Aspartate β-Hydroxylase Serves as a Prognostic Biomarker for Neoadjuvant Chemotherapy in Gastric Cancer
Abstract
:1. Introduction
2. Results
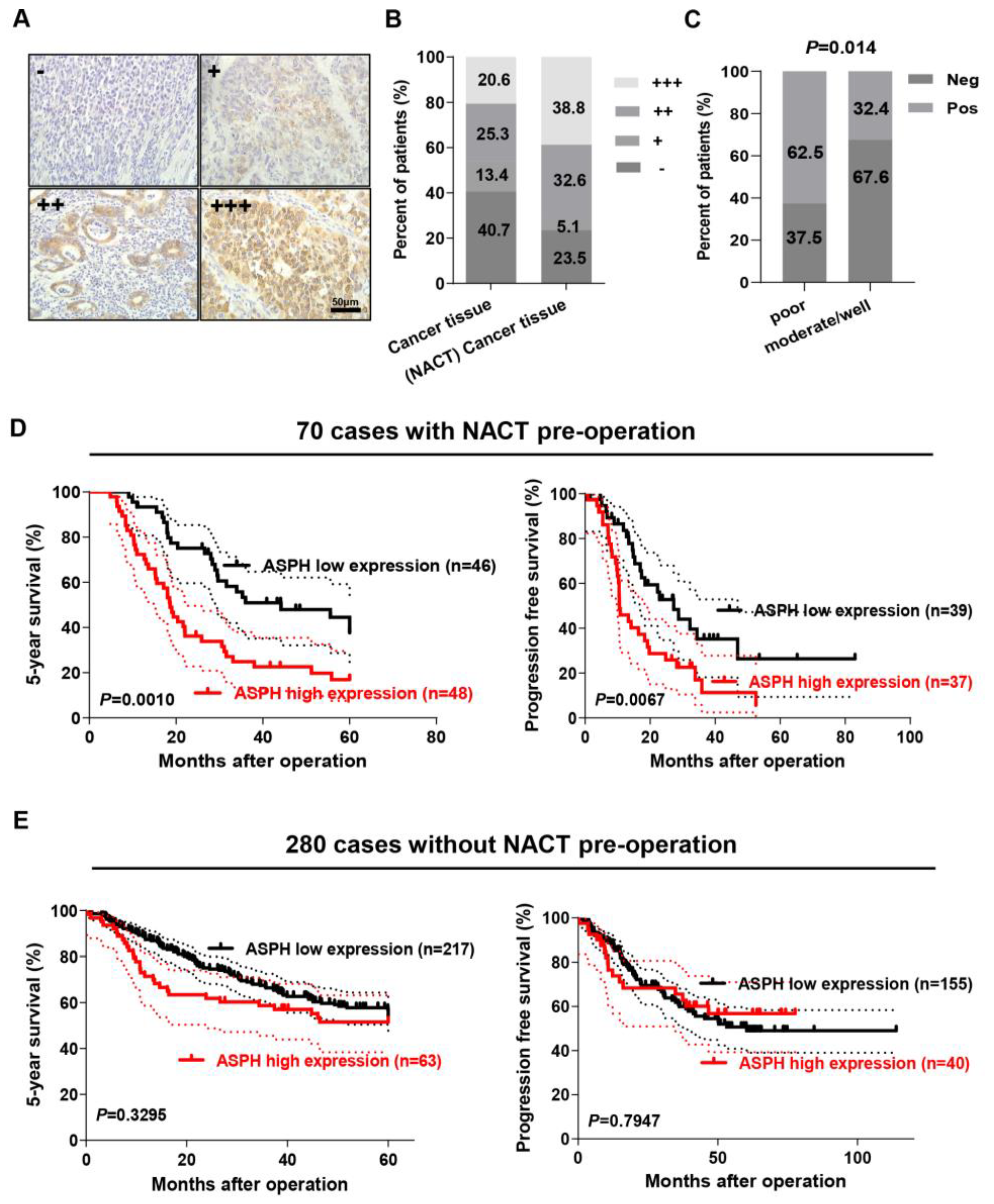
2.1. ASPH Expression Was Correlated with NACT in GC
2.2. ASPH Expression Level Positively Correlated with Poor Prognosis of GC Patients with NACT
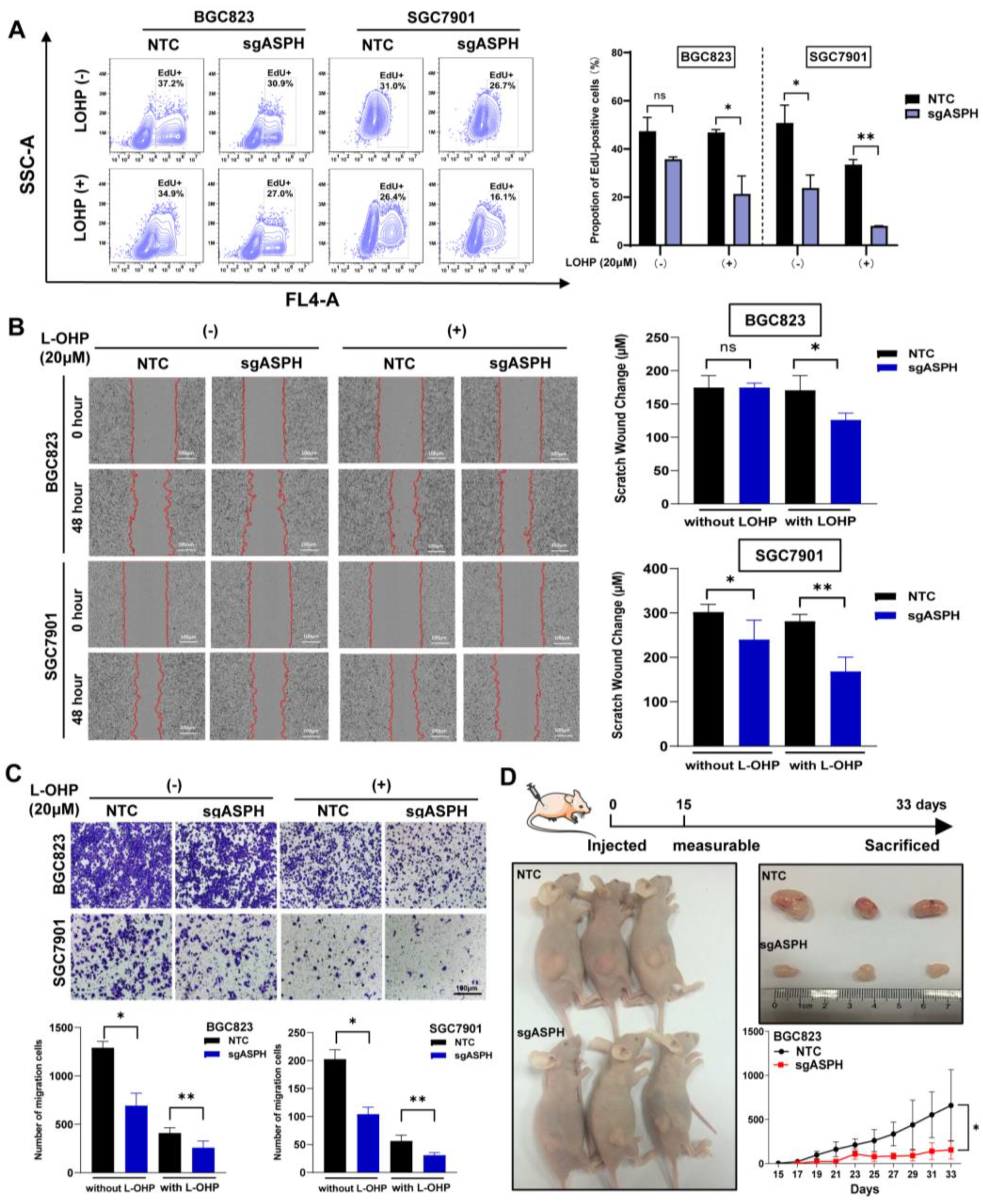
2.3. ASPH Knockout Decreased Malignant Phenotypes of Cancer Cells Especially with Addition of Chemotherapeutics
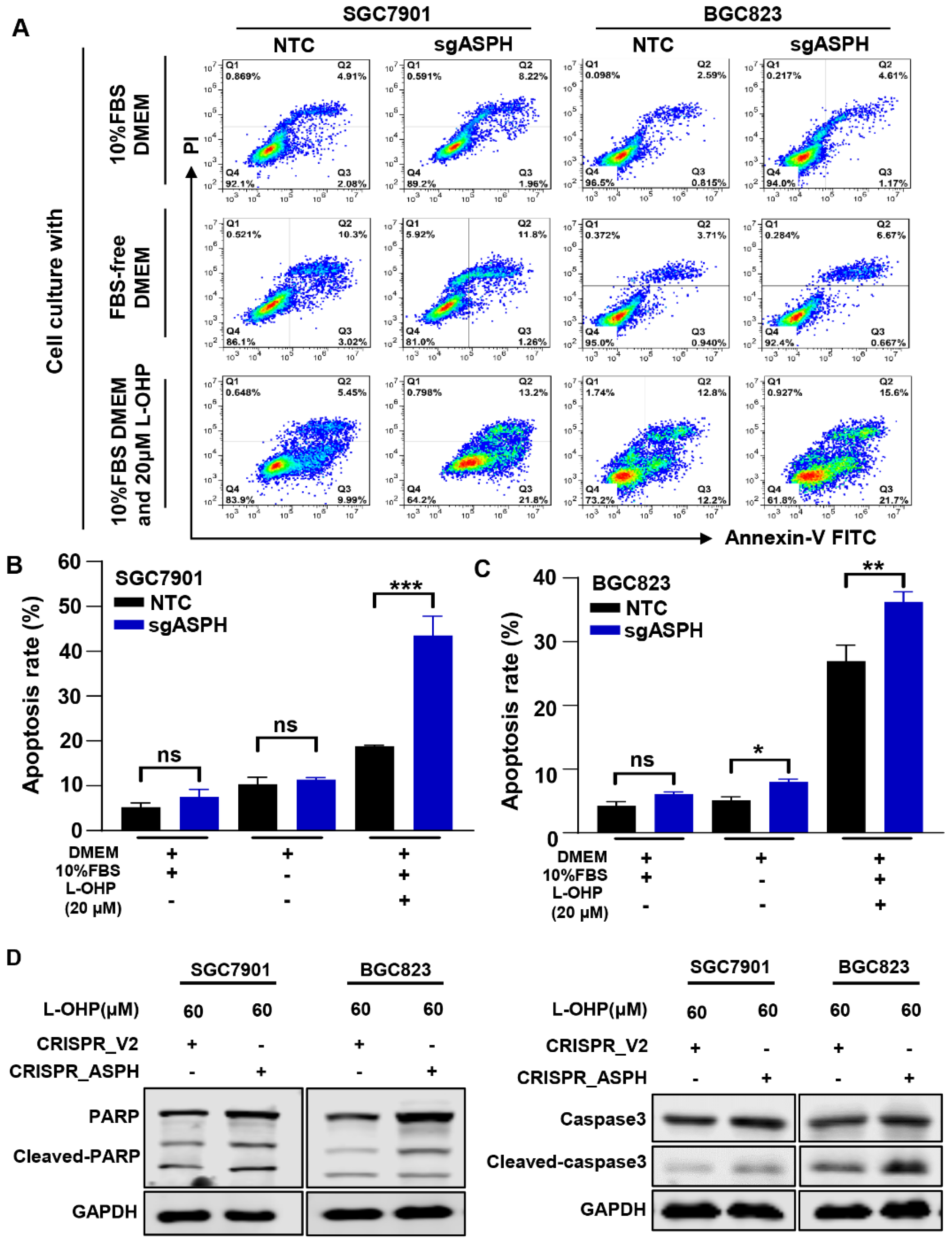
2.4. ASPH Knockout Increased the Sensitivity to L-OHP
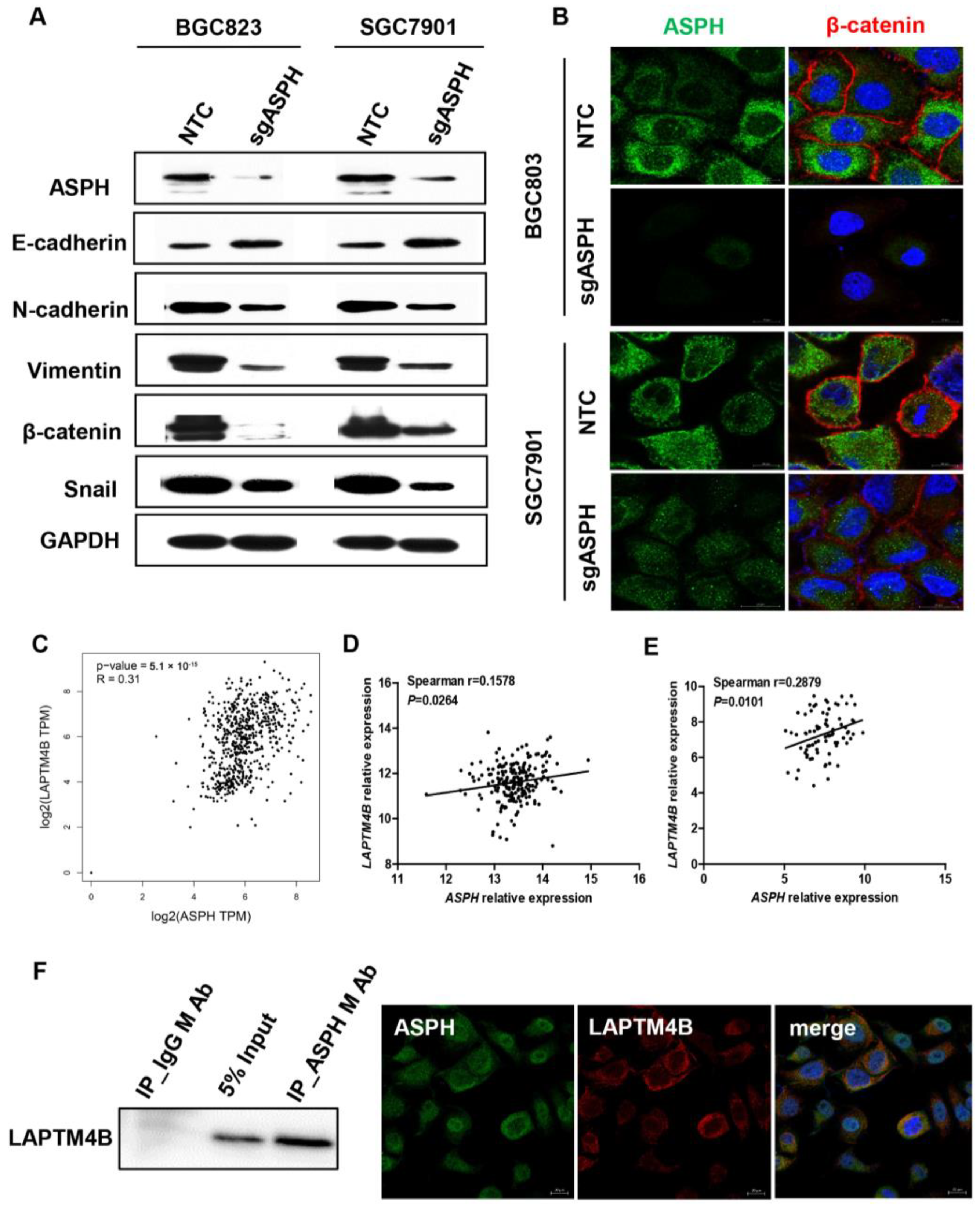
2.5. ASPH Mediated Malignant Phenotypes via Regulating EMT Process
3. Discussion
4. Materials and Methods
4.1. Clinical Specimen Collection
4.2. Immunohistochemistry
4.3. Cells and Cell Culture
4.4. RNA Isolation and RT-qPCR
4.5. Construction of ASPH Knockout Cell Lines by CRISPR-Cas9 Technology
4.6. Western Blotting
4.7. IncuCyte™ Cell Proliferation and Wound Healing Assays
4.8. EdU Assays
4.9. Trans-Well Assay
4.10. Annexin V/PI Assay
4.11. Immunofluorescence Staining
4.12. Xenograft Models
4.13. Co-Immunoprecipitation (Co-IP)
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eto, K.; Hiki, N.; Kumagai, K.; Shoji, Y.; Tsuda, Y.; Kano, Y.; Yasufuku, I.; Okumura, Y.; Tsujiura, M.; Ida, S.; et al. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer 2018, 21, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Qin, J.; Sun, Y.-H.; Liu, T.-S. Neoadjuvant chemotherapy for advanced gastric cancer: A meta-analysis. World J. Gastroenterol. 2010, 16, 5621–5628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Gao, P.; Song, Y.X.; Sun, J.X.; Chen, X.W.; Ma, B.; Yang, Y.C.; Wang, Z.N. Which is better for gastric cancer patients, perioperative or adjuvant chemotherapy: A meta-analysis. BMC Cancer 2016, 16, 631. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Shan, F.; Ying, X.; Zhang, Y.; Jian-Yu, E.; Wang, Y.; Ren, H.; Su, X.; Ji, J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg 2019, 154, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Nagaoka, K.; Bai, X.; Ogawa, K.; Dong, X.; Zhang, S.; Zhou, Y.; Carlson, R.I.; Jiang, Z.G.; Fuller, S.; Lebowitz, M.S.; et al. Anti-tumor activity of antibody drug conjugate targeting aspartate-β-hydroxylase in pancreatic ductal adenocarcinoma. Cancer Lett. 2019, 449, 87–98. [Google Scholar] [CrossRef]
- Aihara, A.; Huang, C.K.; Olsen, M.J.; Lin, Q.; Chung, W.; Tang, Q.; Dong, X.; Wands, J.R. A cell-surface β-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology 2014, 60, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Sturla, L.M.; Tong, M.; Hebda, N.; Gao, J.; Thomas, J.M.; Olsen, M.; Suzanne, M. Aspartate-β-hydroxylase (ASPH): A potential therapeutic target in human malignant gliomas. Heliyon 2016, 2, e00203. [Google Scholar] [CrossRef]
- Wang, J.; Suzanne, M.; Sabo, E.; Kethu, S.; Tavares, R.; Branda, M.; Simao, L.; Wands, J.R.; Resnick, M.B. Prognostic value of humbug gene overexpression in stage II colon cancer. Hum. Pathol. 2007, 38, 17–25. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Yan, Z.L.; Li, J.; Shi, L.H.; Cong, W.M.; Xia, Y.; Zou, Q.F.; Xi, T.; Shen, F.; et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology 2010, 52, 164–173. [Google Scholar] [CrossRef]
- Luu, M.; Sabo, E.; Suzanne, M.; Greaves, W.; Wang, J.; Tavares, R.; Simao, L.; Wands, J.R.; Resnick, M.B.; Wang, L. Prognostic value of aspartyl (asparaginyl)-beta-hydroxylase/humbug expression in non-small cell lung carcinoma. Hum. Pathol. 2009, 40, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Chen, X.; Meng, F.; Ogawa, K.; Li, M.; Song, R.; Zhang, S.; Zhang, Z.; Kong, X.; Xu, Q.; et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol. Cancer 2019, 18, 156. [Google Scholar] [CrossRef]
- Huyan, T.; Li, Q.; Ye, L.J.; Yang, H.; Xue, X.P.; Zhang, M.J.; Huang, Q.S.; Yin, D.C.; Shang, P. Inhibition of human natural killer cell functional activity by human aspartyl β-hydroxylase. Int. Immunopharmacol. 2014, 23, 452–459. [Google Scholar] [CrossRef]
- Shimoda, M.; Tomimaru, Y.; Charpentier, K.P.; Safran, H.; Carlson, R.I.; Wands, J. Tumor progression-related transmembrane protein aspartate-β-hydroxylase is a target for immunotherapy of hepatocellular carcinoma. J. Hepatol. 2012, 56, 1129–1135. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Lin, Q.; Li, L.; Bai, X.; Chen, X.; Chen, H.; Kong, R.; Wang, Y.; Zhu, H.; He, F.; et al. Aspartate β-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway. J. Hematol. Oncol. 2019, 12, 144. [Google Scholar] [CrossRef] [Green Version]
- Iwagami, Y.; Huang, C.K.; Olsen, M.J.; Thomas, J.M.; Jang, G.; Kim, M.; Lin, Q.; Carlson, R.I.; Wagner, C.E.; Dong, X.; et al. Aspartate β-hydroxylase modulates cellular senescence through glycogen synthase kinase 3β in hepatocellular carcinoma. Hepatology 2016, 63, 1213–1226. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Song, K.; Xue, T.; Xue, X.P.; Huyan, T.; Wang, W.; Wang, H. The distribution and expression profiles of human Aspartyl/Asparaginyl beta-hydroxylase in tumor cell lines and human tissues. Oncol. Rep. 2010, 24, 1257–1264. [Google Scholar]
- Lee, J.H. Overexpression of humbug promotes malignant progression in human gastric cancer cells. Oncol. Rep. 2008, 19, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. 5), v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Shao, G.Z.; Zhou, R.-L.; Zhang, Q.-Y.; Zhang, Y.; Liu, J.-J.; Rui, J.-A.; Wei, X.; Ye, D.-X. Molecular cloning and characterization of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma. Oncogene 2003, 22, 5060–5069. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wei, X.H.; Pan, Y.P.; Li, H.C.; Yang, H.; He, Q.H.; Pang, Y.; Shan, Y.; Xiong, F.X.; Shao, G.Z.; et al. LAPTM4B: A novel cancer-associated gene motivates multidrug resistance through efflux and activating PI3K/AKT signaling. Oncogene 2010, 29, 5785–5795. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xiong, F.; Wei, X.; Yang, H.; Zhou, R. LAPTM4B-35, a novel tetratransmembrane protein and its PPRP motif play critical roles in proliferation and metastatic potential of hepatocellular carcinoma cells. Cancer Sci. 2009, 100, 2335–2340. [Google Scholar] [CrossRef]
- Meng, F.; Chen, X.; Song, H.; Lou, G. LAPTM4B down regulation inhibits the proliferation, invasion and angiogenesis of HeLa cells in vitro. Cell Physiol. Biochem. 2015, 37, 890–900. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, F.; Wei, X.; Yang, Y.; McNutt, M.A.; Zhou, R. Overexpression of LAPTM4B-35 promotes growth and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2010, 294, 236–244. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Y.; Thapa, N.; Liao, Y.; Hedman, A.C.; Anderson, R.A. LAPTM4B is a PtdIns(4,5)P2 effector that regulates EGFR signaling, lysosomal sorting, and degradation. EMBO J. 2015, 34, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, M.; Smahel, M.; Olsen, M.; Smahelova, J.; Tachezy, R. Aspartate β-hydroxylase as a target for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 163. [Google Scholar] [CrossRef]
- Spirina, L.V.; Avgustinovich, A.V.; Bakina, O.V.; Afanas’Ev, S.G.; Volkov, M.Y.; Kebekbayeva, A.Y. LC3B, mTOR, AMPK Are Molecular Targets for Neoadjuvant Chemotherapy in Gastric Cancers. Curr. Issues Mol. Biol. 2022, 44, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
| Gastric Cancer | Gastric Cancer | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| Variables | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Age | 0.409 | |||||
| ≤60 vs. >60 | 1.297 | 0.699–2.404 | ||||
| Gender | 0.212 | |||||
| Male vs. Female | 1.578 | 0.771–3.227 | ||||
| Lymph node metastasis | 0.036 | 0.028 | ||||
| No vs. N1+2+3 | 2.404 | 1.060–5.451 | 2.597 | 1.108–6.091 | ||
| Depth of invasion | 0.775 | |||||
| T1+2 vs. T3+4 | 1.146 | 0.449–2.927 | ||||
| Distant metastasis | 0.010 | 0.019 | ||||
| M0 vs. M1 | 2.810 | 1.280–6.168 | 2.670 | 1.178–6.052 | ||
| Differentiation | 0.128 | |||||
| Poor vs. Moderate + Well | 0.620 | 0.335–1.147 | ||||
| Gross type | 0.988 | |||||
| Ulcerative type vs. others | 0.994 | 0.436–2.263 | ||||
| Histology | 0.649 | |||||
| Adenocarcinoma vs. others | 1.272 | 0.452–3.578 | ||||
| Tumor size | 0.796 | |||||
| ≤5.0 cm vs. >5.0 cm | 1.039 | 0.539–2.004 | ||||
| Location | 0.272 | |||||
| Gastric vs. Cardiac + GEJ | 1.399 | 0.768–2.547 | ||||
| Lauren | 0.120 | |||||
| Intestinal vs. Diffused | 1.711 | 0.767–3.815 | 0.189 | |||
| Intestinal vs. Mixed | 2.738 | 0.924–8.112 | 0.069 | |||
| ASPH | 0.006 | 0.002 | ||||
| negative vs. positive | 2.381 | 1.275–4.445 | 2.804 | 1.468–5.356 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, X.; Li, S.; Wang, Y.; Du, H.; Hu, Y.; Xing, X.; Cheng, X.; Yan, Y.; Li, Z. Aspartate β-Hydroxylase Serves as a Prognostic Biomarker for Neoadjuvant Chemotherapy in Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 5482. https://doi.org/10.3390/ijms24065482
Gan X, Li S, Wang Y, Du H, Hu Y, Xing X, Cheng X, Yan Y, Li Z. Aspartate β-Hydroxylase Serves as a Prognostic Biomarker for Neoadjuvant Chemotherapy in Gastric Cancer. International Journal of Molecular Sciences. 2023; 24(6):5482. https://doi.org/10.3390/ijms24065482
Chicago/Turabian StyleGan, Xuejun, Shen Li, Yiding Wang, Hong Du, Ying Hu, Xiaofang Xing, Xiaojing Cheng, Yan Yan, and Ziyu Li. 2023. "Aspartate β-Hydroxylase Serves as a Prognostic Biomarker for Neoadjuvant Chemotherapy in Gastric Cancer" International Journal of Molecular Sciences 24, no. 6: 5482. https://doi.org/10.3390/ijms24065482
APA StyleGan, X., Li, S., Wang, Y., Du, H., Hu, Y., Xing, X., Cheng, X., Yan, Y., & Li, Z. (2023). Aspartate β-Hydroxylase Serves as a Prognostic Biomarker for Neoadjuvant Chemotherapy in Gastric Cancer. International Journal of Molecular Sciences, 24(6), 5482. https://doi.org/10.3390/ijms24065482






