Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage
Abstract
:1. Introduction
2. Results
2.1. Quercetin Increases the Viability and Decreases the Differentiation of LPS-Induced RAW264.7 Cells
2.2. Quercetin Inhibits ROS Production and Pro-Inflammatory Cytokine Expression, and Improves the Antioxidant Capacity of LPS-Induced RAW264.7 Cells
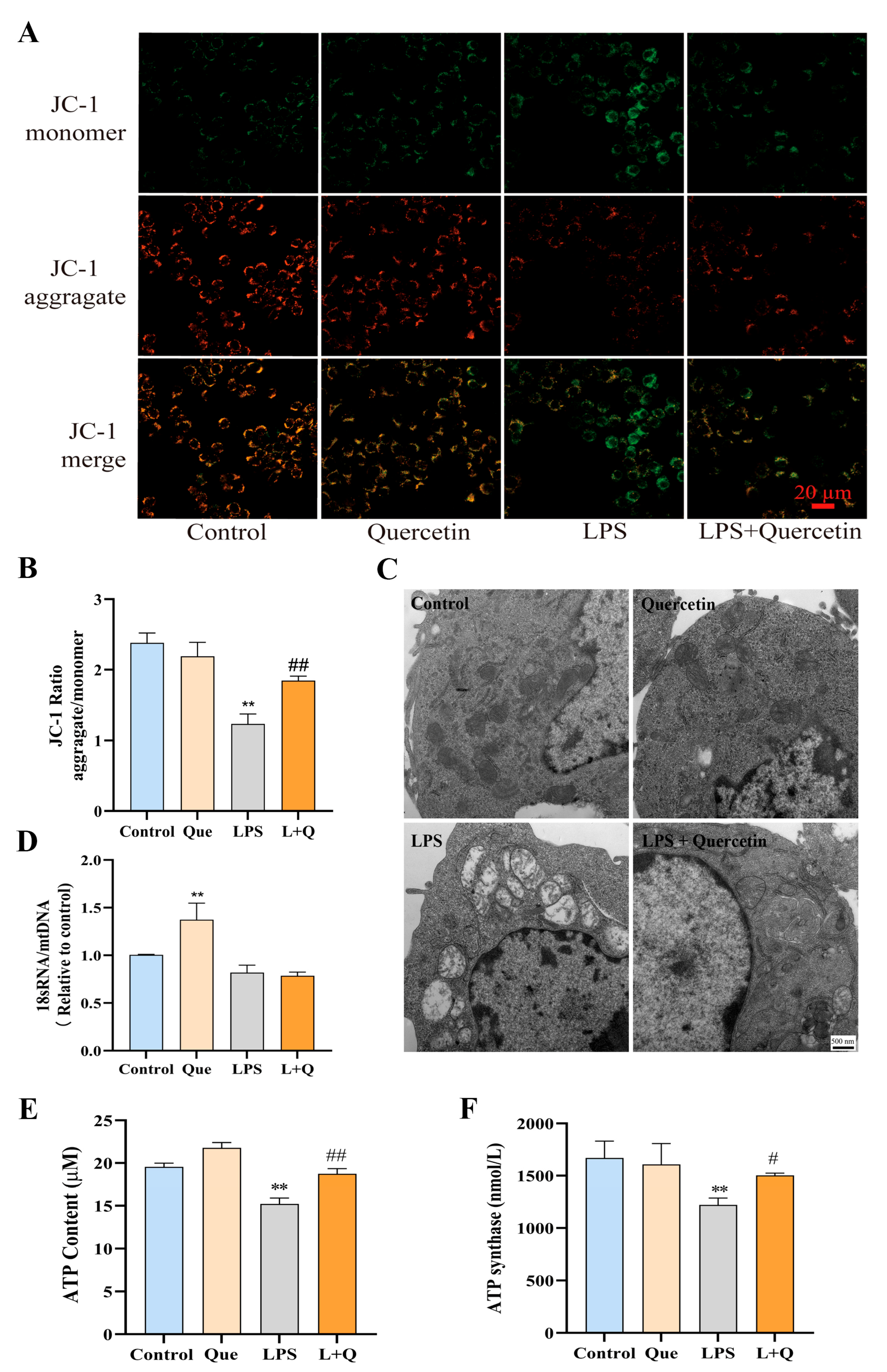
2.3. Quercetin Protects Mitochondrial Function and Prevents LPS-Induced Mitochondrial Morphological Damage
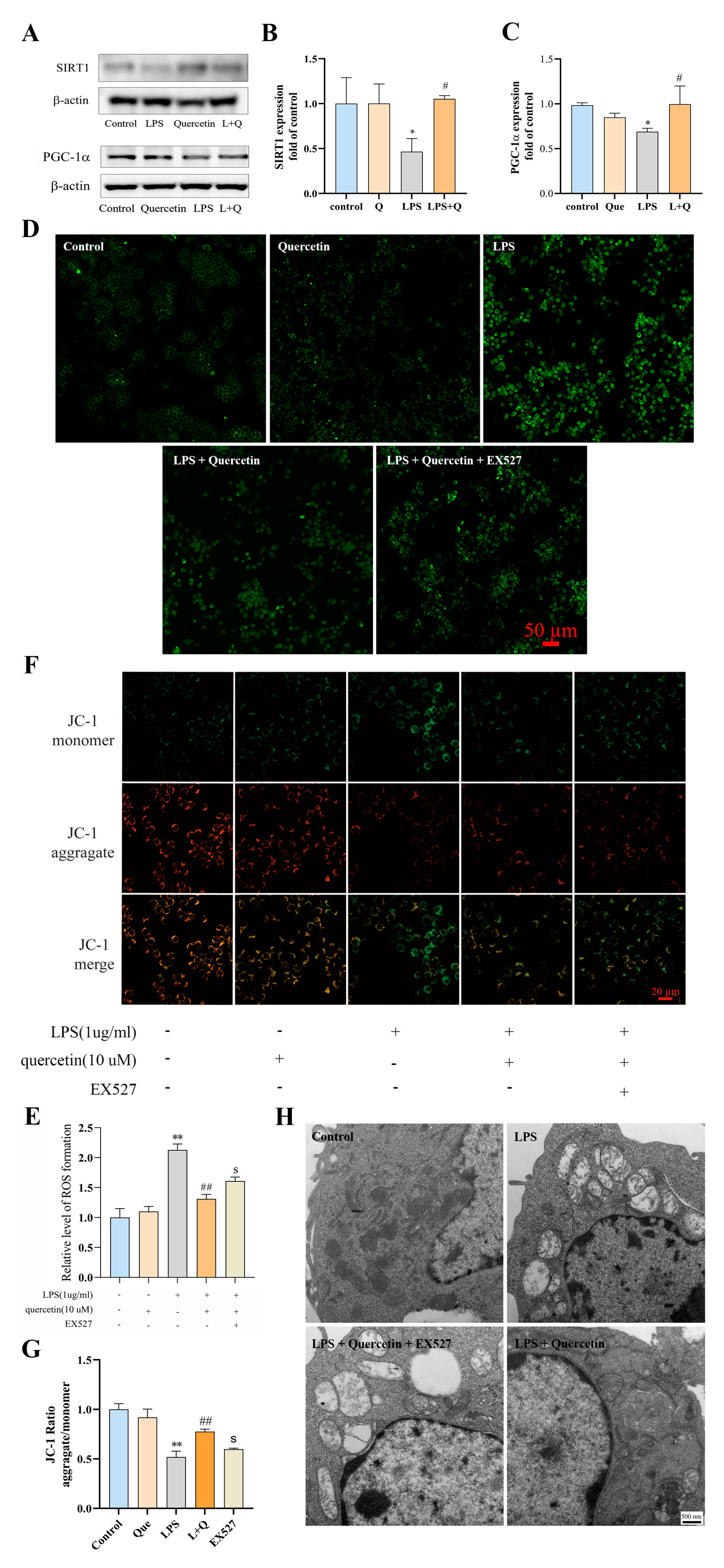
2.4. Quercetin Suppresses LPS-Induced ROS Production and Mitochondrial Damage in RAW264.7 Cells via the SIRT1/PGC-1a Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Observation of Cell Morphological Changes
4.5. Cell Proliferation Assay
4.6. Determination of Cellular ROS Production
4.7. Evaluation of Enzyme Activity
4.8. Detection of mRNA Expression
4.9. Detection of Effects on Mitochondria
4.9.1. Detection of Mitochondrial Membrane Potential
4.9.2. Observation of Mitochondrial Morphology
4.9.3. Determination of Mitochondrial DNA Copy Number
4.9.4. Determination of Mitochondrial Function
ATP Contents
ATP Synthase Content
4.10. Western Blotting Analysis
4.11. SIRT-1 Inhibition Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef]
- Devanneya, N.; Stewarta, A.N.; Gensel, J.C. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp. Neurol. 2020, 329, 113310. [Google Scholar] [CrossRef] [PubMed]
- Piganelli, J.; Flores, S.; Cruz, C.; Koepp, J.; Batinic-haberle, I.; Crapo, J.; Day, B.; Kachadourian, R.; Young, R.; Bradley, B.; et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-Cell clone. Diabetes 2002, 51, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.L.; Deng, H.D.; Cui, H.M.; Fang, J.; Zuo, Z.C.; Deng, J.L.; Li, Y.L.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 4, 480–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.J.; Kundu, J.K.; Na, H.K.; Lee, J.S. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J. Nutr. 2005, 135 (Suppl. S12), 2993S–3001S. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.; Wang, X.L.; Vikash, V.; Ye, Q.; Wu, D.D.; Liu, Y.L.; Dong, W.G. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [Green Version]
- Chelombitko, M.A. Role of reactive oxygen species in inflammation: A minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Ginhoux, F.; Schultze, J.L.; Murray, P.J.; Ochando, J.; Biswas, S.K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 2016, 17, 34–40. [Google Scholar] [CrossRef]
- Geeraerts, X.; Bolli, E.; Fendt, S.M.; Ginderachter, J.A.V. Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front. Immunol. 2017, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.H.; Sluimer, J.C.; Wang, Y.; Subramanian, M.; Brown, K.; Pattison, J.S.; Robbins, J.; Martinez, J.; Tabas, I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012, 15, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennery, P.A. Signaling function of heme oxygenase proteins. Antioxid. Redox. Signal 2014, 20, 1743–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, W.; Itoh, K.; Akutsu, S.; Kishi, H.; Ohhira, S. Nasunin inhibits the lipopolysaccharide-induced pro-inflammatory mediator production in RAW264 mouse macrophages by suppressing ROS-mediated activation of PI3K/Akt/NF-κB and p38 signaling pathways. Biosci. Biotechnol. Biochem. 2017, 81, 1956–1966. [Google Scholar] [CrossRef] [Green Version]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Majewska, M.; Skrzycki, M.; Podsiad, M.; Czeczot, H. Evaluation of antioxidant potential of flavonoids: An in vitro study. Acta Pol. Pharm. 2011, 68, 611–615. [Google Scholar]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Ferreira, I.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. The flavone luteolin inhibits liver x receptor activation. J. Nat. Prod. 2016, 79, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Nijveldt, R.J.; Nood, E.V.; Hoorn, D.E.V.; Boelens, P.G.; Norren, K.V.; Leeuwen, P.A.V. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.R.; Li, G.H.; Zhou, M.X.; Xiang, L.; Ren, D.M.; Lou, H.X.; Wang, X.N.; Shen, T. Discovery of natural flavonoids as activators of Nrf2-mediated defense system: Structure-activity relationship and inhibition of intracellular oxidative insults. Bioorganic Med. Chem. 2018, 26, 5140–5150. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Drent, M.; Boer, V.C.J.; Bast, A.; Haenen, G.R.M. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; Haenen, G.R.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerin, F.; Sener, U.; Erman, H.; Yilmaz, A.; Aydin, B.; Armutcu, F.; Gurel, A. The effects of quercetin on acute lung injury and biomarkers of inflammation and oxidative stress in the rat model of sepsis. Inflammation 2016, 39, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, W.J.; Choi, J.; Frei, B. Quercetin affects glutathione levels and redox ratio in human aortic endothelial cells not through oxidation but formation and cellular export of quercetin-glutathione conjugates and upregulation of glutamate-cysteine ligase. Redox Biol. 2016, 9, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Galleggiante, V.; Santis, D.S.; Liso, M.; Verna, G.; Sommella, E.; Mastronardi, M.; Campiglia, P.; Chieppa, M.; Serino, G. Quercetin-induced miR-369-3p suppresses chronic inflammatory response targeting C/EBP-b. Mol. Nutr. Food Res. 2019, 63, e1801390. [Google Scholar] [CrossRef]
- Tan, R.Z.; Wang, C.; Deng, C.; Zhong, X.; Yan, Y.; Luo, Y.; Lan, H.Y.; He, T.; Wang, L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NFkB signaling maintained macrophage inflammation. Phytother. Res. 2020, 34, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Saccol, R.S.; Silveira, K.L.; Manzoni, A.G.; Abdalla, F.H.; Oliveira, J.S.; Dornelles, G.L.; Barbisan, F.; Passos, D.F.; Casali, E.A.; Andrade, C.M.; et al. Antioxidant, hepatoprotective, genoprotective, and cytoprotective effects of quercetin in a murine model of arthritis. J. Cell Biochem. 2019, 121, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.F.; Dong, Y.Y.; Sun, J.J.; Sun, M.; Hou, Q.H.; Lai, Y.J.; Zhang, B.K. Protective effect of kaempferol on LPS-induced inflammation and barrier dysfunction in a coculture model of intestinal epithelial cells and intestinal microvascular endothelial cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, L.L.; Zhao, W.B.; Dou, Y.; An, H.J.; Tao, H.; Xu, X.Q.; Jia, Y.; Lu, S.; Zhang, J.X.; et al. Targeted therapy of atherosclerosis by a broad-Spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Fazzini, F.; Lamina, C.; Raftopoulou, A.; Koller, A.; Fuchsberger, C.; Pattaro, C.; Del Greco, F.M.; Döttelmayer, P.; Fendt, L.; Fritz, J.; et al. Association of mitochondrial DNA copy number with metabolic syndrome and type 2 diabetes in 14 176 individuals. J. Intern. Med. 2021, 290, 190–202. [Google Scholar] [CrossRef]
- Castracani, C.C.; Longhitano, L.; Distefano, A.; Anfuso, A.; Kalampoka, S.; Spina, E.L.; Astuto, M.; Avola, R.; Caruso, M.; Nicolosi, D.; et al. Role of 17β-estradiol on cell proliferation and mitochondrial fitness in glioblastoma cells. J. Oncol. 2020, 2020, 2314693. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Li, S.Y.; Lv, Y.Y.; Yang, D.Q.; Li, J.Y.; Yang, Q.Y.; Wu, P.F.; Lv, Z.J.; Zhang, Z.G. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019, 10, 5555–5565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, W.W.; Cheng, X.; Xiang, H.R.; He, B.; Zhang, Q.Z.; Peng, W.X. Curcumin attenuates isoniazid-induced hepatotoxicity by upregulating the SIRT1/PGC-1α/NRF1 pathway. J. Appl. Toxicol. 2022, 7, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D., Eds.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Khaper, N.; Bryan, S.; Dhingra, S.; Singal, R.; Bajaj, A.; Pathak, C.M.; Singal, P.K. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid. Redox Signal 2010, 13, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Suzuki, M.; Takashima, S.; Sasaki, T.; Oh-Hashi, K.; Takemori, H. The new live imagers MitoMM1/2 for mitochondrial visualization. Biochem. Biophys. Res. Commun. 2021, 562, 50–54. [Google Scholar] [CrossRef]
- Meo, S.D.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Song, G.H.; Wang, R.L.; Chen, Z.Y.; Zhang, B.; Wang, H.L.; Liu, M.L.; Gao, J.P.; Yan, X.Y. Toxic effects of sodium fluoride on cell proliferation and apoptosis of Leydig cells from young mice. J. Physiol. Biochem. 2014, 70, 761–768. [Google Scholar] [CrossRef]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Gorlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, X.; Chen, Y.; Wang, H.; Zhang, R.; Zhang, Q.; Wei, Y.T.; Shi, S.N.; Li, X. Calreticulin regulated intrinsic apoptosis through mitochondria-dependent and independent pathways mediated by ER stress in arsenite exposed HT-22 cells. Chemosphere 2020, 251, 126466. [Google Scholar] [CrossRef]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.Y.; Li, Y.; Hu, L.M.; Guo, J.Y.; et al. Copper induces oxidative stress and apoptosis through mitochondria mediated pathway in chicken hepatocytes. Toxicol. In Vitro 2019, 54, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Qian, Y.; Chohan, K.R.; Shirley, C.R.; Amidon, W.; Banerjee, S.; Middleton, F.A.; Conkrite, K.L.; Barcza, M.; Gonchoroff, N.; et al. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc. Natl. Acad. Sci. USA 2006, 103, 14813–14818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, K.; Sundquist, J.; Palmer, K.; Ashfaque, M. Role of mitochondrial DNA copy number in incident cardiovascular diseases and the association between cardiovascular disease and type 2 diabetes: A follow-up study on middle-aged women. Atherosclerosis 2022, 341, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Eaton, J.S. Regulation of mtDNA copy number by the ATM/ATR signaling pathway. Faseb J. 2006, 20, A510. [Google Scholar] [CrossRef]
- Galeota-Sprung, B.; Fernandez, A.; Sniegowski, P. Changes to the mtDNA copy number during the course of yeast culture growth. R. Soc. Open Sci. 2022, 9, 211842. [Google Scholar] [CrossRef]
- Haikonen, J. Effects of Mitochondrial DNA Replication Stress and Double-Strand Breaks on DNA Damage Response Pathways and Mitochondrial Gene Expression. Master’s Thesis, University of Eastern Finland, Kuopio, Finland, February 2018. [Google Scholar]
- Ramzan, R.; Dolga, A.M.; Michels, S.; Weber, P.; Culmsee, C.; Rastan, A.J.; Vogt, S. Cytochrome c oxidase inhibition by ATP decreases mitochondrial ROS production. Cells 2022, 11, 992. [Google Scholar] [CrossRef]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H2O2 signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Artika, I.M. Current understanding of structure, function and biogenesis of yeast mitochondrial ATP synthase. J. Bioenerg. Biomembr. 2019, 51, 315–328. [Google Scholar] [CrossRef]
- Srivastava, A.P.; Luo, M.; Zhou, W.; Symersky, J.; Bai, D.; Chambers, M.G.; Faraldo-Gomez, J.D.; Liao, M.; Mueller, D.M. High-resolution cryo-EM analysis of the yeast ATP synthase in a lipid membrane. Science 2018, 360, eaas9699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Pedersen, P.L. ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol. Mol. Biol. Rev. 2008, 72, 590–641. [Google Scholar] [CrossRef] [Green Version]
- Daniele, G.; Massimo, Z. Human diseases associated with defects in assembly of oxphos complexes. Essays Biochem. 2018, 62, 271–286. [Google Scholar] [CrossRef]
- Suwanjang, W.; Ruankham, W.; Chetsawang, B.; Mukda, S.; Ngampramuan, S.; Srisung, S.; Prachayasittikul, V.; Prachayasittikul, S. Spilanthes acmella Murr. ameliorates chronic stress through improving mitochondrial function in chronic restraint stress rats. Neurochem. Int. 2021, 148, 105083. [Google Scholar] [CrossRef] [PubMed]
- Balaji, B.; Cassel, S.L. Mitochondria in innate immune signaling. Transl. Res. 2018, 202, 52–67. [Google Scholar] [CrossRef]
- Alice, R.; Paola, P.; Riccardo, F. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. BBA-Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. BBA-Bioenergetics 2010, 1797, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Li, Y.; Shi, L.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H. Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology 2022, 469, 153136. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Poznyak, A.V.; Sobenin, I.A.; Nikifirov, N.N.; Ivanova, E.A. Mitochondrion as a Selective Target for the Treatment of Atherosclerosis: Role of Mitochondrial DNA Mutations and Defective Mitophagy in the Pathogenesis of Atherosclerosis and Chronic Inflammation. Curr. Neuropharmacol. 2020, 18, 1067–1075. [Google Scholar] [CrossRef]
- Dehghan, E.; Goodarzi, M.; Saremi, B.; Lin, R.; Mirzaei, H. Hydralazine targets cAMP-dependent protein kinase leading to sirtuin1/5 activation and lifespan extension in C. elegans. Nat. Commun. 2019, 10, 4905. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Wu, Y.; Zhai, Y.; Hu, B.; Ma, W.; Yang, W.; Yu, Q.; Chen, Z.; Workman, J.L.; Yu, X.; et al. Exogenous pyruvate represses histone gene expression and inhibits cancer cell proliferation via the NAMPT-NAD+-SIRT1 pathway. Nucleic Acids Res. 2019, 47, 11132–11150. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Baiyun, R.; Lv, Z.; Li, J.; Han, D.; Zhao, W.; Yu, L.; Deng, N.; Liu, Z.; Zhang, Z. Exploring the kidney hazard of exposure to mercuric chloride in mice: Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere 2019, 234, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kageyama, S.; Ke, B.; Fujii, T.; Sosa, R.A.; Reed, E.F.; Datta, N.; Zarrinpar, A.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Sirtuin 1 attenuates inflammation and hepatocellular damage in liver transplant ischemia/reperfusion: From mouse to human. Liver Transpl. 2017, 23, 1282–1293. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhou, M.; Zhu, R.; Zhou, J.; Ni, L.; Wang, Z.; Liu, N.; Zhu, F.; Shi, T.; Deng, Z.; et al. Hydrogen sulfide protects against particle-induced inflammatory response and osteolysis via SIRT1 pathway in prosthesis loosening. Faseb J. 2020, 34, 3743–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Hu, Q.; Ma, Q.; Liu, C.; Wang, G. The protease Omi regulates mitochondrial biogenesis through the GSK3b/PGC-1a pathway. Cell Death Dis. 2014, 5, e1373. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.J.; Lerin, C.; Haas, W.; Gygi, S.P.; Puigserver, P. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation. J. Biol. Chem. 2009, 284, 19945–19952. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver P, P. Metabolic control of mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef]
- Zhang, L.X.; Sun, X.M.; Jia, Y.B.; Liu, X.G.; Dong, M.D.; Xu, Z.P.; Liu, R.T. Nanovaccine’s rapid induction of anti-tumor immunity significantly improves malignant cancer immunotherapy. Nano Today 2020, 35, 100923. [Google Scholar] [CrossRef]
- Ding, X.Y.; Sun, W.; Chen, J.L. IL-2 augments the sorafenib-induced apoptosis in liver cancer by promoting mitochondrial fission and activating the JNK/TAZ pathway. Cancer Cell Int. 2018, 9, 176. [Google Scholar] [CrossRef]
- Chen, K.L.; Li, H.X.; Xu, X.L.; Zhou, G.H. The protective effect of rosmarinic acid on hyperthermia-induced C2C12 muscle cells damage. Mol. Biol. Rep. 2014, 41, 5525–5531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Dong, L.; Yang, L.Q.; Luo, Y.H.; Chen, F. MiR-27a-5p regulates acrylamide-induced mitochondrial dysfunction and intrinsic apoptosis via targeting Btf3 in rats. Food Chem. 2022, 368, 130816. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol | Sequence (5′ → 3′) |
|---|---|
| IL-1β | (F) TCGCTCAGGGTCACAAGAAA |
| (R) CATCAGAGGCAAGGAGGAAAAC | |
| IL-6 | (F) TCTATACCACTTCACAAGTCGGA |
| (R) GAATTGCCATTGCACAACTCTTT | |
| TNF-α | (F) GCCACCACGCTCTTCTGTCT |
| (R) GTCTGGGCCATAGAACTGAT | |
| NF-κB | (F) GAGGTCTCTGGGGGTACCAT |
| (R) TTGCGGAAGGATGTCTCCAC | |
| β-actin | (F) GAAGATCAAGATCATTGCTCCT |
| (R) TGGAAGGTGGACAGTGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 5542. https://doi.org/10.3390/ijms24065542
Peng J, Yang Z, Li H, Hao B, Cui D, Shang R, Lv Y, Liu Y, Pu W, Zhang H, et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. International Journal of Molecular Sciences. 2023; 24(6):5542. https://doi.org/10.3390/ijms24065542
Chicago/Turabian StylePeng, Jing, Zhen Yang, Hao Li, Baocheng Hao, Dongan Cui, Ruofeng Shang, Yanan Lv, Yu Liu, Wanxia Pu, Hongjuan Zhang, and et al. 2023. "Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage" International Journal of Molecular Sciences 24, no. 6: 5542. https://doi.org/10.3390/ijms24065542





