Combining Three Tyrosine Kinase Inhibitors: Drug Monitoring Is the Key
Abstract
1. Introduction
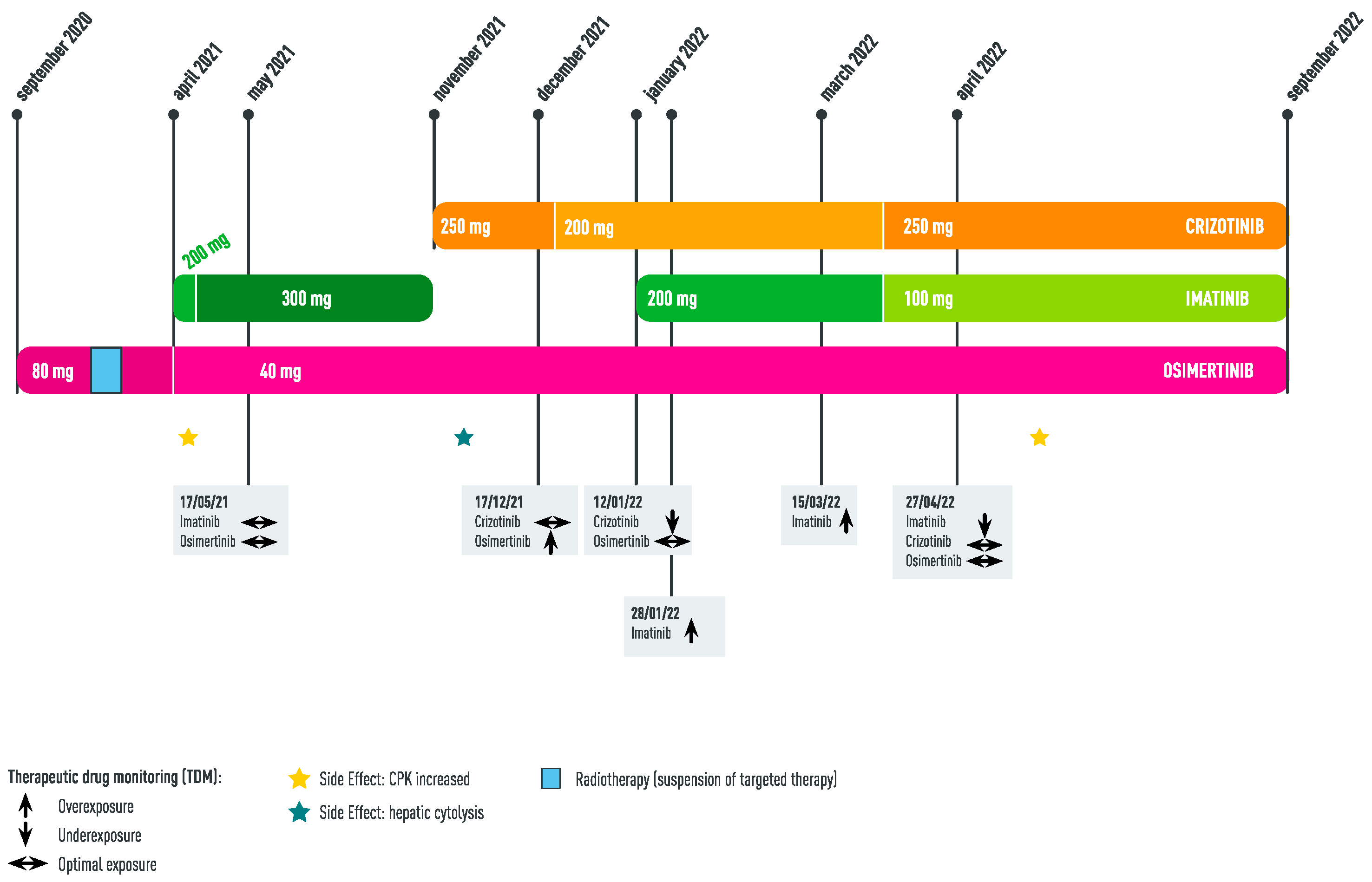
2. Case Report
2.1. Initial Management of GIST
2.2. Diagnosis of EGFR Lung Adenocarcinoma: Concomitant Administration of Imatinib + Osimertinib
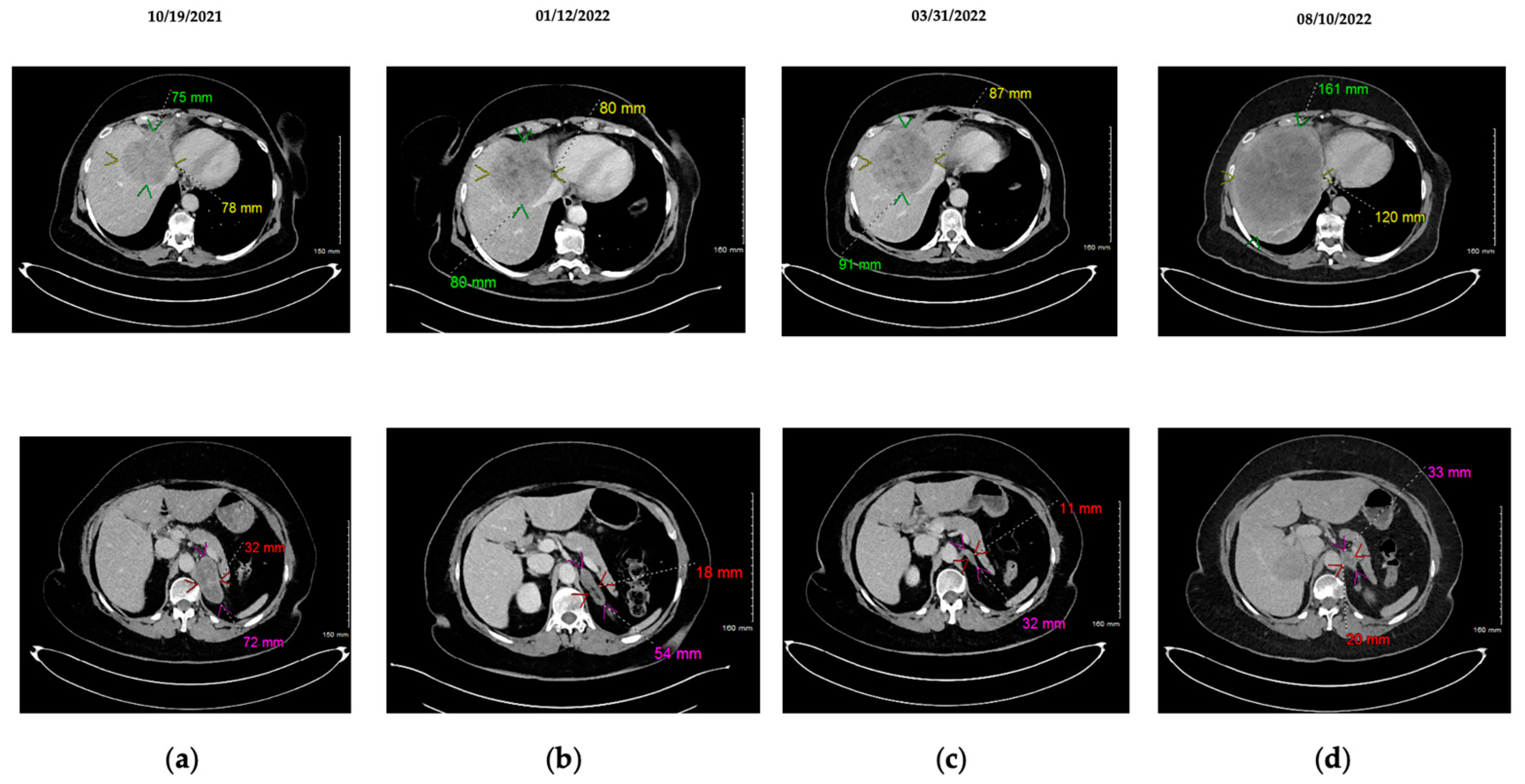
2.3. Appearance of MET Amplification as Resistance Mechanism to Osimertinib: Concomitant Administration of Imatinib + Osimertinib + Crizotinib
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Dagher, R.; Cohen, M.; Williams, G.; Rothmann, M.; Gobburu, J.; Robbie, G.; Rahman, A.; Chen, G.; Staten, A.; Griebel, D.; et al. Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 3034–3038. [Google Scholar]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J. Hematol. Oncol. 2020, 13, 143. [Google Scholar] [CrossRef]
- Ferrara, M.G.; Di Noia, V.; D’argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Moliner, L.; Arriola, E. Acquired Mechanisms of Resistance to Osimertinib-The Next Challenge. Cancers 2022, 14, 1931. [Google Scholar] [CrossRef] [PubMed]
- Girard, N. New Strategies and Novel Combinations in EGFR TKI-Resistant Non-small Cell Lung Cancer. Curr. Treat. Options Oncol. 2022, 23, 1626–1644. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Han, J.-Y.; Ahn, M.-J.; Cho, B.C.; Yu, H.; Kim, S.-W.; Yang, J.C.-H.; Lee, J.S.; Su, W.-C.; Kowalski, D.; et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020, 21, 373–386. [Google Scholar] [CrossRef]
- F Smit, E.; Dooms, C.; Raskin, J.; Nadal, E.; Tho, L.M.; Le, X.; Mazieres, J.; S Hin, H.; Morise, M.; W Zhu, V.; et al. INSIGHT 2: A phase II study of tepotinib plus osimertinib in MET-amplified NSCLC and first-line osimertinib resistance. Future Oncol. Lond. Engl. 2022, 18, 1039–1054. [Google Scholar] [CrossRef]
- Giroux-Leprieur, E.; Dumenil, C.; Chinet, T. Combination of Crizotinib and Osimertinib or Erlotinib Might Overcome MET-Mediated Resistance to EGFR Tyrosine Kinase Inhibitor in EGFR-Mutated Adenocarcinoma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, e232–e234. [Google Scholar] [CrossRef]
- Mancini, M.; Thomas, Q.-D.; Bourdel, S.; Papon, L.; Bousquet, E.; Jalta, P.; La Monica, S.; Travert, C.; Alfieri, R.; Quantin, X.; et al. Generation and Characterization of a New Preclinical Mouse Model of EGFR-Driven Lung Cancer with MET-Induced Osimertinib Resistance. Cancers 2021, 13, 3441. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; van Eerden, R.A.G.; Westerdijk, K.; Meertens, M.; Koolen, S.L.W.; Moes, D.J.A.R.; de Vries, N.; Rosing, H.; Otten, H.; Vulink, A.; et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: A prospective multicenter study. Ann. Oncol. 2022, 33, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Patrikidou, A.; Chabaud, S.; Ray-Coquard, I.; Bui, B.N.; Adenis, A.; Rios, M.; Bertucci, F.; Duffaud, F.; Chevreau, C.; Cupissol, D.; et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Levêque, D.; Veal, G.; Astier, A. Review of therapeutic drug monitoring of anticancer drugs part two—Targeted therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef]
- Rodier, T.; Puszkiel, A.; Cardoso, E.; Balakirouchenane, D.; Narjoz, C.; Arrondeau, J.; Fallet, V.; Khoudour, N.; Guidi, M.; Vidal, M.; et al. Exposure-Response Analysis of Osimertinib in Patients with Advanced Non-Small-Cell Lung Cancer. Pharmaceutics 2022, 14, 1844. [Google Scholar] [CrossRef]
- Groenland, S.L.; Geel, D.R.; Janssen, J.M.; de Vries, N.; Rosing, H.; Beijnen, J.H.; Burgers, J.A.; Smit, E.F.; Huitema, A.D.; Steeghs, N. Exposure–Response Analyses of Anaplastic Lymphoma Kinase Inhibitors Crizotinib and Alectinib in Non-Small Cell Lung Cancer Patients. Clin. Pharmacol. Ther. 2021, 109, 394–402. [Google Scholar] [CrossRef]
- Girard, N.; Basse, C. EGFR-mutant NSCLC: Monitoring the molecular evolution of tumors in 2022. Expert Rev Anticancer Ther. 2022, 22, 1115–1125. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Lu, P.; Yu, Z.; Xu, C.; Zhuang, W.; Song, Z. Crizotinib with or without an EGFR-TKI in treating EGFR-mutant NSCLC patients with acquired MET amplification after failure of EGFR-TKI therapy: A multicenter retrospective study. J. Transl. Med. 2019, 17, 52. [Google Scholar] [CrossRef]
- Malnoë, D.; Fardel, O.; Le Corre, P. Involvement of Transporters in Intestinal Drug–Drug Interactions of Oral Targeted Anticancer Drugs Assessed by Changes in Drug Absorption Time. Pharmaceutics 2022, 14, 2493. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, J.; Chu, M.; Long, X.; Wang, J. Pharmacokinetic-Based Drug-Drug Interactions with Anaplastic Lymphoma Kinase Inhibitors: A Review. Drug Des. Devel. Ther. 2020, 14, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Small-Molecule Inhibitors, Immune Checkpoint Inhibitors, and More: FDA-Approved Novel Therapeutic Drugs for Solid Tumors from 1991 to 2021. Available online: https://pubmed-ncbi-nlm-nih-gov.ezpum.scdi-montpellier.fr/36209184/ (accessed on 30 December 2022).
- Shen, C.; Wang, C.; He, T.; Cai, Z.; Yin, X.; Yin, Y.; Lu, D.; Zhang, B.; Zhou, Z. Long-term survival among patients with gastrointestinal stromal tumors diagnosed after another malignancy: A SEER population-based study. World J. Surg. Oncol. 2020, 18, 88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, Q.D.; Firmin, N.; Mbatchi, L.; Evrard, A.; Quantin, X.; Leenhardt, F. Combining Three Tyrosine Kinase Inhibitors: Drug Monitoring Is the Key. Int. J. Mol. Sci. 2023, 24, 5518. https://doi.org/10.3390/ijms24065518
Thomas QD, Firmin N, Mbatchi L, Evrard A, Quantin X, Leenhardt F. Combining Three Tyrosine Kinase Inhibitors: Drug Monitoring Is the Key. International Journal of Molecular Sciences. 2023; 24(6):5518. https://doi.org/10.3390/ijms24065518
Chicago/Turabian StyleThomas, Quentin Dominique, Nelly Firmin, Litaty Mbatchi, Alexandre Evrard, Xavier Quantin, and Fanny Leenhardt. 2023. "Combining Three Tyrosine Kinase Inhibitors: Drug Monitoring Is the Key" International Journal of Molecular Sciences 24, no. 6: 5518. https://doi.org/10.3390/ijms24065518
APA StyleThomas, Q. D., Firmin, N., Mbatchi, L., Evrard, A., Quantin, X., & Leenhardt, F. (2023). Combining Three Tyrosine Kinase Inhibitors: Drug Monitoring Is the Key. International Journal of Molecular Sciences, 24(6), 5518. https://doi.org/10.3390/ijms24065518





