Transcriptomic Differentiation of Phenotypes in Chronic Rhinosinusitis and Its Implications for Understanding the Underlying Mechanisms
Abstract
:1. Introduction
2. Results
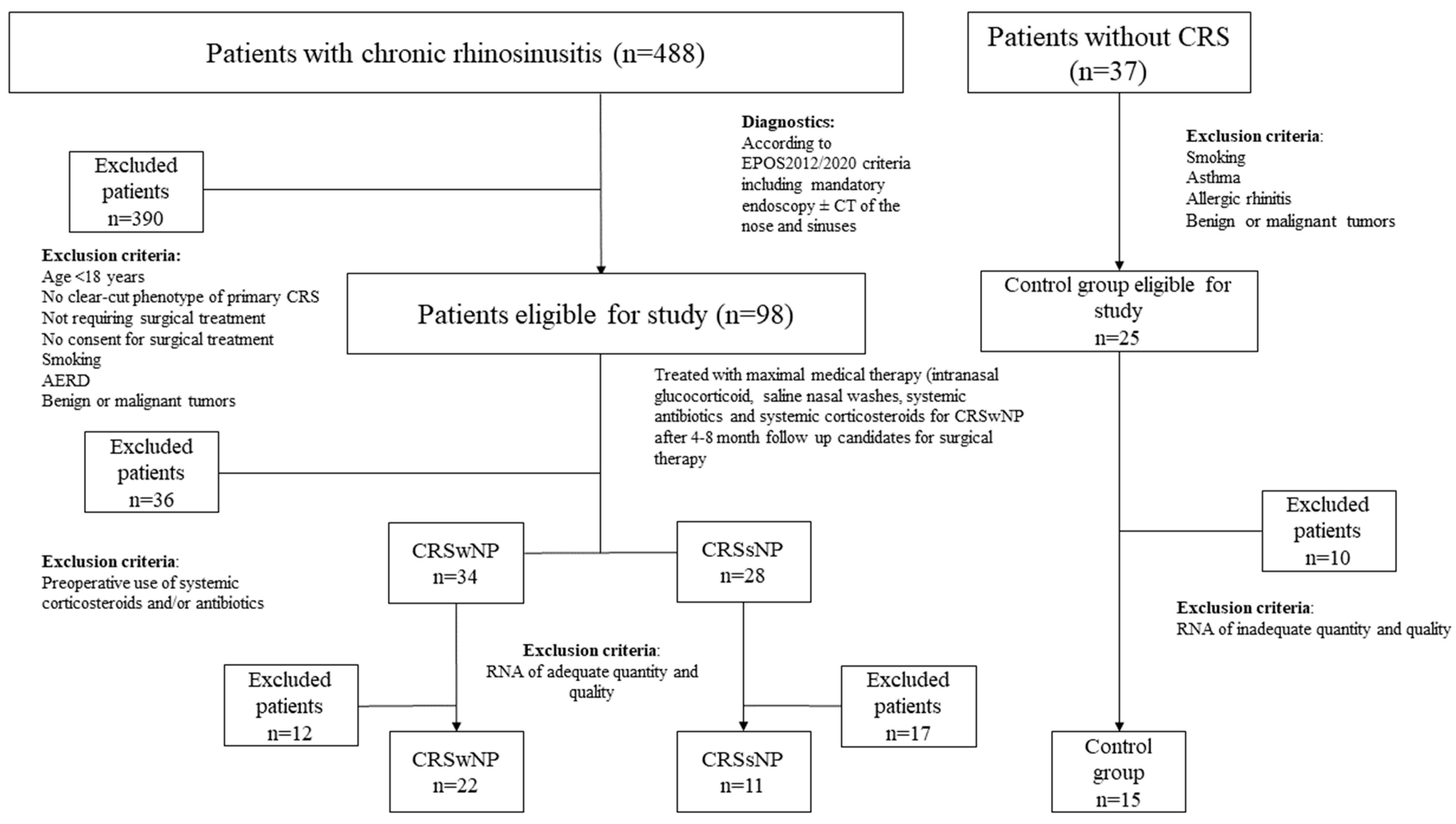
2.1. Characteristics of the Study Cohort of Patients with CRS and Controls
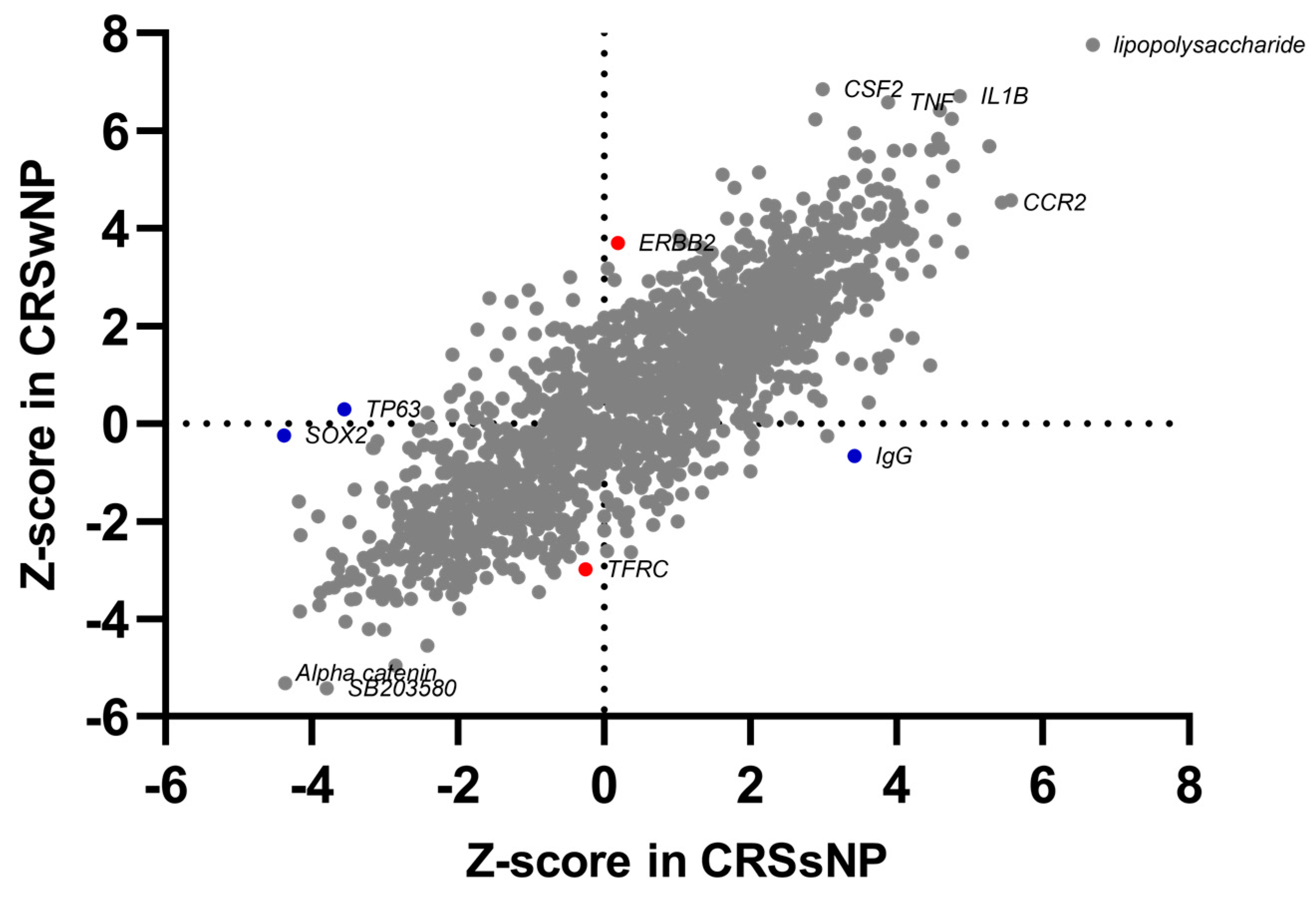
2.2. Common and Phenotype-Specific Transcriptional Alteration in CRS
2.3. Transcriptomic Alterations in CRS Are Associated with Immune Response Modulation, Cellular Movement, Bone Homeostasis, and Inflammation
3. Discussion
4. Materials and Methods
4.1. Study Subjects
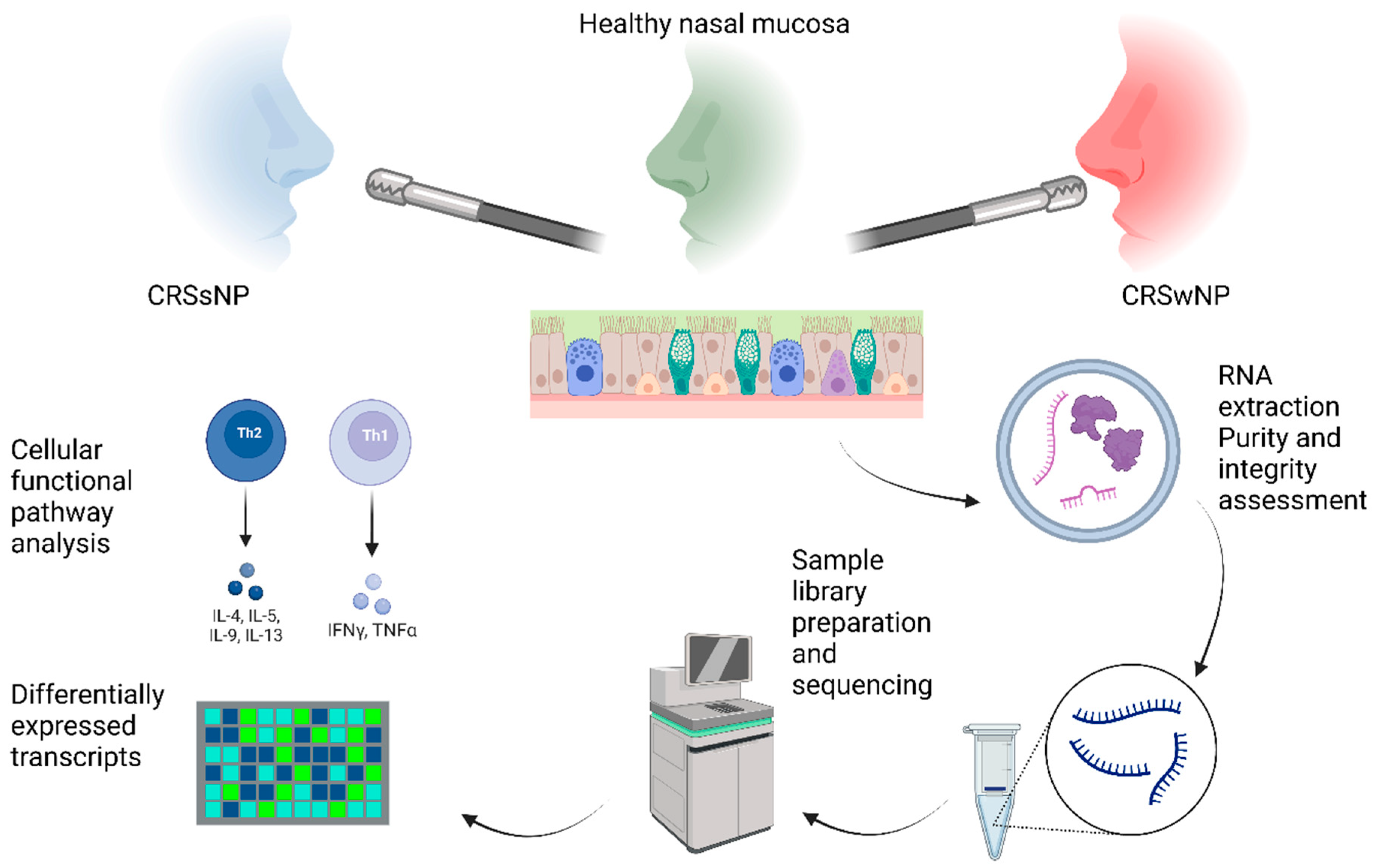
4.2. Sampling Site and Biopsy
4.3. RNA Sequencing
4.3.1. Processing of Tissue Samples and RNA Isolation
4.3.2. Sample Library Preparation and Sequencing
4.3.3. RNAseq Data Analyses
4.3.4. Functional and Pathway Analysis
4.3.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.G.; Peters, A.T.; Kato, A.; Stevens, W.W. Use of Endotypes, Phenotypes, and Inflammatory Markers to Guide Treatment Decisions in Chronic Rhinosinusitis. Ann. Allergy Asthma Immunol. 2020, 124, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of TH Cytokine Profiles in Patients with Chronic Rhinosinusitis: A Multicenter Study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziaber, A.; Holtappels, G.; et al. Inflammatory Endotypes of Chronic Rhinosinusitis Based on Cluster Analysis of Biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456e4. [Google Scholar] [CrossRef] [Green Version]
- Stevens, W.W.; Peters, A.T.; Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Hulse, K.E.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; et al. Associations Between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 2812–2820.e3. [Google Scholar] [CrossRef] [PubMed]
- Klingler, A.I.; Stevens, W.W.; Tan, B.K.; Peters, A.T.; Poposki, J.A.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; Kern, R.C.; et al. Mechanisms and Biomarkers of Inflammatory Endotypes in Chronic Rhinosinusitis without Nasal Polyps. J. Allergy Clin. Immunol. 2021, 3, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Stevens, W.W.; Peters, A.T.; Suh, L.A.; Norton, J.; Carter, R.G.; Hulse, K.E.; Harris, K.E.; et al. Heterogeneous Inflammatory Patterns in Chronic Rhinosinusitis without Nasal Polyps in Chicago, Illinois. J. Allergy Clin. Immunol. 2017, 139, 699–703.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soklic, T.K.; Silar, M.; Rijavec, M.; Koren, A.; Kern, I.; Hocevar-Boltezar, I.; Korosec, P. CD3+CD4−CD8− Mucosal T Cells Are Associated with Uncontrolled Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 2019, 143, 1235–1237.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassiouni, A.; Ou, J.; Schreiber, A.; Geoghegan, J.; Tsykin, A.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. The Global Transcriptomic Signature in Sinonasal Tissues Reveals Roles for Tissue Type and Chronic Rhinosinusitis Disease Phenotype. Rhinology 2020, 58, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Soklic, T.K.; Rijavec, M.; Silar, M.; Koren, A.; Kern, I.; Hocevar-Boltezar, I.; Korosec, P. Transcription Factors Gene Expression in Chronic Rhinosinusitis with and without Nasal Polyps. Radiol. Oncol. 2019, 53, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.; Avila, P.C.; Kern, R.C.; Hayes, M.G.; Schleimer, R.P.; Pinto, J.M. Genetics of Chronic Rhinosinusitis: State of the Field and Directions Forward. J. Allergy Clin. Immunol. 2013, 131, 977–993.e5. [Google Scholar] [CrossRef] [Green Version]
- Laulajainen-Hongisto, A.; Lyly, A.; Hanif, T.; Dhaygude, K.; Kankainen, M.; Renkonen, R.; Donner, K.; Mattila, P.; Jartti, T.; Bousquet, J.; et al. Genomics of Asthma, Allergy and Chronic Rhinosinusitis: Novel Concepts and Relevance in Airway Mucosa. Clin. Transl. Allergy 2020, 10, 45. [Google Scholar] [CrossRef]
- Ishino, T.; Takeno, S.; Takemoto, K.; Yamato, K.; Oda, T.; Nishida, M.; Horibe, Y.; Chikuie, N.; Kono, T.; Taruya, T.; et al. Distinct Gene Set Enrichment Profiles in Eosinophilic and Non-Eosinophilic Chronic Rhinosinusitis with Nasal Polyps by Bulk RNA Barcoding and Sequencing. Int. J. Mol. Sci. 2022, 23, 5653. [Google Scholar] [CrossRef]
- Do, A.N.; Chun, Y.; Grishina, G.; Grishin, A.; Rogers, A.J.; Raby, B.A.; Weiss, S.T.; Vicencio, A.; Schadt, E.E.; Bunyavanich, S. Network Study of Nasal Transcriptome Profiles Reveals Master Regulator Genes of Asthma. J. Allergy Clin. Immunol. 2021, 147, 879–893. [Google Scholar] [CrossRef]
- Dong, X.; Ding, M.; Zhang, J.; Ogülür, I.; Pat, Y.; Akdis, M.; Gao, Y.; Akdis, C.A. Involvement and Therapeutic Implications of Airway Epithelial Barrier Dysfunction in Type 2 Inflammation of Asthma. Chin. Med. J. 2022, 135, 519–531. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Brar, T.; McCabe, C.; Miglani, A.; Marino, M.; Lal, D. Tissue Eosinophilia Is Superior to an Analysis by Polyp Status for the Chronic Rhinosinusitis Transcriptome: An RNA Study. Laryngoscope 2023, 1–10. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.; Bachert, C.; Mullol, J.; Bjermer, L.; Bousquet, J.; Canonica, G.W.; Deneyer, L.; Desrosiers, M.; Diamant, Z.; et al. EUFOREA Consensus on Biologics for CRSwNP with or without Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 2312–2319. [Google Scholar] [CrossRef] [Green Version]
- Hellings, P.W.; Fokkens, W.J.; Akdis, C.; Bachert, C.; Cingi, C.; Dietz de Loos, D.; Gevaert, P.; Hox, V.; Kalogjera, L.; Lund, V.; et al. Uncontrolled Allergic Rhinitis and Chronic Rhinosinusitis: Where Do We Stand Today? Allergy 2013, 68, 1–7. [Google Scholar] [CrossRef]
- Tepeš, I.; Košak Soklič, T.; Urbančič, J. The Agreement of the Endoscopic Modified Lund-Kennedy Scoring in a Clinical Research Group: An Observational Study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 139, 185–188. [Google Scholar] [CrossRef]
- Steinke, J.W.; Smith, A.R.; Carpenter, D.J.; Patrie, J.T.; Payne, S.C.; Borish, L. Lack of Efficacy of Symptoms and Medical History in Distinguishing the Degree of Eosinophilia in Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2017, 5, 1582–1588.e3. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Xie, S.; Wang, F. Identification of Key Genes and Pathways in Chronic Rhinosinusitis with Nasal Polyps Using Bioinformatics Analysis. Am. J. Otolaryngol. 2019, 40, 191–196. [Google Scholar] [CrossRef]
- Lu, X.; Wang, N.; Long, X.B.; You, X.J.; Cui, Y.H.; Liu, Z. The Cytokine-Driven Regulation of Secretoglobins in Normal Human Upper Airway and Their Expression, Particularly That of Uteroglobin-Related Protein 1, in Chronic Rhinosinusitis. Respir. Res. 2011, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Orysiak, J.; Lenczowska, M.J.; Multanowski, B.M. Expression of SCGB1C1 Gene as a Potential Marker of Susceptibility to Upper Respiratory Tract Infections in Elite Athletes—A Pilot Study. Biol. Sport 2016, 33, 107–110. [Google Scholar] [CrossRef] [Green Version]
- De Cid, R.; Riveira-Munoz, E.; Zeeuwen, P.L.J.M.; Robarge, J.; Liao, W.; Dannhauser, E.N.; Giardina, E.; Stuart, P.E.; Nair, R.; Helms, C.; et al. Deletion of the Late Cornified Envelope LCE3B and LCE3C Genes as a Susceptibility Factor for Psoriasis. Nat. Genet. 2009, 41, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Chen, M.; Yin, M.; Feng, H. Identifying the Potential Therapeutic Targets for Atopic Dermatitis through the Immune Infiltration Analysis and Construction of a Cerna Network. Clin. Cosmet. Investig. Dermatol. 2021, 14, 437–453. [Google Scholar] [CrossRef]
- Niesler, B.; Kapeller, J.; Hammer, C.; Rappold, G. Serotonin Type 3 Receptor Genes: HTR3A, B, C, D, E. Pharmacogenomics 2008, 9, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Yánez, D.C.; Ross, S.; Crompton, T. The IFITM Protein Family in Adaptive Immunity. Immunology 2020, 159, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Song, Z.; Liu, A.; Dahmen, U.; Yang, X.; Fang, H. Effects of Lipopolysaccharide-Binding Protein (LBP) Single Nucleotide Polymorphism (SNP) in Infections, Inflammatory Diseases, Metabolic Disorders and Cancers. Front. Immunol. 2021, 12, 681810. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, C.; Yi, X.; Xie, S.; Sun, H. Comparative Analysis of Inflammatory Signature Profiles in Eosinophilic and Noneosinophilic Chronic Rhinosinusitis with Nasal Polyposis. Biosci. Rep. 2020, 40, BSR20193101. [Google Scholar] [CrossRef] [Green Version]
- Lewis, T.C.; Henderson, T.A.; Carpenter, A.R.; Ramirez, I.A.; Mchenry, C.L.; Goldsmith, A.M.; Ren, X.; Mentz, G.B.; Mukherjee, B.; Robins, T.G.; et al. Nasal Cytokine Responses to Natural Colds in Asthmatic Children. Clin. Exp. Allergy 2012, 42, 1734–1744. [Google Scholar] [CrossRef] [Green Version]
- Brunner, P.M.; Glitzner, E.; Reininger, B.; Klein, I.; Stary, G.; Mildner, M.; Uhrin, P.; Sibilia, M.; Stingl, G. CCL7 Contributes to the TNF-Alpha-Dependent Inflammation of Lesional Psoriatic Skin. Exp. Dermatol. 2015, 24, 522–528. [Google Scholar] [CrossRef]
- Erbek, S.S.; Yurtcu, E.; Erbek, S.; Atac, F.B.; Sahin, F.I.; Cakmak, O. Proinflammatory Cytokine Single Nucleotide Polymorphisms in Nasal Polyposis. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Endam, L.M.; Bossé, Y.; Filali-Mouhim, A.; Cormier, C.; Boisvert, P.; Boulet, L.-P.; Hudson, T.J.; Desrosiers, M. Polymorphisms in the Interleukin-22 Receptor Alpha-1 Gene Are Associated with Severe Chronic Rhinosinusitis. Otolaryngol. Neck Surg. 2009, 140, 741–747. [Google Scholar] [CrossRef]
- Brett, T.J. CLCA1 and TMEM16A: The Link towards a Potential Cure for Airway Diseases. Expert Rev. Respir. Med. 2015, 9, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Li, N.; Zhang, J.; Jiang, Y. IL-13 Regulates Human Nasal Epithelial Cell Differentiation via H3K4me3 Modification. J. Inflamm. Res. 2017, 10, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Kook, J.H.; Kang, K.R.; Oh, D.J.; Kim, T.H.; Lee, S.H. Increased Expression of HCLCA1 in Chronic Rhinosinusitis and Its Contribution to Produce MUC5AC. Laryngoscope 2016, 126, E347–E355. [Google Scholar] [CrossRef]
- Hu, H.; Wang, S.; Wang, J.; Huang, R.; Dong, P.; Sun, Z. UPA Affects the CRSsNP Nasal Mucosa Epithelium Apoptosis by Regulating WIF1. Exp. Cell Res. 2019, 377, 75–85. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.Q.; Xiao, Q.; Liu, Y.B.; Song, J.; Liang, Y.; Ruan, J.W.; Wang, Z.Z.; Li, J.X.; Pan, L.; et al. Defective STING Expression Potentiates IL-13 Signaling in Epithelial Cells in Eosinophilic Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 2021, 147, 1692–1703. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, K.J.; Choi, I.H.; Han, H.M.; Kim, T.H.; Lee, S.H. Decreased Expression of Type I (IFN-β) and Type III (IFN-λ) Interferons and Interferon-Stimulated Genes in Patients with Chronic Rhinosinusitis with and without Nasal Polyps. J. Allergy Clin. Immunol. 2019, 144, 1551–1565.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karatzanis, A.D.; Samara, K.D.; Antoniou, K.M.; Lymbouridou, R.; Chatzakis, N.; Spandidos, D.A.; Velegrakis, G.A.; Siafakas, N.M. Investigation of Angiogenetic Pathways in Nasal Polyposis. Mol. Med. Rep. 2012, 5, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Divekar, R.; Rank, M.; Squillace, D.; Kita, H.; Lal, D. Unsupervised Network Mapping of Commercially Available Immunoassay Yields Three Distinct Chronic Rhinosinusitis Endotypes. Int. Forum Allergy Rhinol. 2017, 7, 373–379. [Google Scholar] [CrossRef]
- Hassan, A.; Bagu, E.T.; Levesque, M.; Patten, S.A.; Benhadjeba, S.; Edjekouane, L.; Villemure, I.; Tremblay, A.; Moldovan, F. The 17β-Estradiol Induced Upregulation of the Adhesion G-Protein Coupled Receptor (ADGRG7) Is Modulated by ESRα and SP1 Complex. Biol. Open 2019, 8, bio037390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Cao, R.; Chen, J.; Xie, X. Screening and Identification of Biomarkers Associated with Clinicopathological Parameters and Prognosis in Oral Squamous Cell Carcinoma. Exp. Ther. Med. 2019, 18, 3579–3587. [Google Scholar] [CrossRef]
- Ozdogan, S.; Sonmez, C.; Yolcu, D.; Gungormus, M. Tear Opiorphin Levels in Ocular Pain Caused by Corneal Foreign Body. Cornea 2020, 39, 1377–1380. [Google Scholar] [CrossRef]
- Harcha, P.A.; López-López, T.; Palacios, A.G.; Sáez, P.J. Pannexin Channel Regulation of Cell Migration: Focus on Immune Cells. Front. Immunol. 2021, 12, 5124. [Google Scholar] [CrossRef]
- Zhang, P.; Ishikawa, M.; Rhodes, C.; Doyle, A.; Ikeuchi, T.; Nakamura, K.; Chiba, Y.; He, B.; Yamada, Y. Pannexin-3 Deficiency Delays Skin Wound Healing in Mice Due to Defects in Channel Functionality. J. Investig. Dermatol. 2019, 139, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Wang, C.; Zhang, L. Epithelial Physical Barrier Defects in Chronic Rhinosinusitis. Expert Rev. Clin. Immunol. 2019, 15, 679–688. [Google Scholar] [CrossRef]
- Guerra, G.; Testa, D.; Salzano, F.A.; Tafuri, D.; Hay, E.; Schettino, A.; Iovine, R.; Marcuccio, G.; Motta, G. Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Chronic Rhinosinusitis with Nasal Polyps: Etiopathogenesis and Recurrence. Ear Nose Throat J. 2021, 100, 597S–605S. [Google Scholar] [CrossRef] [Green Version]
- Updegraff, B.L.; Zhou, X.; Guo, Y.; Padanad, M.S.; Chen, P.H.; Yang, C.; Sudderth, J.; Rodriguez-Tirado, C.; Girard, L.; Minna, J.D.; et al. Transmembrane Protease TMPRSS11B Promotes Lung Cancer Growth by Enhancing Lactate Export and Glycolytic Metabolism. Cell Rep. 2018, 25, 2223–2233.e6. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.; Zhao, S.; Le, H.T.; Wang, J.; Neurath, M.F.; Neufert, C.; Fiocchi, C.; Rieder, F. IL-36 in Chronic Inflammation and Fibrosis—Bridging the Gap? J. Clin. Investig. 2021, 131, e144336. [Google Scholar] [CrossRef]
- Scheibe, K.; Kersten, C.; Schmied, A.; Vieth, M.; Primbs, T.; Carlé, B.; Knieling, F.; Claussen, J.; Klimowicz, A.C.; Zheng, J.; et al. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice With Chronic Intestinal Inflammation. Gastroenterology 2019, 156, 1082–1097.e11. [Google Scholar] [CrossRef] [Green Version]
- Okada, N.; Nakayama, T.; Asaka, D.; Inoue, N.; Tsurumoto, T.; Takaishi, S.; Otori, N.; Kojima, H.; Matsuda, A.; Oboki, K.; et al. Distinct Gene Expression Profiles and Regulation Networks of Nasal Polyps in Eosinophilic and Non-Eosinophilic Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2018, 8, 592–604. [Google Scholar] [CrossRef]
- Workman, A.D.; Nocera, A.L.; Mueller, S.K.; Otu, H.H.; Libermann, T.A.; Bleier, B.S. Translating Transcription: Proteomics in Chronic Rhinosinusitis with Nasal Polyps Reveals Significant Discordance with Messenger RNA Expression. Int. Forum Allergy Rhinol. 2019, 9, 776–786. [Google Scholar] [CrossRef]
- Karjalainen, J.; Joki-Erkkila, V.-P.; Hulkkonen, J.; Pessi, T.; Nieminen, M.M.; Aromaa, A.; Klaukka, T.; Hurme, M. The IL1A Genotype Is Associated with Nasal Polyposis in Asthmatic Adults. Allergy 2003, 58, 393–396. [Google Scholar] [CrossRef]
- Karnati, R.; Talla, V.; Peterson, K.; Laurie, G.W. Lacritin and Other Autophagy Associated Proteins in Ocular Surface Health HHS Public Access Graphical Abstract. Exp. Eye Res. 2016, 144, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Kousvelari, E.E.; Baratz, R.S.; Burke, B.; Oppenheim, F.G. Immunochemical Identification and Determination of Proline-Rich Proteins in Salivary Secretions, Enamel Pellicle, and Glandular Tissue Specimens. J. Dent. Res. 1980, 59, 1430–1438. [Google Scholar] [CrossRef]
- Posch, B.; Irsara, C.; Gamper, F.S.; Herrmann, M.; Bindreither, D.; Fuchs, D.; Reider, N.; Redl, B.; Heufler, C. Allergenic Can f 1 and Its Human Homologue Lcn-1 Direct Dendritic Cells to Induce Divergent Immune Responses. J. Cell. Mol. Med. 2015, 19, 2375–2384. [Google Scholar] [CrossRef]
- Sifuentes-Dominguez, L.; Li, H.; Llano, E.; Liu, Z.; Singla, A.; Patel, A.S.; Kathania, M.; Khoury, A.; Norris, N.; Rios, J.J.; et al. SCGN Deficiency Results in Colitis Susceptibility. Elife 2019, 8, e49910. [Google Scholar] [CrossRef]
- Urbaniak, A.; Jablonska, K.; Podhorska-Okolow, M.; Ugorski, M.; Dziegiel, P. Prolactin-Induced Protein (PIP)-Characterization and Role in Breast Cancer Progression. Am. J. Cancer Res. 2018, 8, 2150–2164. [Google Scholar] [PubMed]
- Caputo, E.; Camarca, A.; Moharram, R.; Tornatore, P.; Thatcher, B.; Guardiola, J.; Martin, B.M. Structural Study of GCDFP-15/Gp17 in Disease versus Physiological Conditions Using a Proteomic Approach. Biochemistry 2003, 42, 6169–6178. [Google Scholar] [CrossRef]
- Ellis, A.K.; Tenn, M.W. Advances in Rhinitis: Models and Mechanisms. Ann. Allergy Asthma Immunol. 2018, 121, 61–64. [Google Scholar] [CrossRef]
- Trzeciak, M.; Sakowicz-Burkiewicz, M.; Wesserling, M.; Gleń, J.; Dobaczewska, D.; Bandurski, T.; Nowicki, R.; Pawelczyk, T. Altered Expression of Genes Encoding Cornulin and Repetin in Atopic Dermatitis. Int. Arch. Allergy Immunol. 2017, 172, 11–19. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, R.; Bo, M.; Fan, E.; Duan, S.; Zhang, L. The Expression of Epithelial Intercellular Junctional Proteins in the Sinonasal Tissue of Subjects with Chronic Rhinosinusitis: A Histopathologic Study. Orl 2014, 76, 110–119. [Google Scholar] [CrossRef]
- Wu, N.; Song, Y.; Pang, L.; Chen, Z. CRCT1 Regulated by MicroRNA-520 g Inhibits Proliferation and Induces Apoptosis in Esophageal Squamous Cell Cancer. Tumor Biol. 2016, 37, 8271–8279. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Azouz, N.P.; Ynga-Durand, M.A.; Caldwell, J.M.; Jain, A.; Rochman, M.; Fischesser, D.M.; Ray, L.M.; Bedard, M.C.; Mingler, M.K.; Forney, C.; et al. The Antiprotease SPINK7 Serves as an Inhibitory Checkpoint for Esophageal Epithelial Inflammatory Responses. Sci. Transl. Med. 2018, 10, eaap9736. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.J.; Liu, H.B. Identification and Validation of Ferroptosis-Related Genes for Chronic Rhinosinusitis with Nasal Polyps. Eur. Arch. Oto-Rhino-Laryngol. 2022, 280, 1501–1508. [Google Scholar] [CrossRef]
- Liu, T.; Xu, P.; Ke, S.; Dong, H.; Zhan, M.; Hu, Q.; Li, J. Histone Methyltransferase SETDB1 Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition in Pulmonary Fibrosis by Regulating SNAI1 Expression and the Ferroptosis Signaling Pathway. Arch. Biochem. Biophys. 2022, 715, 109087. [Google Scholar] [CrossRef]
- Ryu, G.; Mo, J.-H.; Shin, H.-W. Epithelial-to-Mesenchymal Transition in Neutrophilic Chronic Rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Bailey, M.; Zaunders, J.; Mrad, N.; Sacks, R.; Sewell, W.; Harvey, R.J. Cellular Comparison of Sinus Mucosa vs Polyp Tissue from a Single Sinus Cavity in Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Mackay, I.S. Staging in Rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Psaltis, A.J.; Li, G.; Vaezeafshar, R.; Cho, K.-S.; Hwang, P.H. Modification of the Lund-Kennedy Endoscopic Scoring System Improves Its Reliability and Correlation with Patient-Reported Outcome Measures. Laryngoscope 2014, 124, 2216–2223. [Google Scholar] [CrossRef]
- Urbančič, J.; Soklič Košak, T.; Jenko, K.; Iglič, Č.; Matos, A.; Rainer, K.; Gluvajić, D.; Kordiš, Š.; Škafar, I.; Božanić Urbančič, N.; et al. SNOT-22: Ovrednotenje Vprašalnika in Ocena Zdravljenja Kroničnega Rinosinuzitisa. Med. Razgl. 2016, 55, 39–45. [Google Scholar]
- Liu, C.; Di, Y.P. Molecular Toxicology Protocols; Keohavong, P., Singh, K.P., Gao, W., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2102, ISBN 978-1-0716-0222-5. [Google Scholar]



| Controls | Patients with CRSwNP | Patients with CRSsNP | p | |
|---|---|---|---|---|
| Subjects, n (%) | 15 (31) | 22 (46) | 11 (23) | - |
| Age (mean, min.–max., SD; years) | 45.7 (18–79, SD 18.8) | 54.2 (18–82, SD 16.9) | 55.6 (33–78, SD 15.1) | 0.21 1 |
| Females, n (%) | 4 (27) | 9 (41) | 5 (46) | 0.59 2 |
| Family history of CRS, n (%) | 0 (0) | 4 (18) | 2 (18) | 0.24 2 |
| Allergy, n (%) | - | 12 (54) | 4 (36) | 0.46 3 |
| Asthma, n (%) | - | 9 (41) | 2 (18) | 0.26 3 |
| GERD, n (%) | 0 (0) | 4 (18) | 7 (64) | 0.02 3 |
| GERD on therapy, n (%) | 0 (0) | 3 (14) | 5 (45) | 0.08 3 |
| Smell subjectively good, n (%) | 15 (100) | 11 (50) | 11 (100) | <0.001 1 |
| Smell SS 12 score (mean, min.–max., SD) | 9.56 (8–12, SD 1.5) | 5.2 (2–7, SD 1.7) | 9 (6–11, SD 1.8) | <0.001 1,5 |
| Patients with eosinophilia in tissue samples, n (%) | - | 22 (100) | 1 (9) | <0.001 3 |
| Eosinophilia in tissue samples (mean, min.–max., SD) No./HPF | - | 30 (10–60, SD 12.2) | 10 | - |
| Patients with eosinophilia in blood, n (%) | - | 8 (36) | 1 (9) | 0.21 3 |
| No. of past surgeries (mean, min.–max., SD) | - | 0.6 (0–3, SD 0.9) | 0.5 (0–2, SD 0.7) | 0.72 4 |
| Time from last surgery (mean, min–max, SD; years) | - | 5.8 (2–11, SD 3) | 5.5 (0.8–16, SD 7) | 0.33 4 |
| Patients with a need for systemic steroids (>2 times in past year), n (%) | - | 10 (45) | - | - |
| Lund–Mackay score (range 0–24) mean, min.–max., SD) | - | 18.1 (13–22, SD 2.2) | 12.4 (7–20, SD 3.8) | <0.001 4 |
| Modified Kennedy–Lund score [21] (range 0–12), (mean, min.–max., SD) | - | 9.7 (4–12, SD 2.4) | 6 (2–8, SD 2.1) | <0.001 4 |
| Total polyp score (range 0–8) (mean, min.–max., SD) | - | 5.9 (2–8, SD 2.2) | 0 | - |
| CRS symptoms VAS score (range 0–10); (mean, min.–max., SD) | 4.9 (1–9, SD 2.3) | 7.4 (4–9, SD 1.3) | 6.3 (3–8, SD 1.4) | 0.02 4 |
| SNOT-22 score (mean, min.–max., SD) | 7.3 (2–15, SD 3.7) | 43.9 (18–59, SD 12.3) | 41.7 (21–70, SD 13.9) | 0.31 4 |
| Patients with T2 clinical endotype, n (%) | 22 (100) | 1 (9) | <0.001 3 | |
| Patients with non-T2 clinical endotype, n (%) | 0 | 10 (91) | <0.0013 |
| Full Gene Name | Gene | CRSsNP vs. Control—Log2 FC | CRSsNP vs. Control—FDR | CRSwNP vs. CRSsNP—Log2 FC | CRSwNP vs. CRSsNP—FDR | CRSwNP vs. Control—Log2 FC | CRSwNP vs. Control—FDR |
|---|---|---|---|---|---|---|---|
| Chloride Channel Accessory 1 | CLCA1 | −1.85 | 3.56 × 10−1 | 10.52 | 5.10 × 10−12 | 8.70 | 1.00 × 10−10 |
| Lipopolysaccharide-binding protein | LBP | 9.32 | 5.31 × 10−13 | −6.94 | 4.11 × 10−9 | 2.42 | 4.17 × 10−2 |
| Lacritin | LACRT | −0.14 | 9.52 × 10−1 | −6.53 | 1.96 × 10−8 | −6.64 | 1.00 × 10−10 |
| Pannexin 3 | PANX3 | 7.58 | 9.51 × 10−10 | −5.43 | 1.90 × 10−9 | 2.10 | 2.13 × 10−1 |
| C-X-C Motif Chemokine Ligand 13 | CXCL13 | 5.21 | 2.19 × 10−7 | −5.33 | 9.48 × 10−10 | −0.04 | 9.79 × 10−1 |
| Lipocalin 1 | LCN1 | −2.16 | 2.08 × 10−1 | −5.08 | 4.30 × 10−7 | −7.17 | 1.35 × 10−13 |
| Prolactin-induced protein | PIP | −1.12 | 3.79 × 10−1 | −4.87 | 7.64 × 10−7 | −5.94 | 1.00 × 10−10 |
| Cystatin D | CST5 | −1.99 | 1.84 × 10−1 | −4.13 | 7.76 × 10−4 | −6.11 | 1.63 × 10−11 |
| Osteocrin | OSTN | 4.96 | 4.62 × 10−5 | −4.10 | 9.03 × 10−5 | 0.81 | 6.61 × 10−1 |
| Matrix metallopeptidase 9 | MMP9 | 5.07 | 1.00 × 10−10 | −2.63 | 3.92 × 10−3 | 2.50 | 2.92 × 10−4 |
| C-C motif chemokine ligand 7 | CCL7 | 3.68 | 1.22 × 10−2 | 2.85 | 9.32 × 10−2 | 6.64 | 3.58 × 10−8 |
| G protein-coupled receptor G7 | ADGRG7 | 0.38 | 8.65 × 10−1 | 6.00 | 1.96 × 10−4 | 6.67 | 2.53 × 10−7 |
| Desmoglein−1 | DSG1 | −9.24 | 3.37 × 10−8 | 6.24 | 8.18 × 10−4 | −2.91 | 6.17 × 10−2 |
| MT-RNR2 like 9 pseudogene | MTRNR2L9 | 0.31 | 9.12 × 10−1 | 7.07 | 1.40 × 10−4 | 7.28 | 5.26 × 10−7 |
| Small proline-rich protein 1A | SPRR1A | −8.33 | 1.40 × 10−13 | 7.89 | 1.02 × 10−10 | −0.50 | 8.44 × 10−1 |
| small proline-rich protein 2D | SPRR2D | −8.51 | 1.27 × 10−11 | 8.45 | 1.45 × 10−9 | −0.06 | 9.84 × 10−1 |
| Late cornified envelope 3D | LCE3D | −11.07 | 1.18 × 10−8 | 9.02 | 1.87 × 10−5 | −1.96 | 3.48 × 10−1 |
| Serine peptidase inhibitor kazal type 7 | SPINK7 | −11.33 | 1.00 × 10−10 | 9.33 | 8.05 × 10−9 | −1.95 | 3.77 × 10−1 |
| Involucrin | IVL | −11.55 | 1.00 × 10−10 | 10.08 | 5.94 × 10−11 | −1.48 | 5.03 × 10−1 |
| Endotypes 1 | Cytokine 1 | Full Gene Name | Gene | CRSsNP vs. Control—Log2 FC | CRSsNP vs. Control—FDR | CRSwNP vs. CRSsNP—Log2 FC | CRSwNP vs. CRSsNP—FDR | CRSwNP vs. Control—Log2 FC | CRSwNP vs. Control—FDR |
|---|---|---|---|---|---|---|---|---|---|
| T1 | IFN-γ | Interferon gamma | IFNG | 1.84 | 2.12 × 10−2 | −1.49 | 1.10 × 10−1 | 0.39 | 7.52 × 10−1 |
| IL-12 | Interleukin 12B | IL12B | 2.16 | 2.07 × 10−2 | −1.74 | 5.28 × 10−2 | 0.47 | 7.72 × 10−1 | |
| T2 | IL-5 | Interleukin 5 | IL5 | 0.40 | 8.29 × 10−1 | 3.82 | 2.57 × 10−4 | 4.23 | 4.66 × 10−7 |
| IL-13 | Interleukin 13 | IL13 | 0.64 | 5.95 × 10−1 | 2.96 | 1.81 × 10−3 | 3.61 | 1.49 × 10−7 | |
| T3 | IL-17 | Interleukin 17B | IL17B | 0.16 | 9.01 × 10−1 | −2.24 | 1.65 × 10−2 | −2.02 | 3.27 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbančič, J.; Košak Soklič, T.; Demšar Luzar, A.; Hočevar Boltežar, I.; Korošec, P.; Rijavec, M. Transcriptomic Differentiation of Phenotypes in Chronic Rhinosinusitis and Its Implications for Understanding the Underlying Mechanisms. Int. J. Mol. Sci. 2023, 24, 5541. https://doi.org/10.3390/ijms24065541
Urbančič J, Košak Soklič T, Demšar Luzar A, Hočevar Boltežar I, Korošec P, Rijavec M. Transcriptomic Differentiation of Phenotypes in Chronic Rhinosinusitis and Its Implications for Understanding the Underlying Mechanisms. International Journal of Molecular Sciences. 2023; 24(6):5541. https://doi.org/10.3390/ijms24065541
Chicago/Turabian StyleUrbančič, Jure, Tanja Košak Soklič, Ajda Demšar Luzar, Irena Hočevar Boltežar, Peter Korošec, and Matija Rijavec. 2023. "Transcriptomic Differentiation of Phenotypes in Chronic Rhinosinusitis and Its Implications for Understanding the Underlying Mechanisms" International Journal of Molecular Sciences 24, no. 6: 5541. https://doi.org/10.3390/ijms24065541
APA StyleUrbančič, J., Košak Soklič, T., Demšar Luzar, A., Hočevar Boltežar, I., Korošec, P., & Rijavec, M. (2023). Transcriptomic Differentiation of Phenotypes in Chronic Rhinosinusitis and Its Implications for Understanding the Underlying Mechanisms. International Journal of Molecular Sciences, 24(6), 5541. https://doi.org/10.3390/ijms24065541







