposed to chronic intermittent hypoxia may be HIF-1α dependent. Oral Dis. 2013, 19, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Jaffuel, D.; Mallet, J.P.; Dauvilliers, Y.; Bourdin, A. Is the muscle the only potential target of desipramine in obstructive sleep apnea syndrome? Am. J. Respir. Crit. Care Med. 2017, 195, 1677–1678. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Sands, S.A.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. Is the muscle the only potential target of desipramine in obstructive sleep apnea syndrome? Reply. Am. J. Respir. Crit. Care Med. 2017, 195, 1678–1679. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A.; Wellman, A.; White, D.P. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014, 37, 811–819. [Google Scholar] [CrossRef]
- Carley, D.W.; Prasad, B.; Reid, K.J.; Malkani, R.; Attarian, H.; Abbott, S.M.; Vern, B.; Xie, H.; Yuan, C.; Zee, P.C. Pharmacotherapy of apnea by cannabimimetic enhancement, the PACE clinical trial: Effects of dronabinol in obstructive sleep apnea. Sleep 2018, 41, zsx184. [Google Scholar] [CrossRef] [PubMed]
- Roizenblatt, S.; Guilleminault, C.; Poyares, D.; Cintra, F.; Kauati, A.; Tufik, S. A double-blind, placebo-controlled, crossover study of sildenafil in obstructive sleep apnea. Arch. Intern. Med. 2006, 166, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Taranto-Montemurro, L.; Messineo, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity a randomized, placebo-controlled, double-blind crossover trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Meng, Y.; Wang, Y.; Fan, S.; Liu, Y.; Zhang, X.; Ran, C.; Wang, H.; Lu, M. Protective effect of Astragaloside IV on chronic intermittent hypoxia-induced vascular endothelial dysfunction through the calpain-1/SIRT1/AMPK signaling pathway. Front. Pharmacol. 2022, 13, 920977. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, H.; Cui, Z.; Tai, X.; Chu, Y.; Guo, X. Tauroursodeoxycholic acid attenuates endoplasmic reticulum stress and protects the liver from chronic intermittent hypoxia induced injury. Exp. Ther. Med. 2017, 14, 2461–2468. [Google Scholar] [CrossRef]
- Inamoto, S.; Yoshioka, T.; Yamashita, C.; Miyamura, M.; Mori, T.; Ukimura, A.; Matsumoto, C.; Matsumura, Y.; Kitaura, Y.; Hayashi, T. Pitavastatin reduces oxidative stress and attenuates intermittent hypoxia-induced left ventricular remodeling in lean mice. Hypertens. Res. 2010, 33, 579–586. [Google Scholar] [CrossRef]
- Williams, A.L.; Chen, L.; Scharf, S.M. Effects of allopurinol on cardiac function and oxidant stress in chronic intermittent hypoxia. Sleep Breath. 2010, 14, 51–57. [Google Scholar] [CrossRef]
- Fleming, W.E.; Holty, J.C.; Bogan, R.K.; Hwang, D.; Ferouz-Colborn, A.S.; Budhiraja, R.; Redline, S.; Mensah-Osman, E.; Osman, N.I.; Li, Q.; et al. Use of blood biomarkers to screen for obstructive sleep apnea. Nat. Sci. Sleep 2018, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Z.; Tang, T.; Wu, J.; Liu, F.; Gu, L. 5-HTR2A and IL-6 polymorphisms and obstructive sleep apnea-hypopnea syndrome. Biomed. Rep. 2016, 4, 203–208. [Google Scholar] [CrossRef]
- Lin, S.W.; Tsai, C.N.; Lee, Y.S.; Chu, S.F.; Chen, N.H. Gene expression profiles in peripheral blood mononuclear cells of asian obstructive sleep apnea patients. Biomed. J. 2014, 37, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Strausz, S.; Ruotsalainen, S.; Ollila, H.M.; Karjalainen, J.; Kiiskinen, T.; Reeve, M.; Kurki, M.; Mars, N.; Havulinna, A.S.; Luonsi, E.; et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur. Respir. J. 2021, 57, 2003091. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, Q.; Sun, H.; Yang, Y.; Jiao, X.; Yang, S.; Yu, H.; Qin, Y. Combined Association Between ADIPOQ, PPARG, and TNF Genes Variants and Obstructive Sleep Apnea in Chinese Han Population. Nat. Sci. Sleep 2022, 14, 363–372. [Google Scholar] [CrossRef]
- Tanizawa, K.; Chin, K. Genetic factors in sleep-disordered breathing. Respir. Investig. 2018, 56, 111–119. [Google Scholar] [CrossRef]
- Wang, H.; Cade, B.E.; Sofer, T.; Sands, S.A.; Chen, H.; Browning, S.R.; Stilp, A.M.; Louie, T.L.; Thornton, T.A.; Johnson, W.C.; et al. Admixture mapping identifies novel loci for obstructive sleep apnea in Hispanic/Latino Americans. Hum. Mol. Genet. 2019, 28, 675–687. [Google Scholar] [CrossRef]
- Kalra, M.; Pal, P.; Kaushal, R.; Amin, R.S.; Dolan, L.M.; Fitz, K.; Kumar, S.; Sheng, X.; Guha, S.; Mallik, J.; et al. Association of ApoE genetic variants with obstructive sleep apnea in children. Sleep Med. 2008, 9, 260–265. [Google Scholar] [CrossRef]
- Gozal, D.; Khalyfa, A.; Capdevila, O.S.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Kim, J. Cognitive function in prepubertal children with obstructive sleep apnea: A modifying role for NADPH oxidase p22 subunit gene polymorphisms? Antioxid. Redox Signal. 2012, 16, 171–177. [Google Scholar] [CrossRef]
- Cade, B.E.; Chen, H.; Stilp, A.M.; Gleason, K.J.; Sofer, T.; Ancoli-Israel, S.; Arens, R.; Bell, G.I.; Below, J.E.; Bjonnes, A.C.; et al. Genetic associations with obstructive sleep apnea traits in Hispanic/Latino Americans. Am. J. Respir. Crit. Care Med. 2016, 194, 886–897. [Google Scholar] [CrossRef]
- Chen, W.; Ye, J.; Han, D.; Yin, G.; Wang, B.; Zhang, Y. Association of prepro-orexin polymorphism with obstructive sleep apnea/hypopnea syndrome. Am. J. Otolaryngol.-Head Neck Med. Surg. 2012, 33, 31–36. [Google Scholar] [CrossRef]
- Pinilla, L.; Barbé, F.; de Gonzalo-Calvo, D. MicroRNAs to guide medical decision-making in obstructive sleep apnea: A review. Sleep Med. Rev. 2021, 59, 101458. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine 2017, 96, e7917. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, G.; Huang, J.; Chen, G.; Huang, X.; Lin, Q. Expression profile of long non-coding RNAs in rat models of OSA-induced cardiovascular disease: New insight into pathogenesis. Sleep Breath. 2019, 23, 795–804. [Google Scholar] [CrossRef]
- Li, K.; Chen, Z.; Qin, Y.; Wei, Y. MiR-664a-3p expression in patients with obstructive sleep apnea: A potential marker of atherosclerosis. Medicine 2018, 97, e9813. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, X.; Xiao, Y.; Lin, K.; Chen, X. MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: Potential diagnostic and early warning markers 11 Medical and Health Sciences 1102 Cardiorespiratory Medicine and Haematology. Respir. Res. 2018, 19, 194. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Li, Q.Y.; Li, M.; Wan, H.Y. Levels of thioredoxin are related to the severity of obstructive sleep apnea: Based on oxidative stress concept. Sleep Breath. 2013, 17, 311–316. [Google Scholar] [CrossRef]
- Takahashi, K.I.; Chin, K.; Nakamura, H.; Morita, S.; Sumi, K.; Oga, T.; Matsumoto, H.; Niimi, A.; Fukuhara, S.; Yodoi, J.; et al. Plasma thioredoxin, a novel oxidative stress marker, in patients with obstructive sleep apnea before and after nasal continuous positive airway pressure. Antioxid. Redox Signal. 2008, 10, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A.; BaHammam, A.S.O. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: A pilot study. Sleep Breath. 2005, 9, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.; Cohrs, S.; Degner, D.; Meier, A.; Rodenbeck, A.; Mayer, G.; Pilz, J.; Rüther, E.; Kornhuber, J.; Bleich, S. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J. Neural Transm. 2006, 113, 239–254. [Google Scholar] [CrossRef]
- Cofta, S.; Wysocka, E.; Piorunek, T.; Rzymkowska, M.; Batura-Gabryel, H.; Torlinski, L. Oxidative stress markers in the blood of persons with different stages of obstructive sleep apnea syndrome. J. Physiol. Pharmacol. 2008, 59, 183–190. [Google Scholar]
- Mancuso, M.; Bonanni, E.; LoGerfo, A.; Orsucci, D.; Maestri, M.; Chico, L.; DiCoscio, E.; Fabbrini, M.; Siciliano, G.; Murri, L. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med. 2012, 13, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Simiakakis, M.; Kapsimalis, F.; Chaligiannis, E.; Loukides, S.; Sitaras, N.; Alchanatis, M. Lack of effect of sleep apnea on oxidative stress in obstructive sleep apnea syndrome (OSAS) patients. PLoS ONE 2012, 7, e39172. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, K.; Kontakiotis, T.; Spanogiannis, D.; Vlachogiannis, E.; Kougioulis, M.; Gerou, S.; Daskalopoulou, E. Total antioxidant status in patients with obstructive sleep apnea without comorbidities: The role of the severity of the disease. Sleep Breath. 2011, 15, 861–866. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links with Obstructive Sleep Apnea. Front. Physiol. 2020, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, L.; Benítez, I.D.; Santamaria-Martos, F.; Targa, A.; Moncusí-Moix, A.; Dalmases, M.; Mínguez, O.; Aguilà, M.; Jové, M.; Sol, J.; et al. Plasma profiling reveals a blood-based metabolic fingerprint of obstructive sleep apnea. Biomed. Pharmacother. 2022, 145, 112425. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. Molecular mechanisms of cardiovascular disease in OSAHS: The oxidative stress link. Eur. Respir. J. 2009, 33, 1467–1484. [Google Scholar] [CrossRef]
- Kim, J.; Bhattacharjee, R.; Snow, A.B.; Capdevila, O.S.; Kheirandish-Gozal, L.; Gozal, D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur. Respir. J. 2010, 35, 843–850. [Google Scholar] [CrossRef]
- Barbato, C. MicroRNA-Mediated Silencing Pathways in the Nervous System and Neurological Diseases. Cells 2022, 11, 2375. [Google Scholar] [CrossRef]
- Liang, S.; Li, N.; Heizhati, M.; Yao, X.; Abdireim, A.; Wang, Y.; Abulikemu, Z.; Zhang, D.; Chang, G.; Kong, J.; et al. What do changes in concentrations of serum surfactant proteins A and D in OSA mean? Sleep Breath. 2015, 19, 955–962. [Google Scholar] [CrossRef]
- Kong, W.; Zheng, Y.; Xu, W.; Gu, H.; Wu, J. Biomarkers of Alzheimer’s disease in severe obstructive sleep apnea–hypopnea syndrome in the Chinese population. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 865–872. [Google Scholar] [CrossRef]
- Gao, H.; Han, Z.; Huang, S.; Bai, R.; Ge, X.; Chen, F.; Lei, P. Intermittent hypoxia caused cognitive dysfunction to relate to miRNAs dysregulation in hippocampus. Behav. Brain Res. 2017, 335, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.R.; Lien, C.F.; Jeng, J.R.; Hsieh, J.C.; Chang, C.W.; Lin, J.H.; Yang, K.T. Intermittent Hypoxia Inhibits Na + -H + Exchange-Mediated Acid Extrusion Via Intracellular Na + Accumulation in Cardiomyocytes. Cell Physiol. Biochem. 2018, 46, 1252–1262. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Polecka, A.; Walasek, M.; Olszewska, E. Potential Diagnostic and Monitoring Biomarkers of Obstructive Sleep Apnea–Umbrella Review of Meta-Analyses. J. Clin. Med. 2023, 12, 60. [Google Scholar] [CrossRef]
- Riccardi, G.; Bellizzi, M.G.; Fatuzzo, I.; Zoccali, F.; Cavalcanti, L.; Greco, A.; Vincentiis, M.d.; Ralli, M.; Fiore, M.; Petrella, C.; et al. Salivary Biomarkers in Oral Squamous Cell Carcinoma: A Proteomic Overview. Proteomes 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
| Potential Biomarkers | References |
|---|---|
| glycated hemoglobin, c-reactive protein, erythropoietin | [101] |
| 5-hydroxytryptamine receptor 2A | [102] |
| ADAM29, SLC18A3, FLRT2 | [103] |
| miR-664a-3p, miR-126-3p, miR-26a-5p, miR-107 | [115,116] |
| thioredoxin, malondialdehyde, superoxide dismutase | [117,118,119] |
| TBARS | [120,121] |
| FRAP | [122,123] |
| TAS | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meliante, P.G.; Zoccali, F.; Cascone, F.; Di Stefano, V.; Greco, A.; de Vincentiis, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea. Int. J. Mol. Sci. 2023, 24, 5478. https://doi.org/10.3390/ijms24065478
Meliante PG, Zoccali F, Cascone F, Di Stefano V, Greco A, de Vincentiis M, Petrella C, Fiore M, Minni A, Barbato C. Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea. International Journal of Molecular Sciences. 2023; 24(6):5478. https://doi.org/10.3390/ijms24065478
Chicago/Turabian StyleMeliante, Piero Giuseppe, Federica Zoccali, Francesca Cascone, Vanessa Di Stefano, Antonio Greco, Marco de Vincentiis, Carla Petrella, Marco Fiore, Antonio Minni, and Christian Barbato. 2023. "Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea" International Journal of Molecular Sciences 24, no. 6: 5478. https://doi.org/10.3390/ijms24065478
APA StyleMeliante, P. G., Zoccali, F., Cascone, F., Di Stefano, V., Greco, A., de Vincentiis, M., Petrella, C., Fiore, M., Minni, A., & Barbato, C. (2023). Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea. International Journal of Molecular Sciences, 24(6), 5478. https://doi.org/10.3390/ijms24065478










