Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Cell Death and Cell Viability Assay
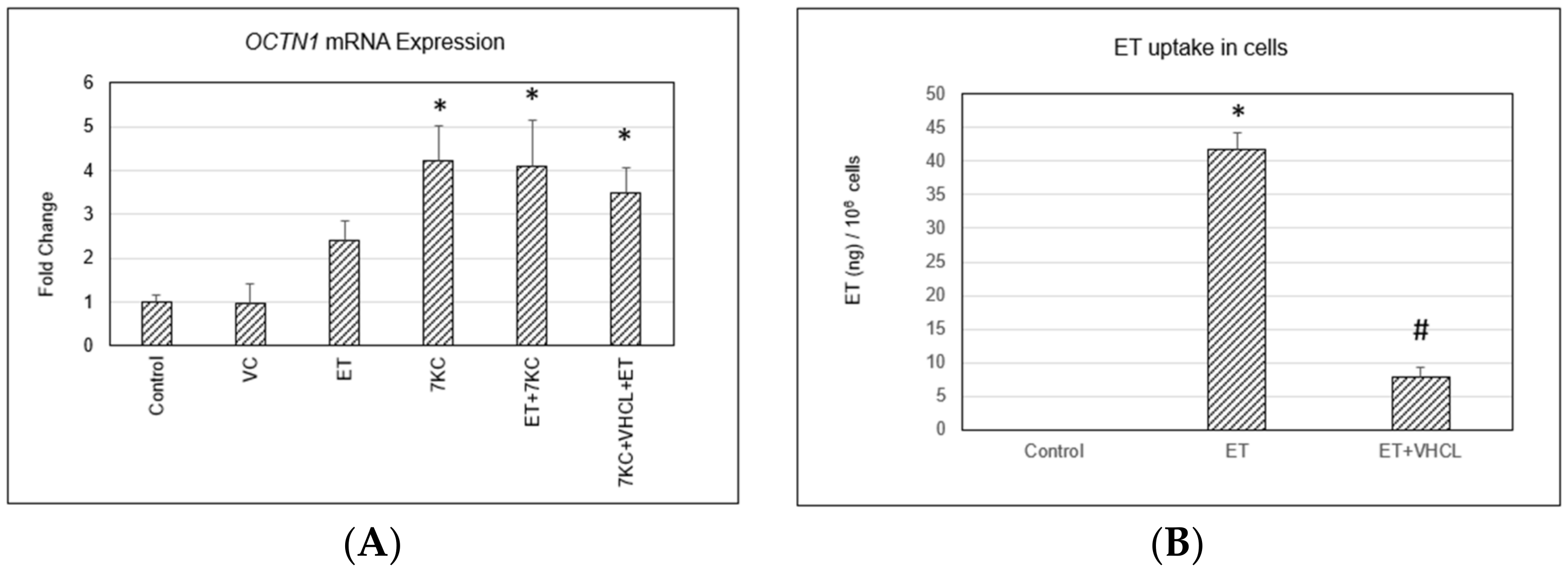
2.2. OCTN1 Gene (SLC22A4) Expression and ET Uptake in Cells
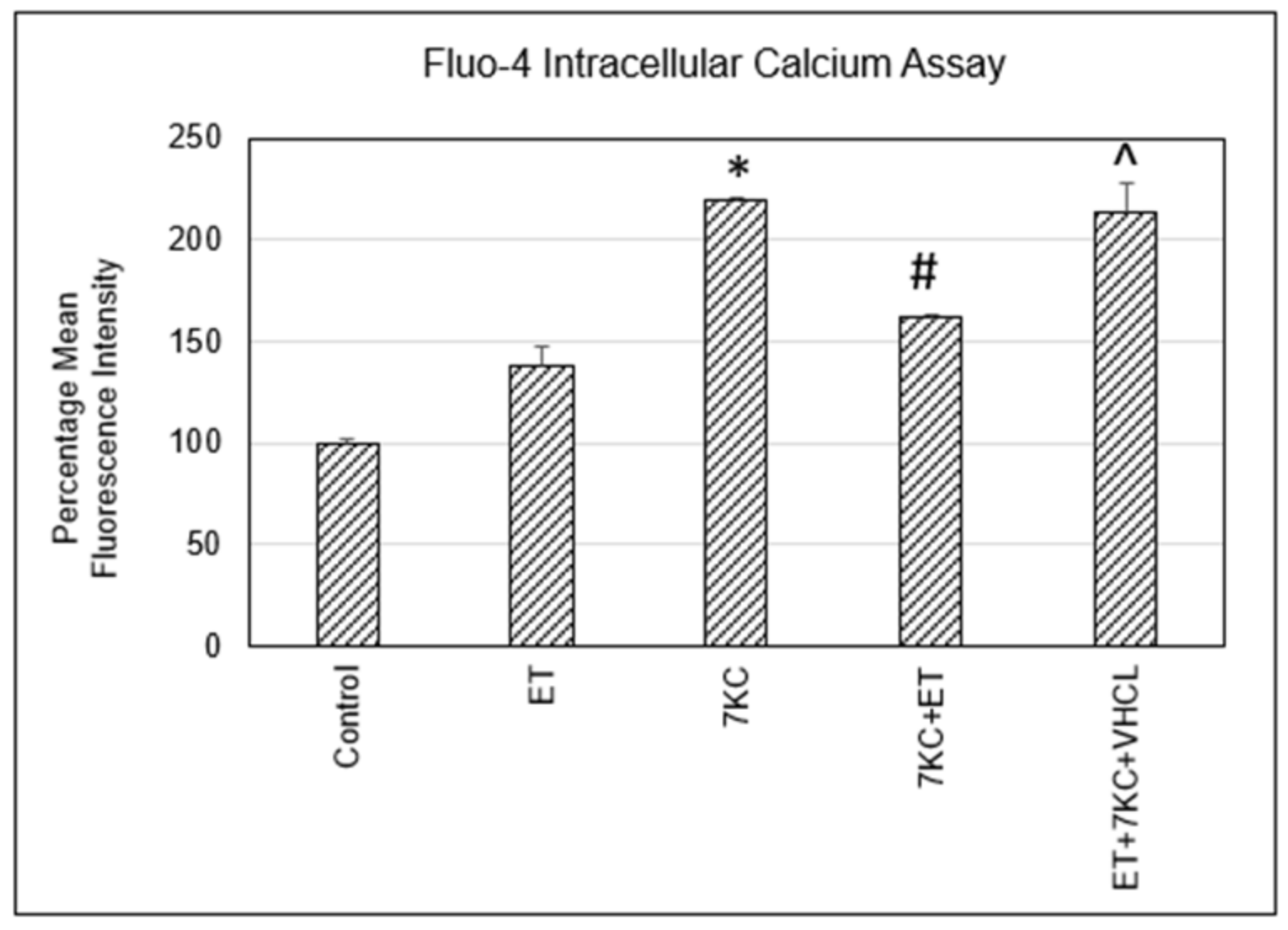
2.3. Intracellular Free Calcium Levels
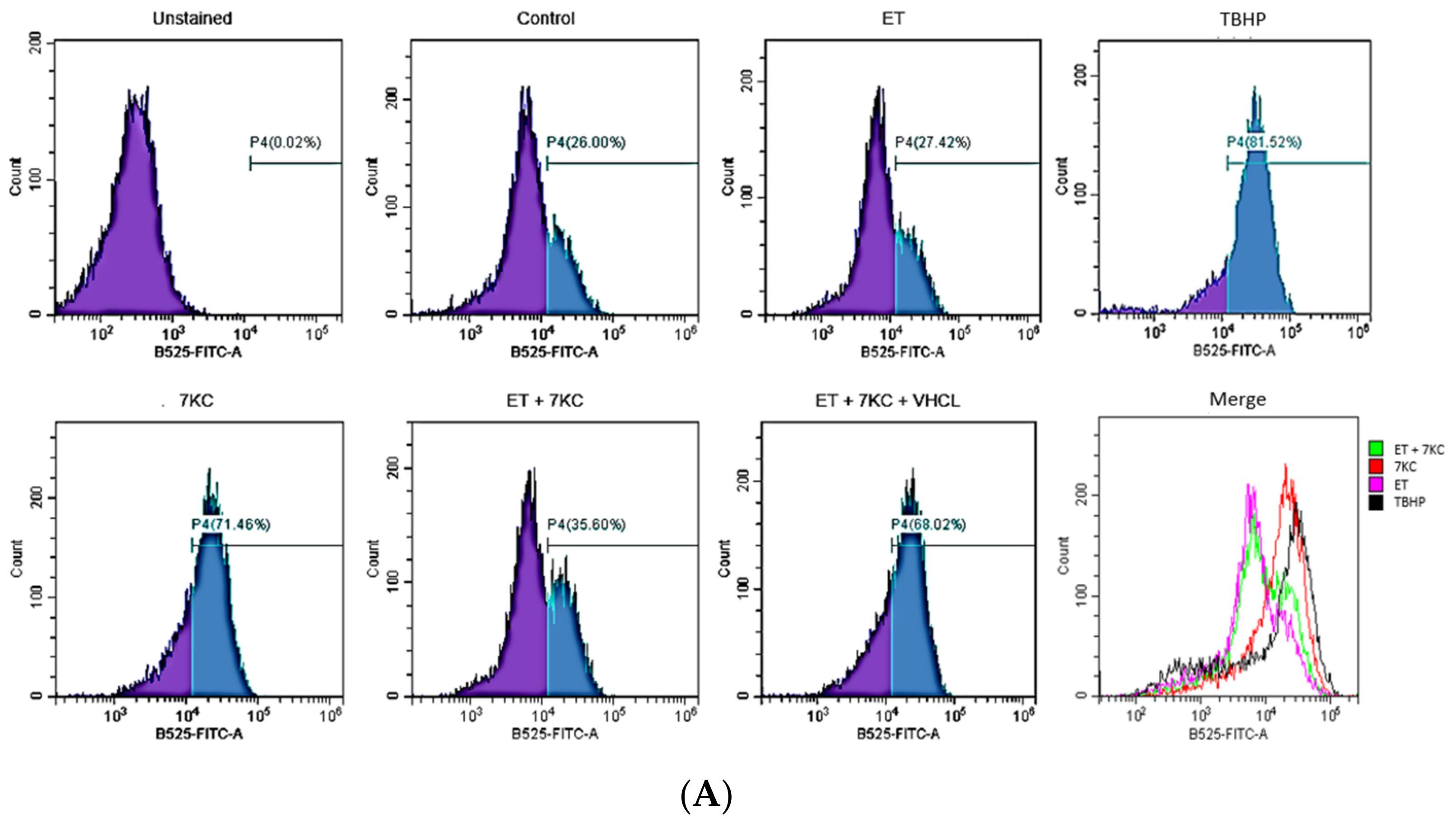
2.4. Overall ROS Production
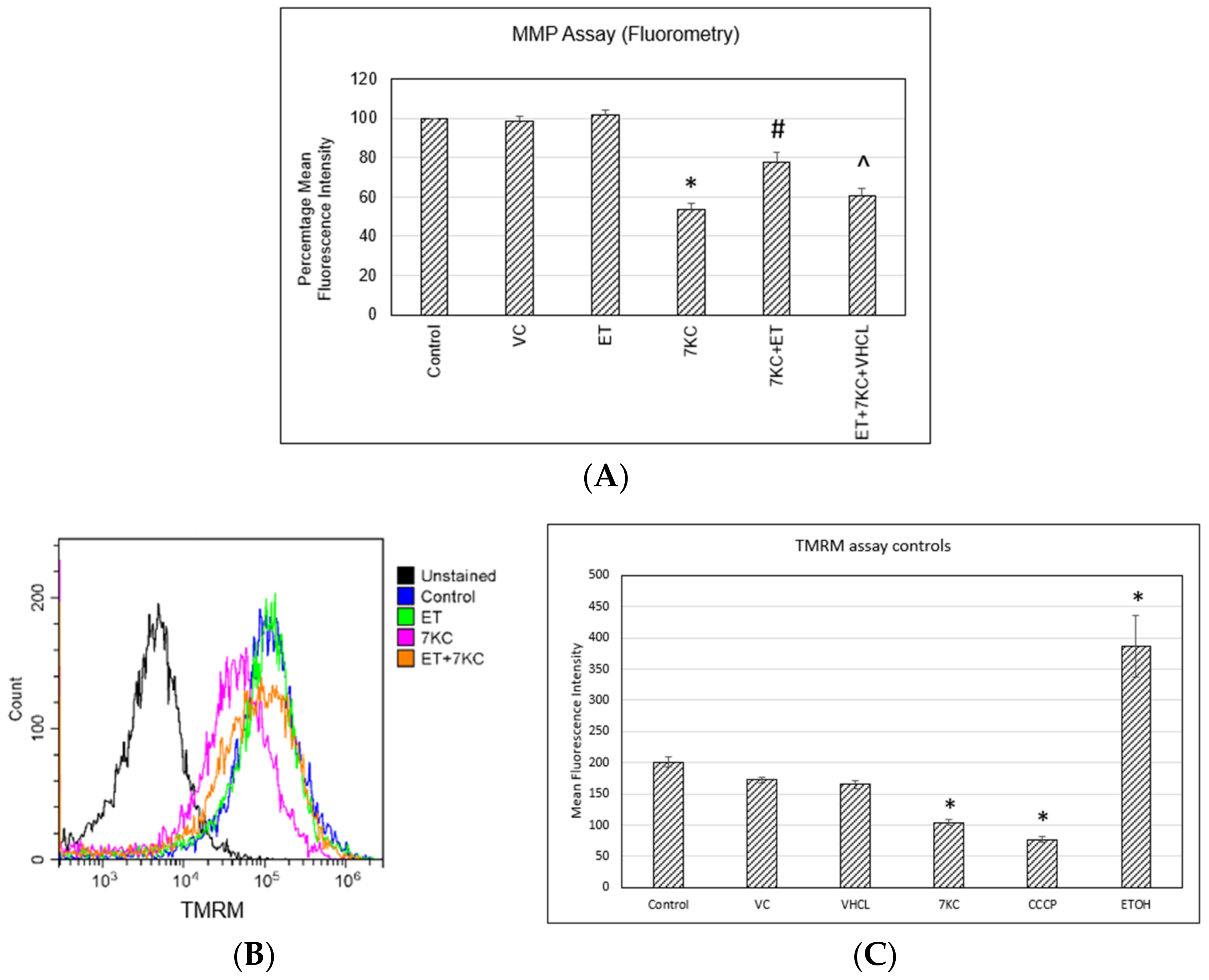
2.5. Mitochondrial Membrane Potential
2.6. ATP Levels
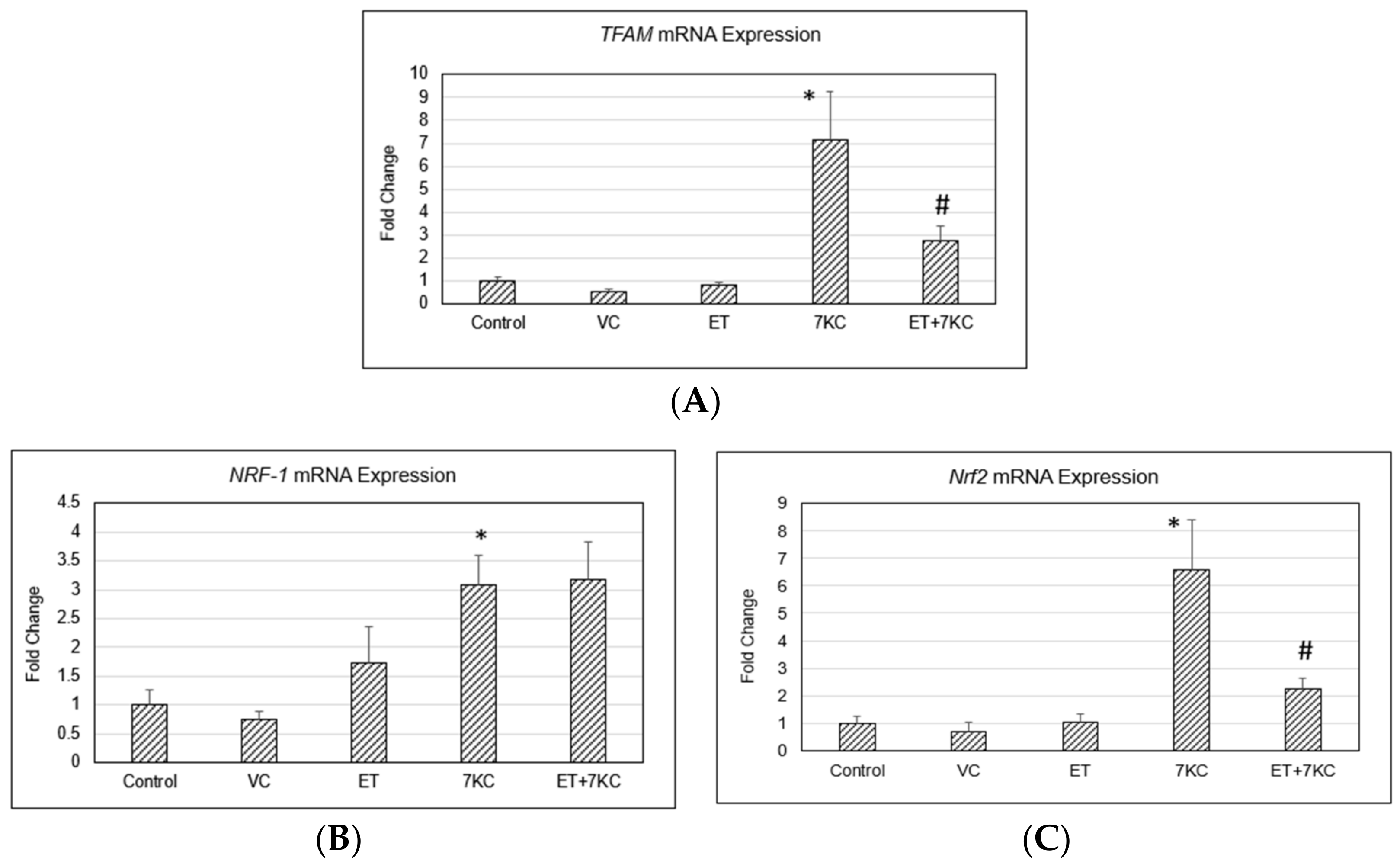
2.7. TFAM, NRF-1 and Nrf2 Gene Expression
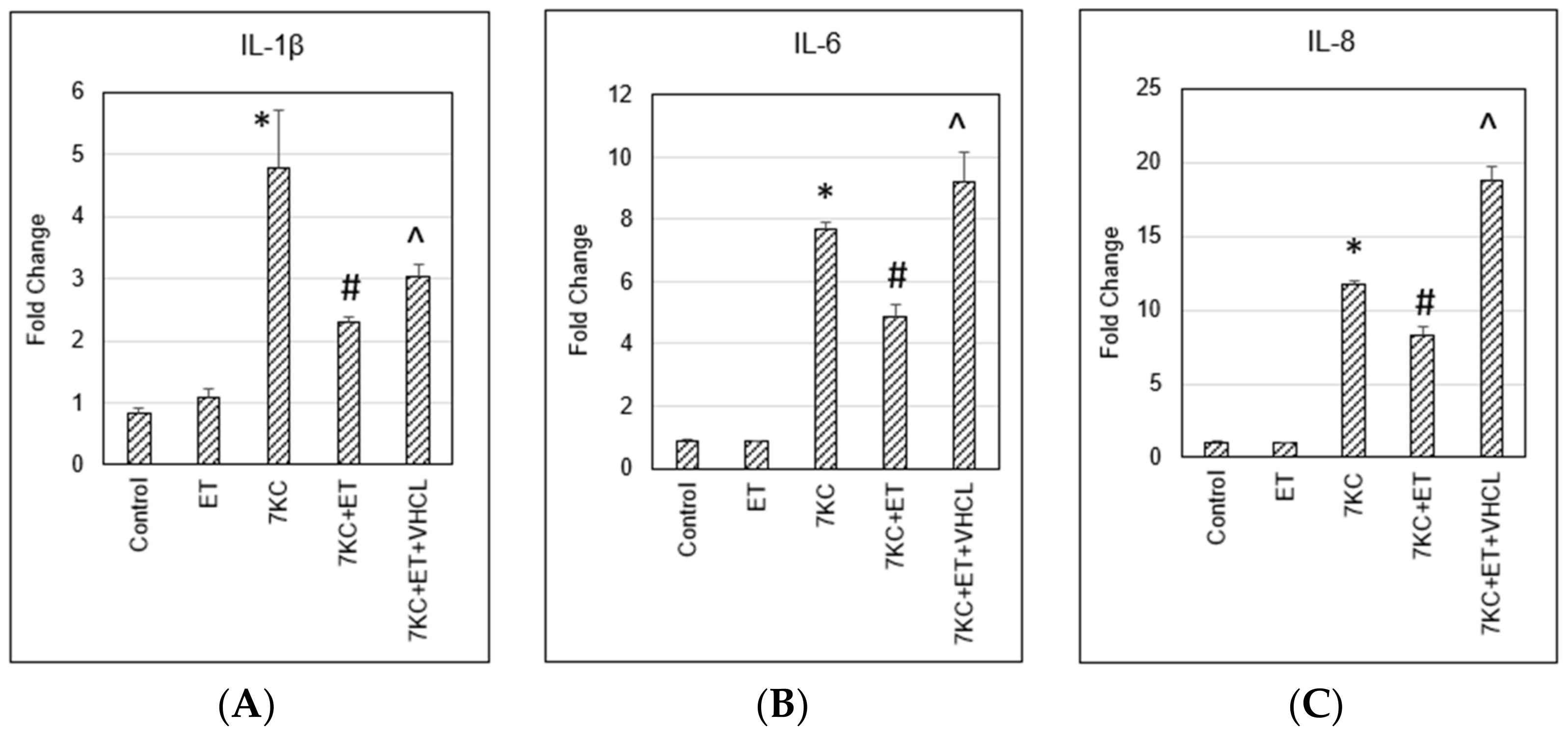
2.8. Pro-Inflammatory Gene Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Trypan Blue Cell Viability Assay
4.4. MTS Assay
4.5. Cellular ET Uptake and Liquid Chromatography Mass Spectrometry
4.6. Measurement of Intracellular Free Calcium, Mitochondrial Membrane Potential, and Cellular/Mitochondrial Reactive Oxygen Species (ROS)
4.7. ATP Assay
4.8. Quantitative RT-PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyons, M.A.; Brown, A.J. 7-Ketocholesterol. Int. J. Biochem. Cell Biol. 1999, 31, 369–375. [Google Scholar]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Jerome, W.G.; O’Connor, M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2019, 29, 101380. [Google Scholar] [CrossRef]
- Ravi, S.; Duraisamy, P.; Krishnan, M.; Martin, L.C.; Manikandan, B.; Raman, T.; Sundaram, J.; Arumugam, M.; Ramar, M. An insight on 7- ketocholesterol mediated inflammation in atherosclerosis and potential therapeutics. Steroids 2021, 172, 108854. [Google Scholar] [CrossRef]
- Larrayoz, I.M.; Huang, J.-D.; Lee, J.W.; Pascual, I.; Rodríguez, I.R. 7-Ketocholesterol–Induced Inflammation: Involvement of Multiple Kinase Signaling Pathways via NF-κB but Independently of Reactive Oxygen Species Formation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4942–4955. [Google Scholar] [CrossRef] [Green Version]
- Ong, W.-Y.; Goh, E.W.-S.; Lu, X.-R.; Farooqui, A.A.; Patel, S.C.; Halliwell, B. Increase in Cholesterol and Cholesterol Oxidation Products, and Role of Cholesterol Oxidation Products in Kainate-induced Neuronal Injury. Brain Pathol. 2006, 13, 250–262. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Kim, J.-H.; He, X.; Chen, P.; Farooqui, A.A.; Jenner, A.M. Changes in Brain Cholesterol Metabolome After Excitotoxicity. Mol. Neurobiol. 2010, 41, 299–313. [Google Scholar] [CrossRef]
- Gosselet, F.; Saint-Pol, J.; Fenart, L. Effects of oxysterols on the blood–brain barrier: Implications for Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2014, 446, 687–691. [Google Scholar] [CrossRef]
- Saeed, A.A.; Genové, G.; Li, T.; Lütjohann, D.; Olin, M.; Mast, N.; Pikuleva, I.A.; Crick, P.; Wang, Y.; Griffiths, W.; et al. Effects of a Disrupted Blood-Brain Barrier on Cholesterol Homeostasis in the Brain. J. Biol. Chem. 2014, 289, 23712–23722. [Google Scholar] [CrossRef] [Green Version]
- Samadi, A.; Gurlek, A.; Sendur, S.N.; Karahan, S.; Akbiyik, F.; Lay, I. Oxysterol species: Reliable markers of oxidative stress in diabetes mellitus. J. Endocrinol. Investig. 2018, 42, 7–17. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Halliday, G.M.; et al. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 2011, 6, e17299. [Google Scholar]
- Endo, K.; Oyama, T.; Saiki, A.; Ban, N.; Ohira, M.; Koide, N.; Murano, T.; Watanabe, H.; Nishii, M.; Miura, M.; et al. Determination of serum 7-ketocholesterol concentrations and their relationships with coronary multiple risks in diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 80, 63–68. [Google Scholar] [CrossRef]
- Lizard, G.; Monier, S.; Cordelet, C.; Gesquiere, L.; Deckert, V.; Gueldry, S.; Lagrost, L.; Gambert, P. Characterization and comparison of the mode of cell death, apoptosis versus necrosis, induced by 7beta-hydroxycholesterol and 7-ketocholesterol in the cells of the vascular wall. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1190–1200. [Google Scholar]
- Ma, M.-T.; Zhang, J.; Farooqui, A.A.; Chen, P.; Ong, W.-Y. Effects of cholesterol oxidation products on exocytosis. Neurosci. Lett. 2010, 476, 36–41. [Google Scholar] [CrossRef]
- Yammine, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Greige-Gerges, H.; Auezova, L.; Lizard, G. Prevention of 7-Ketocholesterol-Induced Overproduction of Reactive Oxygen Species, Mitochondrial Dysfunction and Cell Death with Major Nutrients (Polyphenols, ω3 and ω9 Unsaturated Fatty Acids) of the Mediterranean Diet on N2a Neuronal Cells. Molecules 2020, 25, 2296. [Google Scholar]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529 Pt 1, 57–68. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 784–793. [Google Scholar]
- Cheah, I.K.; Feng, L.; Tang, R.M.; Lim, K.H.; Halliwell, B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar]
- Wu, L.-Y.; Kan, C.N.; Cheah, I.K.; Chong, J.R.; Xu, X.; Vrooman, H.; Hilal, S.; Venketasubramanian, N.; Chen, C.P.; Halliwell, B.; et al. Low Plasma Ergothioneine Predicts Cognitive and Functional Decline in an Elderly Cohort Attending Memory Clinics. Antioxidants 2022, 11, 1717. [Google Scholar]
- Li, R.W.; Yang, C.; Sit, A.S.; Kwan, Y.W.; Lee, S.M.; Hoi, M.P.; Chan, S.-W.; Hausman, M.; Vanhoutte, P.M.; Leung, G.P.H. Uptake and protective effects of ergothioneine in human endothelial cells. J. Pharmacol. Exp. Ther. 2014, 350, 691–700. [Google Scholar]
- Servillo, L.; D’Onofrio, N.; Casale, R.; Cautela, D.; Giovane, A.; Castaldo, D.; Balestrieri, M.L. Ergothioneine products derived by superoxide oxidation in endothelial cells exposed to high-glucose. Free Radic. Biol. Med. 2017, 108, 8–18. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Giovane, A.; Casale, R.; Vitiello, M.; Marfella, R.; Paolisso, G.; Balestrieri, M.L. Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: Involvement of SIRT1 and SIRT6. Free Radic. Biol. Med. 2016, 96, 211–222. [Google Scholar] [CrossRef]
- Martin, K.R. The Bioactive Agent Ergothioneine, a Key Component of Dietary Mushrooms, Inhibits Monocyte Binding to Endothelial Cells Characteristic of Early Cardiovascular Disease. J. Med. Food 2010, 13, 1340–1346. [Google Scholar] [CrossRef]
- Koh, S.S.; Ooi, S.C.-Y.; Lui, N.M.-Y.; Qiong, C.; Ho, L.T.-Y.; Cheah, I.K.-M.; Halliwell, B.; Herr, D.R.; Ong, W.-Y. Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury. NeuroMol. Med. 2020, 23, 184–198. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Mitochondrial Transcription Factor A Induction by Redox Activation of Nuclear Respiratory Factor 1. J. Biol. Chem. 2006, 281, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Weksler, B.; A Romero, I.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.C.; Sonninen, T.M.; Peltonen, S.; Koistinaho, J.; Lehtonen, Š. Blood-Brain Barrier and Neurodegenerative Diseases-Modeling with iPSC-Derived Brain Cells. Int. J. Mol. Sci. 2021, 22, 7710. [Google Scholar]
- Tai, L.M.; Holloway, K.A.; Male, D.K.; Loughlin, A.J.; Romero, I. Amyloid-beta-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation. J. Cell. Mol. Med. 2010, 14, 1101–1112. [Google Scholar]
- Qosa, H.; LeVine, H.; Keller, J.N.; Kaddoumi, A. Mixed oligomers and monomeric amyloid-β disrupts endothelial cells integrity and reduces monomeric amyloid-β transport across hCMEC/D3 cell line as an in vitro blood–brain barrier model. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Mursaleen, L.; Noble, B.; Somavarapu, S.; Zariwala, M. Micellar Nanocarriers of Hydroxytyrosol Are Protective against Parkinson’s Related Oxidative Stress in an In Vitro hCMEC/D3-SH-SY5Y Co-Culture System. Antioxidants 2021, 10, 887. [Google Scholar] [CrossRef]
- Kuan, W.-L.; Bennett, N.; He, X.; Skepper, J.N.; Martynyuk, N.; Wijeyekoon, R.; Moghe, P.V.; Williams-Gray, C.H.; Barker, R.A. α-Synuclein pre-formed fibrils impair tight junction protein expression without affecting cerebral endothelial cell function. Exp. Neurol. 2016, 285, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar]
- Halliwell, B.; Cheah, I.K.; Tang, R.M. Diet-Derived Antioxidants: The Special Case of Ergothioneine. Annu. Rev. Food Sci. Technol. 2023, 14, 3357–3366. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.; Ye, P.; Yew, T.S.; Lim, K.H.; Halliwell, B. Liver ergothioneine accumulation in a guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic. Res. 2016, 50, 14–25. [Google Scholar]
- Shinozaki, Y.; Furuichi, K.; Toyama, T.; Kitajima, S.; Hara, A.; Iwata, Y.; Sakai, N.; Shimizu, M.; Kaneko, S.; Isozumi, N.; et al. Impairment of the carnitine/organic cation transporter 1–ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1356–1369. [Google Scholar] [CrossRef]
- Kruman, I.; Guo, Q.; Mattson, M.P. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J. Neurosci. Res. 1998, 51, 293–308. [Google Scholar] [CrossRef]
- Tombal, B.; Denmeade, S.; Isaacs, J. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium 1999, 25, 19–28. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Zhou, Q.; Wasowicz, E.; Handler, B.; Fleischer, L.; A Kummerow, F. An excess concentration of oxysterols in the plasma is cytotoxic to cultured endothelial cells. Atherosclerosis 2000, 149, 191–197. [Google Scholar] [CrossRef]
- Naito, Y.; Shimozawa, M.; Manabe, H.; Nakabe, N.; Katada, K.; Kokura, S.; Yoshida, N.; Ichikawa, H.; Kon, T.; Yoshikawa, T. Azelnidipine, a new calcium channel blocker, inhibits endothelial inflammatory response by reducing intracellular levels of reactive oxygen species. Eur. J. Pharmacol. 2006, 546, 11–18. [Google Scholar] [CrossRef]
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef]
- Akanmu, D.; Cecchini, R.; Aruoma, O.I.; Halliwell, B. The antioxidant action of ergothioneine. Arch. Biochem. Biophys. 1991, 288, 10–16. [Google Scholar] [CrossRef]
- Motohashi, N.; Mori, I.; Sugiura, Y.; Tanaka, H. Metal Complexes of Ergothioneine. Chem. Pharm. Bull. 1974, 22, 654–657. [Google Scholar]
- Zhu, B.-Z.; Mao, L.; Fan, R.-M.; Zhu, J.-G.; Zhang, Y.-N.; Wang, J.; Kalyanaraman, B.; Frei, B. Ergothioneine Prevents Copper-Induced Oxidative Damage to DNA and Protein by Forming a Redox-Inactive Ergothioneine−Copper Complex. Chem. Res. Toxicol. 2010, 24, 30–34. [Google Scholar] [CrossRef]
- Ruef, P.; Ehrhard, M.; Frommhold, D.; Koch, L.; Fritzsching, B.; Poeschl, J. Lipid A decreases human erythrocytes deformability by increasing intracellular Ca2+: Effects of verapamil, staurosporine and the rho-kinase inhibitor Y-27632. Clin. Hemorheol. Microcirc. 2011, 49, 315–322. [Google Scholar]
- Gramajo, A.L.; Zacharias, L.; Neekhra, A.; Luthra, S.; Atilano, S.; Chwa, M.; Brown, D.J.; Kuppermann, B.D.; Kenney, M.C. Mitochondrial DNA Damage Induced by 7-Ketocholesterol in Human Retinal Pigment Epithelial Cells In Vitro. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1164–1170. [Google Scholar] [CrossRef]
- Aon, M.; Cortassa, S.; O’Rourke, B. Redox-optimized ROS balance: A unifying hypothesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Leoni, V.; Nury, T.; Vejux, A.; Zarrouk, A.; Caccia, C.; Debbabi, M.; Fromont, A.; Sghaier, R.; Moreau, T.; Lizard, G. Mitochondrial dysfunctions in 7-ketocholesterol-treated 158N oligodendrocytes without or with α-tocopherol: Impacts on the cellular profil of tricarboxylic cycle-associated organic acids, long chain saturated and unsaturated fatty acids, oxysterols, cholesterol and cholesterol precursors. J. Steroid Biochem. Mol. Biol. 2017, 169, 96–110. [Google Scholar]
- Fjell, A.M.; McEvoy, L.; Holland, D.; Dale, A.M.; Walhovd, K.B.; Alzheimer’s Disease Neuroimaging Initiative. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 2014, 117, 20–40. [Google Scholar]
- Blonz, E.R. Alzheimer’s Disease as the Product of a Progressive Energy Deficiency Syndrome in the Central Nervous System: The Neuroenergetic Hypothesis. J. Alzheimer’s Dis. 2017, 60, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Błaszczyk, J.W. The Emerging Role of Energy Metabolism and Neuroprotective Strategies in Parkinson’s Disease. Front. Aging Neurosci 2018, 10, 301. [Google Scholar]
- Rho, M.-C.; Kim, Y.K.; Chang, J.S.; Baek, J.A.; Chung, M.Y.; Lee, H.; Rhim, B.Y.; Reidy, M.A. 7-Ketocholesterol predisposes human aorta smooth muscle cells to Fas-mediated death. J. Mol. Cell. Cardiol. 2005, 39, 823–832. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, M.; Guo, Z.; Brisbon, W.; Hoover, R.; Yang, H. Different Cytotoxic Injuries Induced by Lysophosphatidylcholine and 7-Ketocholesterol in Mouse Endothelial Cells. Endothelium 2006, 13, 213–226. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, D.; Wang, S.; Chai, L.Y.A.; Xu, S.; Lam, K.-P. Glycolysis and Oxidative Phosphorylation Play Critical Roles in Natural Killer Cell Receptor-Mediated Natural Killer Cell Functions. Front. Immunol. 2020, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Miranda, S.; Foncea, R.; Guerrero, J.; Leighton, F. Oxidative Stress and Upregulation of Mitochondrial Biogenesis Genes in Mitochondrial DNA-Depleted HeLa Cells. Biochem. Biophys. Res. Commun. 1999, 258, 44–49. [Google Scholar] [CrossRef]
- Koh, J.-H.; Kim, Y.-W.; Seo, D.-Y.; Sohn, T.-S. Mitochondrial TFAM as a Signaling Regulator between Cellular Organelles: A Perspective on Metabolic Diseases. Diabetes Metab. J. 2021, 45, 853–865. [Google Scholar] [CrossRef]
- Yu, J.-W.; Lee, M.-S. Mitochondria and the NLRP3 inflammasome: Physiological and pathological relevance. Arch. Pharm. Res. 2016, 39, 1503–1518. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef]
- Koka, S.; Xia, M.; Chen, Y.; Bhat, O.M.; Yuan, X.; Boini, K.M.; Li, P.-L. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 2017, 13, 336–344. [Google Scholar] [CrossRef]
- Yuan, X.; Bhat, O.M.; Meng, N.; Lohner, H.; Li, P.-L. Protective Role of Autophagy in Nlrp3 Inflammasome Activation and Medial Thickening of Mouse Coronary Arteries. Am. J. Pathol. 2018, 188, 2948–2959. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Bhat, O.M.; Samidurai, A.; Das, A.; Zhang, Y.; Li, P.-L. Reversal of Endothelial Extracellular Vesicle-Induced Smooth Muscle Phenotype Transition by Hypercholesterolemia Stimulation: Role of NLRP3 Inflammasome Activation. Front. Cell Dev. Biol. 2020, 8, 597423. [Google Scholar] [CrossRef]
- Weksler, B.B.; Subileau, E.A.; Perriere, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar]
- Badreddine, A.; Zarrouk, A.; Meddeb, W.; Nury, T.; Rezig, L.; Debbabi, M.; Bessam, F.Z.; Brahmi, F.; Vejux, A.; Mejri, M.; et al. Antioxidant and neuroprotective properties of Mediterranean oils: Argan oil, olive oil, and milk thistle seed oil. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 10; pp. 143–154. [Google Scholar]
- Suwalsky, M.; Muñoz, M.; Mennickent, S.; Sotomayor, C.P.; Bolognin, S.; Zatta, P. Structural Effects Of Verapamil On Cell Membranes And Molecular Models. J. Chil. Chem. Soc. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Yang, N.-C. A Convenient One-Step Extraction of Cellular ATP Using Boiling Water for the Luciferin–Luciferase Assay of ATP. Anal. Biochem. 2002, 306, 323–327. [Google Scholar]
- Ong, W.-Y.; Kao, M.-H.; Cheung, W.-M.; Leow, D.M.-K.; Cheah, I.K.-M.; Lin, T.-N. Protective Effect of Ergothioneine Against Stroke in Rodent Models. NeuroMol. Med. 2022. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leow, D.M.-K.; Cheah, I.K.-M.; Fong, Z.W.-J.; Halliwell, B.; Ong, W.-Y. Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 5498. https://doi.org/10.3390/ijms24065498
Leow DM-K, Cheah IK-M, Fong ZW-J, Halliwell B, Ong W-Y. Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(6):5498. https://doi.org/10.3390/ijms24065498
Chicago/Turabian StyleLeow, Damien Meng-Kiat, Irwin Kee-Mun Cheah, Zachary Wei-Jie Fong, Barry Halliwell, and Wei-Yi Ong. 2023. "Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells" International Journal of Molecular Sciences 24, no. 6: 5498. https://doi.org/10.3390/ijms24065498
APA StyleLeow, D. M.-K., Cheah, I. K.-M., Fong, Z. W.-J., Halliwell, B., & Ong, W.-Y. (2023). Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. International Journal of Molecular Sciences, 24(6), 5498. https://doi.org/10.3390/ijms24065498




