Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study
Abstract
:1. Introduction
2. Results
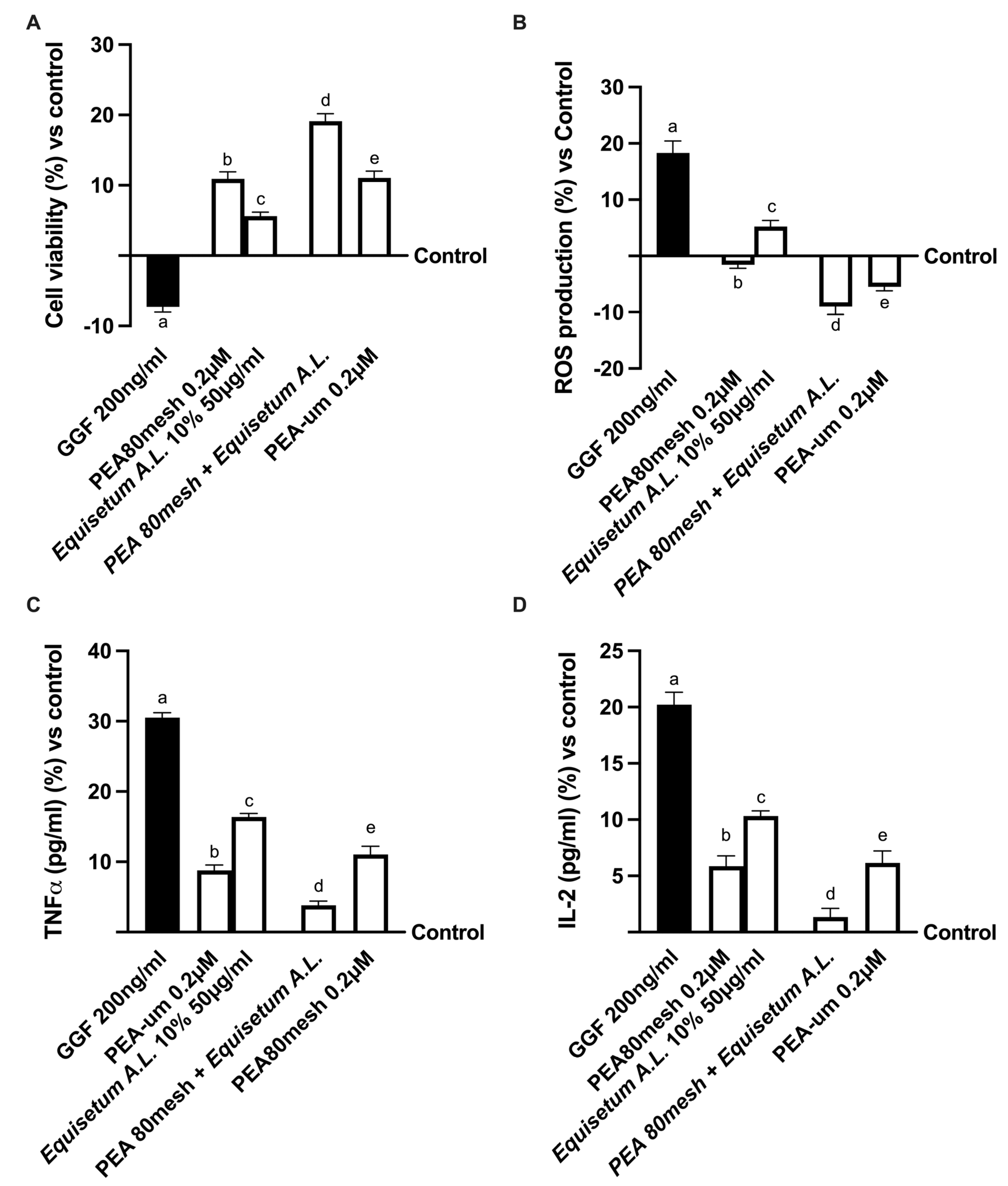
2.1. Safety Analysis of Different Concentrations of PEA and Equisetum on CaCo-2 Cells
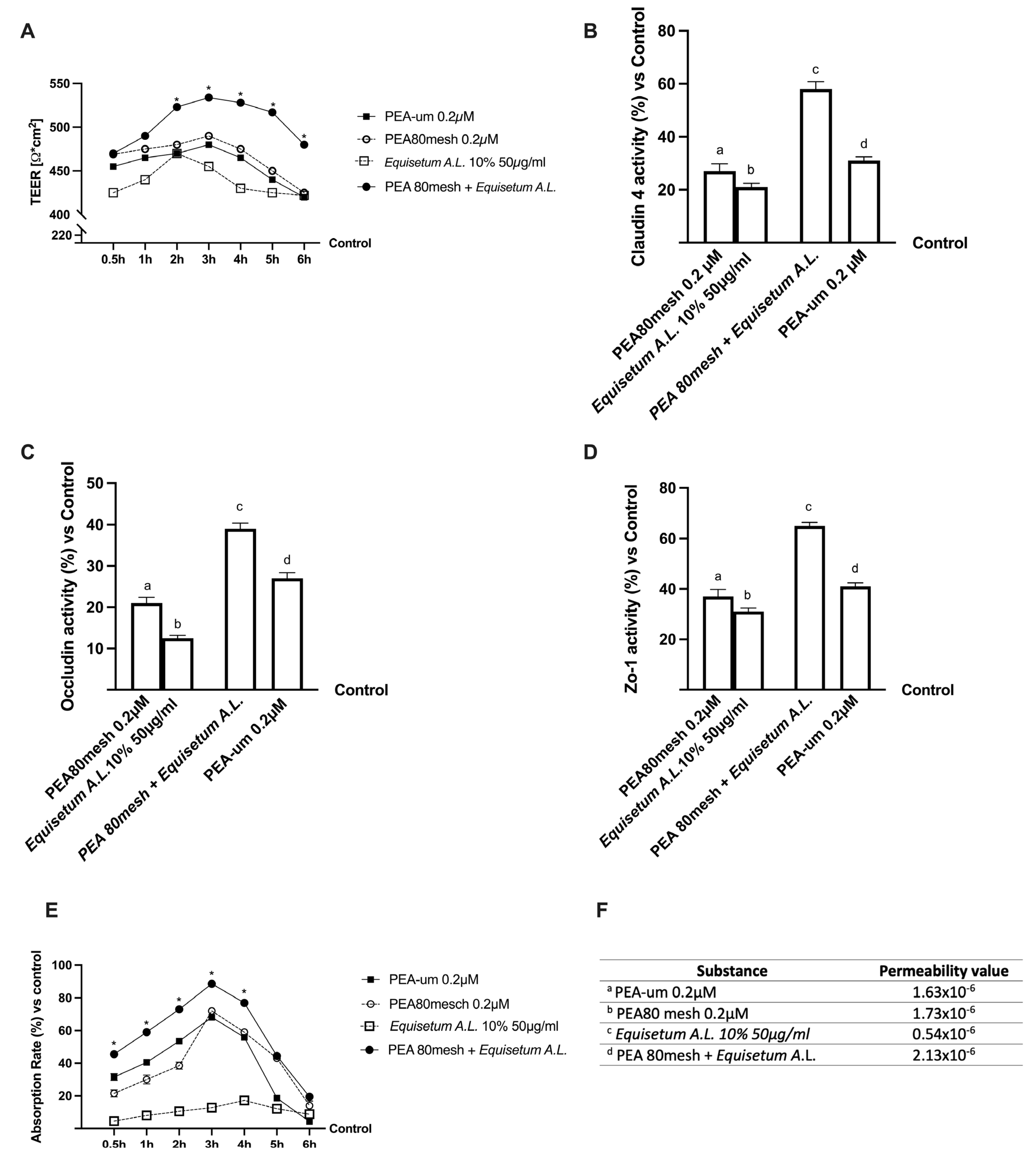
2.2. Permeability and Absorption Mechanisms Analyzed in an In Vitro Intestinal Barrier Model
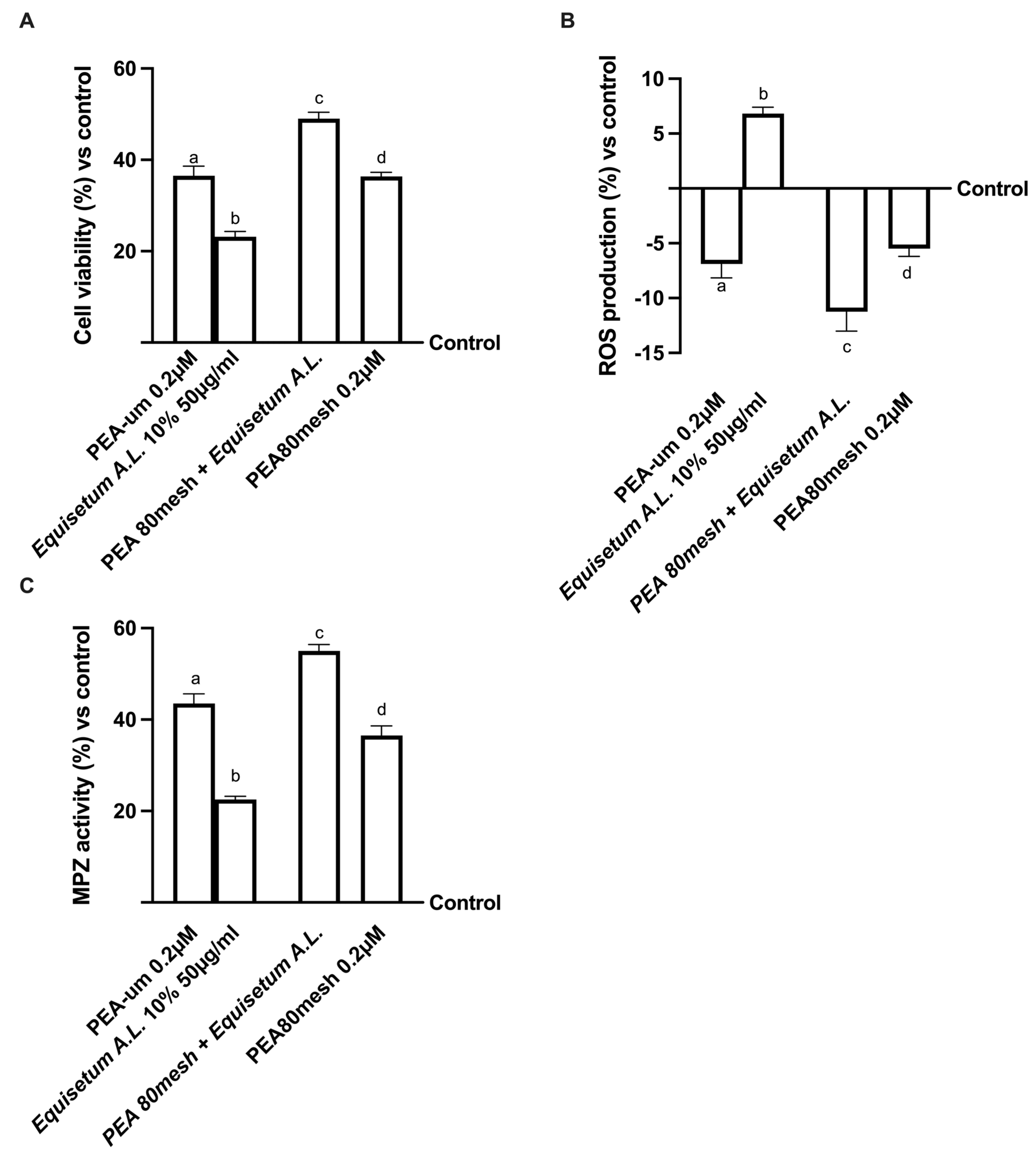
2.3. Effect of the Combination of PEA 80Mesh and Equisetum arvense on 3D EngNT Co-Cultures
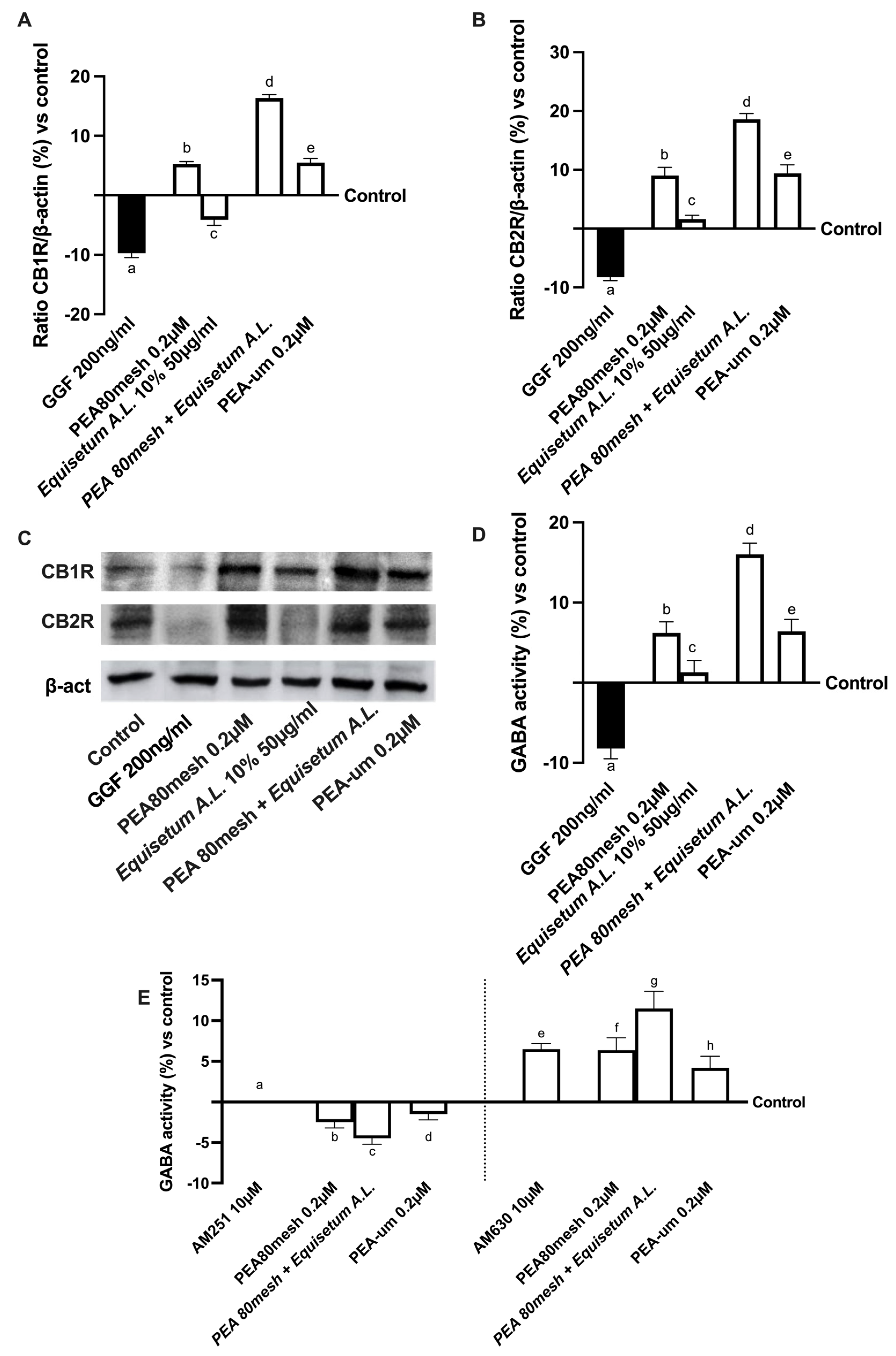
2.4. Biological Effects of the Combination PEA 80Mesh Plus Equisetum A.L. on an In Vitro Model of Peripheral Nervous Injury (PNI)
3. Discussion
4. Materials and Methods
4.1. Agents Preparation
4.2. Cell Culture
4.3. Experimental Protocol
4.4. In Vitro Intestinal Barrier Model
4.5. D EngNT Co-Cultures Setup
4.6. Cell Viability
4.7. ROS Production
4.8. Occludin Quantification Assay
4.9. Claudin-1 ELISA Kit
4.10. Human Tight Junction Protein 1 (ZO-1) Analysis
4.11. TNFα Assay
4.12. Interleukin-2 Assay
4.13. NRG1 Assay
4.14. Myelin Protein Zero Assay
4.15. NGFR Assay
4.16. Human Beta-NGF Assay
4.17. Estrogen Receptor Beta Assay
4.18. Gamma-Aminobutyric Acid Assay
4.19. Western Blot
4.20. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D EngNT | 3D Engineered neural tissue |
| ATCC | American Type Culture Collection |
| BDNF | Brain-derived neurotrophic factor |
| CB1R | cannabinoid receptor 1 |
| CB2R | cannabinoid receptor 2 |
| COX-2 | Cyclooxygenase-2 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMA | European Medicines Agency |
| ERb | Estrogen Receptor beta |
| Equisetum A.L. | Equisetum arvense L. |
| FAAH | Fatty acid amide hydrolase |
| FBS | foetal bovine serum |
| FDA | US Food and Drug Administration |
| GABA | gamma-amino butyric acid |
| GAD | Glutamic acid decarboxylase |
| GGF | glial growth factor 2 |
| IASP | International Association for the Study of Pain |
| IL-1β | Interleukin-1 beta |
| IL-2 | I nterleukin-2 |
| MEM | Minimum Essential Medium |
| MPZ | Myelin protein zero |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NGF | Nerve growth factor |
| NP | neuropathic pain |
| NRG1 | Neuregulin 1 |
| OS | oxidative stress |
| Papp | apparent permeability coefficient |
| PBS | phosphate buffered saline |
| PEA | palmitoylethanolamide |
| PEA-um | ultra-micronized PEA |
| PEAm | micronized PEA |
| PNI | peripheral nervous injury |
| PNS | peripheral nervous system |
| PVDF | polyvinylidene difluoride |
| RNS | Radical nitrogen species |
| ROS | radical oxygen species |
| RPMI | Roswell Park Memorial Institute-1640 |
| RSC-96 | Rat-derived Schwann |
| SIRT1 | sirtuin 1 |
| SIRT3 | sirtuin 3 |
| TEER | transepithelial electrical resistance |
| TJ | Tigh Junction |
| TNFα | Tumour Necrosis Factor alpha |
| ZO-1 | Human Tight Junction Protein 1 |
References
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Moka, E.; Orrillo, E.; Aurilio, C.; Vadalouca, A.; Paladini, A.; Varrassi, G. Neuropathic Pain in Neurologic Disorders: A Narrative Review. Cureus 2022, 14, e22419. [Google Scholar]
- D’Egidio, F.; Lombardozzi, G.; Kacem Ben Haj M’Barek, H.E.; Mastroiacovo, G.; Alfonsetti, M.; Cimini, A. The Influence of Dietary Supplementations on Neuropathic Pain. Life 2022, 12, 1125. [Google Scholar]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar]
- Mishra, G.; Singh, P.; Molla, M.; Yimer, Y.S.; Ewunetie, A.; Tadesse, T.Y.; Ayele, T.M.; Kefale, B. Nutraceuticals: A source of benefaction for neuropathic pain and fibromyalgia. J. Funct. Foods 2022, 97, 105260. [Google Scholar]
- El Soury, M.; Fornasari, B.E.; Carta, G.; Zen, F.; Haastert-Talini, K.; Ronchi, G. The Role of Dietary Nutrients in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7417. [Google Scholar]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar]
- Campos, R.M.P.; Aguiar, A.F.L.; Paes-Colli, Y.; Trindade, P.M.P.; Ferreira, B.K.; de Melo Reis, R.A.; Sampaio, L.S. Cannabinoid Therapeutics in Chronic Neuropathic Pain: From Animal Research to Human Treatment. Front. Physiol. 2021, 12, 785176. [Google Scholar]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar]
- Hampe, C.S.; Mitoma, H.; Manto, M. GABA and Glutamate: Their transmitter role in the CNS and pancreatic islets. In U: GABA and Glutamate-New Developments in Neurotransmission Research; IntechOpen: London, UK, 2018; pp. 65–90. [Google Scholar]
- Li, C.; Lei, Y.; Tian, Y.; Xu, S.; Shen, X.; Wu, H.; Bao, S.; Wang, F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol. Pain 2019, 15, 1744806919847366. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Res. 2016, 70, 315–347. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Q.; Shu, B.; Tiwari, V.; He, S.Q.; Vera-Portocarrero, L.P.; Dong, X.; Linderoth, B.; Raja, S.N.; Wang, Y.; et al. Activation of cannabinoid CB1 receptor contributes to suppression of spinal nociceptive transmission and inhibition of mechanical hypersensitivity by Aβ-fiber stimulation. Pain 2016, 157, 2582–2593. [Google Scholar] [CrossRef]
- Starowicz, K.; Finn, D.P. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv. Pharmacol. 2017, 80, 437–475. [Google Scholar]
- Petzke, F.; Tölle, T.; Fitzcharles, M.A.; Häuser, W. Cannabis-Based Medicines and Medical Cannabis for Chronic Neuropathic Pain. CNS Drugs 2022, 36, 31–44. [Google Scholar] [CrossRef]
- Szewczyk, A.K.; Jamroz-Wiśniewska, A.; Haratym, N.; Rejdak, K. Neuropathic pain and chronic pain as an underestimated interdisciplinary problem. Int. J. Occup. Med. Environ. Health 2022, 35, 249–264. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2022, 98, 780–795. [Google Scholar] [CrossRef]
- Szymaszkiewicz, A.; López-Gómez, L.; Zielińska, M.; Abalo, R. Nutraceuticals and peripheral glial cells: A possible link? J. Integr. Neurosci. 2022, 21, 1. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Caballero-García, A.; Pérez-Valdecantos, D.; Roche, E.; Noriega-González, D.C. Peripheral Neuropathies Derived from COVID-19: New Perspectives for Treatment. Biomedicines 2022, 10, 1051. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. BioMed Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Morsanuto, V.; Galla, R.; Molinari, C.; Uberti, F. A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sci. 2022, 10, 457. [Google Scholar] [CrossRef]
- Ghonghadze, M.; Pachkoria, K.; Okujava, M.; Antelava, N.; Gongadze, N. Endocannabinoids receptors mediated central and peripheral effects (Review). Georgian Med. News 2020, 298, 137–143. [Google Scholar]
- Befort, K. Interactions of the opioid and cannabinoid systems in reward: Insights from knockout studies. Front. Pharmacol. 2015, 6, 6. [Google Scholar]
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef]
- Beggiato, S.; Tomasini, M.C.; Cassano, T.; Ferraro, L. Chronic Oral Palmitoylethanolamide Administration Rescues Cognitive Deficit and Reduces Neuroinflammation, Oxidative Stress, and Glutamate Levels in A Transgenic Murine Model of Alzheimer’s Disease. J. Clin. Med. 2020, 9, 428. [Google Scholar] [CrossRef]
- Clayton, P.; Subah, S.; Venkatesh, R.; Hill, M.; Bogoda, N. Palmitoylethanolamide: A Potential Alternative to Cannabidiol. J. Diet. Suppl. 2021, 1–26, Advance Online Publication. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Grillo, S.L.; Keereetaweep, J.; Grillo, M.A.; Chapman, K.D.; Koulen, P. N-Palmitoylethanolamine depot injection increased its tissue levels and those of other acylethanolamide lipids. Drug Des Devel Ther. 2013, 7, 747–752. [Google Scholar]
- Hegedűs, C.; Muresan, M.; Badale, A.; Bombicz, M.; Varga, B.; Szilágyi, A.; Sinka, D.; Bácskay, I.; Popoviciu, M.; Magyar, I.; et al. SIRT1 Activation by Equisetum Arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats. Molecules 2020, 25, 2541. [Google Scholar] [CrossRef]
- Cetojević-Simin, D.D.; Canadanović-Brunet, J.M.; Bogdanović, G.M.; Djilas, S.M.; Cetković, G.S.; Tumbas, V.T.; Stojiljković, B.T. Antioxidative and antiproliferative activities of different horsetail (Equisetum arvense L.) extracts. J. Med. Food 2010, 13, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Jurca, T.; Pasca, B.; Sirbu, V.; Honiges, A.N.A.; Costuleanu, M. Analysis of phenolic compounds composition by HPLC and assessment of antioxidant capacity in Equisetum arvense L. extracts. Rev. Chim. 2016, 67, 1623–1627. [Google Scholar]
- Pallag, A.; Filip, G.A.; Olteanu, D.; Clichici, S.; Baldea, I.; Jurca, T.; Micle, O.; Vicaş, L.; Marian, E.; Soriţău, O.; et al. Equisetum arvense L. Extract Induces Antibacterial Activity and Modulates Oxidative Stress, Inflammation, and Apoptosis in Endothelial Vascular Cells Exposed to Hyperosmotic Stress. Oxid. Med. Cell Longev. 2018, 2018, 3060525. [Google Scholar] [CrossRef]
- Song, F.H.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. SIRT1: A promising therapeutic target for chronic pain. CNS Neurosci. Ther. 2022, 28, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.M.; Silvia, M.G.; Vinitha, K.; Moses, J.A.; Anandharamakrishnan, C. Synergistic potential of nutraceuticals: Mechanisms and prospects for futuristic medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, L.; Li, L.; Feng, W. Dihydromyricetin attenuates neuropathic pain via enhancing the transition from M1 to M2 phenotype polarization by potentially elevating ALDH2 activity in vitro and vivo. Ann. Transl. Med. 2020, 8, 1151. [Google Scholar] [CrossRef]
- Heary, K.O.; Wong, A.W.K.; Lau, S.C.L.; Dengler, J.; Thompson, M.R.; Crock, L.W.; Novak, C.B.; Philip, B.A.; Mackinnon, S.E. Quality of Life and Psychosocial Factors as Predictors of Pain Relief Following Nerve Surgery. Hand 2022, 17, 193–199. [Google Scholar] [CrossRef]
- Chen, Y.; Hamidu, S.; Yang, X.; Yan, Y.; Wang, Q.; Li, L.; Oduro, P.K.; Li, Y. Dietary Supplements and Natural Products: An Update on Their Clinical Effectiveness and Molecular Mechanisms of Action During Accelerated Biological Aging. Front. Genet. 2022, 13, 880421. [Google Scholar] [CrossRef]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef]
- Luongo, L.; Maione, S.; Di Marzo, V. Endocannabinoids and neuropathic pain: Focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur. J. Neurosci. 2014, 39, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xiang, H.C.; Li, H.P.; Lin, L.X.; Hu, X.F.; Zhang, H.; Meng, W.Y.; Liu, L.; Chen, C.; Shu, Y.; et al. Inhibition of GABAergic Neurons and Excitation of Glutamatergic Neurons in the Ventrolateral Periaqueductal Gray Participate in Electroacupuncture Analgesia Mediated by Cannabinoid Receptor. Front. Neurosci. 2019, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Poe, A.R.; Wiese, B.; Kibaly, C.; Lueptow, L.; Garcia, J.; Anand, P.; Cahill, C.; Morón, J.A. Effects of inflammatory pain on CB1 receptor in the midbrain periaqueductal gray. Pain Rep. 2021, 6, e897. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Longo, G.; Osikowicz, M.; Ribeiro-da-Silva, A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to pain-related behavior in arthritis. J. Neurosci. 2013, 33, 10066–10074. [Google Scholar] [CrossRef]
- LaBranche, T.P.; Bendele, A.M.; Omura, B.C.; Gropp, K.E.; Hurst, S.I.; Bagi, C.M.; Cummings, T.R.; Grantham, L.E., 2nd; Shelton, D.L.; Zorbas, M.A. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann. Rheum. Dis. 2017, 76, 295–302. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- Pan, P.; Dobrowsky, R.T. Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy. Acta Neuropathol. Commun. 2013, 1, 39. [Google Scholar] [CrossRef]
- Bessa Pereira, C.; Gomes, P.S.; Costa-Rodrigues, J.; Almeida Palmas, R.; Vieira, L.; Ferraz, M.P.; Lopes, M.A.; Fernandes, M.H. Equisetum arvense hydromethanolic extracts in bone tissue regeneration: In vitro osteoblastic modulation and antibacterial activity. Cell Prolif. 2012, 45, 386–396. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef]
- Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection-An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8114. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Morsanuto, V.; Ruga, S.; Galla, R.; Farghali, M.; Notte, F.; Bozzo, C.; Magnani, C.; Nardone, A.; Molinari, C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients 2020, 12, 573. [Google Scholar] [CrossRef] [PubMed]
- Ceriotti, L.; Meloni, M. La valutazione dell’assorbimento intestinale in vitro. L’integratore Nutr. 2014, 17, 62–65. [Google Scholar]
- Rayner, M.L.D.; Laranjeira, S.; Evans, R.E.; Shipley, R.J.; Healy, J.; Phillips, J.B. Developing an In Vitro Model to Screen Drugs for Nerve Regeneration. Anat. Rec. 2018, 301, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Vetrugno, C.; Cossa, L.G.; Marsigliante, S. TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J. Neurochem. 2020, 153, 525–538. [Google Scholar] [CrossRef]
- Chua, P.; Lim, W.K. Optimisation of a PC12 cell-based in vitro stroke model for screening neuroprotective agents. Sci. Rep. 2021, 11, 8096. [Google Scholar] [CrossRef]
- Lahiani, A.; Brand-Yavin, A.; Yavin, E.; Lazarovici, P. Neuroprotective Effects of Bioactive Compounds and MAPK Pathway Modulation in “Ischemia”-Stressed PC12 Pheochromocytoma Cells. Brain Sci. 2018, 8, 32. [Google Scholar] [CrossRef]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- Park, S.E.; Ahn, J.; Jeong, H.E.; Youn, I.; Huh, D.; Chung, S. A three-dimensional in vitro model of the peripheral nervous system. NPG Asia Mater. 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Cappellano, G.; Uberti, F.; Caimmi, P.P.; Pietronave, S.; Mary, D.A.; Dianzani, C.; Grossini, E. Different expression and function of the endocannabinoid system in human epicardial adipose tissue in relation to heart disease. Can. J. Cardiol. 2013, 29, 499–509. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron bioavailability from commercially available iron supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Fda.Gov. Available online: https://www.fda.gov/media/117974/download (accessed on 12 May 2021).
- Ema.Eu. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 6 August 2018).
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.M.A.P.; Day, A.G.E.; Redl, H.; Phillips, J. An Optimized Collagen-Fibrin Blend Engineered Neural Tissue Promotes Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Day, A.; Dimiou, S.; Ataç, A.F.; Kayal, C.; Park, H.; Nazhat, S.N.; Phillips, J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural. Eng. 2020, 17, 046036. [Google Scholar] [CrossRef]
- Uberti, F.; Ruga, S.; Farghali, M.; Galla, R.; Molinari, C. A Combination of α-Lipoic Acid (ALA) and Palmitoylethanolamide (PEA) Blocks Endotoxin-Induced Oxidative Stress and Cytokine Storm: A Possible Intervention for COVID-19. J. Diet. Suppl. 2023, 1–23, Advance Online Publication. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Luan, J.; Jiang, R.P.; Liu, J.; Ma, Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int. J. Immunopathol. Pharm. 2020, 34, 2058738420954948. [Google Scholar] [CrossRef]
- Endo, T.; Kadoya, K.; Kawamura, D.; Iwasaki, N. Evidence for cell-contact factor involvement in neurite outgrowth of dorsal root ganglion neurons stimulated by Schwann cells. Exp. Physiol. 2019, 104, 1447–1454. [Google Scholar] [CrossRef]
- Rzemieniec, J.; Litwa, E.; Wnuk, A.; Lason, W.; Gołas, A.; Krzeptowski, W.; Kajta, M. Neuroprotective action of raloxifene against hypoxia-induced damage in mouse hippocampal cells depends on ERα but not ERβ or GPR30 signalling. J. Steroid Biochem. Mol. Biol. 2015, 146, 26–37. [Google Scholar] [CrossRef]
- Al Suhaibani, A.; Ben Bacha, A.; Alonazi, M.; Bhat, R.S.; El-Ansary, A. Testing the combined effects of probiotics and prebiotics against neurotoxic effects of propionic acid orally administered to rat pups. Food Sci. Nutr. 2021, 9, 4440–4451. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| PEA-um | PEA 80mesh | Equisetum A.L. | PEA 80mesh + Equisetum A.L. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 0.1 µM | 0.2 µM | 0.4 µM | 0.1 µM | 0.2 µM | 0.4 µM | 25 µg/mL | 50 µg/mL | 100 µg/mL | 0.2 µM + 50 µg/mL | |
| Time | |||||||||||
| 3 h | 80 ± 1.71 | 90 ± 1.21 | 72 ± 1.46 | 85 ± 0.83 | 92 ± 1.23 | 70 ± 1.48 | 45 ± 0.85 | 49 ± 1.48 | 46 ± 1.57 | 94 ± 1.33 | |
| 6 h | 76 ± 1.48 | 87 ± 1.67 | 70 ± 1.11 | 78 ± 1.74 | 90 ± 1.26 | 68 ± 1.27 | 30 ± 1.27 | 44 ± 1.22 | 40 ± 1.57 | 91 ± 1.07 | |
| PEA-um | PEA 80mesh | Equisetum A.L. | PEA 80mesh + Equisetum A.L. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 0.1 µM | 0.2 µM | 0.4 µM | 0.1 µM | 0.2 µM | 0.4 µM | 25 µg/mL | 50 µg/mL | 100 µg/mL | 0.2 µM + 50 µg/mL | |
| Time | |||||||||||
| 3 h | 4 ± 1.14 | 5 ± 1.67 | 7 ± 1.48 | 3 ± 1.78 | 2 ± 1.74 | 1 ± 1.19 | −8 ± 1.74 | −9 ± 1.46 | −9 ± 1.71 | −15 ± 0.89 | |
| 6 h | 8 ± 1.15 | 7 ± 1.56 | 10 ± 1.22 | 7 ± 1.41 | 6 ± 1.38 | 5 ± 1.47 | −9 ± 1.38 | −9 ± 1.17 | −9 ± 1.48 | −12 ± 1.23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruga, S.; Galla, R.; Ferrari, S.; Invernizzi, M.; Uberti, F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5503. https://doi.org/10.3390/ijms24065503
Ruga S, Galla R, Ferrari S, Invernizzi M, Uberti F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study. International Journal of Molecular Sciences. 2023; 24(6):5503. https://doi.org/10.3390/ijms24065503
Chicago/Turabian StyleRuga, Sara, Rebecca Galla, Sara Ferrari, Marco Invernizzi, and Francesca Uberti. 2023. "Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study" International Journal of Molecular Sciences 24, no. 6: 5503. https://doi.org/10.3390/ijms24065503
APA StyleRuga, S., Galla, R., Ferrari, S., Invernizzi, M., & Uberti, F. (2023). Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study. International Journal of Molecular Sciences, 24(6), 5503. https://doi.org/10.3390/ijms24065503








