Interest of Procalcitonin in ANCA Vasculitides for Differentiation between Flare and Infections
Abstract
:1. Introduction
2. Results
2.1. Demographic, Clinical, and Laboratory Characteristics of Patients at Diagnosis of AAV
2.2. Characteristics of Relapses and Infections
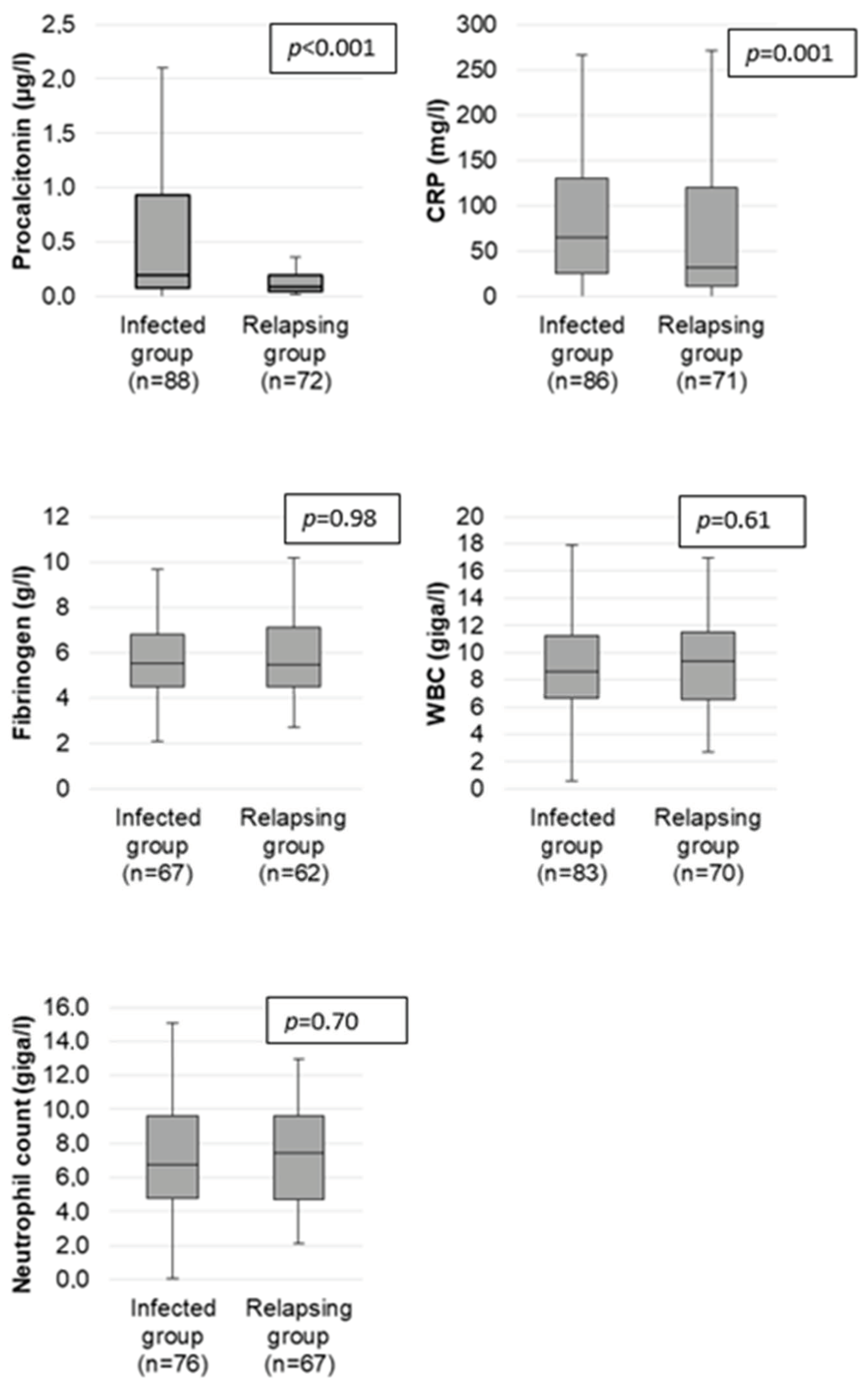
2.3. Clinical and Laboratory Markers in the Infected and Relapsing Group
2.4. PCT and CRP Performance for Diagnosis of Infection
2.5. Analysis of the Relative Risk of Infection According to Different Clinical and Biological Parameters
2.6. PCT in Remission and in the Relapsing Group
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Data Collection
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flossmann, O.; Berden, A.; de Groot, K.; Hagen, C.; Harper, L.; Heijl, C.; Höglund, P.; Jayne, D.; Luqmani, R.; Mahr, A.; et al. Long-term patient survival in ANCA-associated vasculitis. Ann. Rheum. Dis. 2011, 70, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, S.; Singh, H.; Chaturvedi, S.; Mishra, R.; Rai, M.K.; Jain, A.; Misra, D.P.; Agarwal, V. Utility of neutrophil CD64 and serum TREM-1 in distinguishing bacterial infection from disease flare in SLE and ANCA-associated vasculitis. Clin. Rheumatol. 2019, 38, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Kettelhack, C.; Hohenberger, P.; Schulze, G.; Kilpert, B.; Schlag, P.M. Induction of systemic serum procalcitonin and cardiocirculatory reactions after isolated limb perfusion with recombinant human tumor necrosis factor-alpha and melphalan. Crit. Care Med. 2000, 28, 1040–1046. [Google Scholar] [CrossRef]
- Nijsten, M.W.; Olinga, P.; The, H.T.; de Vries, E.G.; Koops, H.S.; Groothuis, G.M.; Limburg, P.C.; Duis, H.J.T.; Moshage, H.; Hoekstra, H.J.; et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit. Care Med. 2000, 28, 458–461. [Google Scholar] [CrossRef]
- Herrmann, K.; Schinke, S.; Csernok, E.; Moosig, F.; Holle, J.U. Diagnostic Value of Procalcitonin in ANCA—Associated Vasculitis (AAV) to Differentiate Between Disease Activity, Infection and Drug Hypersensitivity. Open Rheumatol. J. 2015, 9, 71–76. [Google Scholar] [CrossRef]
- Assicot, M.; Gendrel, D.; Carsin, H.; Raymond, J.; Guilbaud, J.; Bohuon, C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet Lond. Engl. 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Müller, B. Procalcitonin in bacterial infections—hype, hope, more or less? Swiss Med. Wkly. 2005, 135, 451–460. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Müller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet Lond. Engl. 2004, 363, 600–607. [Google Scholar] [CrossRef]
- Eberhard, O.K.; Haubitz, M.; Brunkhorst, F.M.; Kliem, V.; Koch, K.M.; Brunkhorst, R. Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis) and invasive bacterial infection. Arthritis Rheum. 1997, 40, 1250–1256. [Google Scholar] [CrossRef]
- Schwenger, V.; Sis, J.; Breitbart, A.; Andrassy, K. CRP levels in autoimmune disease can be specified by measurement of procalcitonin. Infection 1998, 26, 274–276. [Google Scholar] [CrossRef]
- Moosig, F.; Csernok, E.; Reinhold-Keller, E.; Schmitt, W.; Gross, W.L. Elevated procalcitonin levels in active Wegener’s granulomatosis. J. Rheumatol. 1998, 25, 1531–1533. [Google Scholar] [PubMed]
- Shin, K.C.; Lee, Y.J.; Kang, S.W.; Baek, H.J.; Lee, E.B.; Kim, H.A.; Song, Y.W. Serum procalcitonin measurement for detection of intercurrent infection in febrile patients with SLE. Ann. Rheum. Dis. 2001, 60, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.Y.; Park, J.S.; Park, Y.-B.; Lee, S.-K.; Ha, Y.-J.; Lee, S.-W. Delta neutrophil index as a marker for differential diagnosis between flare and infection in febrile systemic lupus erythematosus patients. Lupus 2013, 22, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- El-Serougy, E.; Zayed, H.S.; Ibrahim, N.M.; Maged, L.A. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: The controversy continues. Lupus 2019, 28, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tanabe, N.; Murasawa, A.; Otaki, Y.; Sakai, T.; Sugaya, T.; Ito, S.; Otani, H.; Abe, A.; Ishikawa, H.; et al. Procalcitonin is a specific marker for detecting bacterial infection in patients with rheumatoid arthritis. J. Rheumatol. 2012, 39, 1517–1523. [Google Scholar] [CrossRef]
- Iankova, I.; Thompson-Leduc, P.; Kirson, N.Y.; Rice, B.; Hey, J.; Krause, A.; Schonfeld, S.A.; DeBrase, C.R.; Bozzette, S.; Schuetz, P. Efficacy and Safety of Procalcitonin Guidance in Patients With Suspected or Confirmed Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 691–698. [Google Scholar] [CrossRef]
- Kim, H.-A.; Jeon, J.-Y.; An, J.-M.; Koh, B.-R.; Suh, C.-H. C-reactive protein is a more sensitive and specific marker for diagnosing bacterial infections in systemic lupus erythematosus compared to S100A8/A9 and procalcitonin. J. Rheumatol. 2012, 39, 728–734. [Google Scholar] [CrossRef]
- Yu, J.; Xu, B.; Huang, Y.; Zhao, J.; Wang, S.; Wang, H.; Yang, N. Serum procalcitonin and C-reactive protein for differentiating bacterial infection from disease activity in patients with systemic lupus erythematosus. Mod. Rheumatol. 2014, 24, 457–463. [Google Scholar] [CrossRef]
- Bador, K.; Intan, S.; Hussin, S.; Gafor, A. Serum procalcitonin has negative predictive value for bacterial infection in active systemic lupus erythematosus. Lupus 2012, 21, 1172–1177. [Google Scholar] [CrossRef]
- Meisner, M.; Lohs, T.; Huettemann, E.; Schmidt, J.; Hueller, M.; Reinhart, K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur. J. Anaesthesiol. 2001, 18, 79–87. [Google Scholar] [CrossRef]
- Level, C.; Chauveau, P.; Delmas, Y.; Lasseur, C.; Pellé, G.; Peuchant, E.; Montaudon, D.; Combe, C. Procalcitonin: A new marker of inflammation in haemodialysis patients? Nephrol. Dial. Transpl. 2001, 16, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M.; Schmidt, J.; Hüttner, H.; Tschaikowsky, K. The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive Care Med. 2000, 26 (Suppl. S2), S212–S216. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.; Turner, R.M. Use of procalcitonin in patients with various degrees of chronic kidney disease including renal replacement therapy. Clin. Infect. Dis. 2014, 59, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.H.; Jang, H.R.; Kim, M.-J.; Jung, S.-H.; Lee, J.E.; Huh, W.; Kim, Y.-G.; Kim, D.J.; Oh, H.Y. Clinical relevance of procalcitonin and C-reactive protein as infection markers in renal impairment: A cross-sectional study. Crit. Care Lond. Engl. 2014, 18, 640. [Google Scholar] [CrossRef]
- Dipalo, M.; Guido, L.; Micca, G.; Pittalis, S.; Locatelli, M.; Motta, A.; Bianchi, A.; Callegari, T.; Aloe, R.; Rin, G.D.; et al. Multicenter comparison of automated procalcitonin immunoassays. Pract. Lab. Med. 2015, 2, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Perren, A.; Cerutti, B.; Lepori, M.; Senn, V.; Capelli, B.; Duchini, F.; Domenighetti, G. Influence of steroids on procalcitonin and C-reactive protein in patients with COPD and community-acquired pneumonia. Infection 2008, 36, 163–166. [Google Scholar] [CrossRef]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar]
- Seo, K.; Kitazawa, T.; Yoshino, Y.; Koga, I.; Ota, Y. Characteristics of serum endocan levels in infection. PLoS ONE 2015, 10, e0123358. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Fanizza, C.; Thomae, R.; Bernasconi, R.; Noto, A.; Oggioni, R.; Pasetti, G.S.; Romero, M.; Tognoni, G.; et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2015, 41, 12–20. [Google Scholar] [CrossRef]
- Bloch, D.A.; Michel, B.A.; Hunder, G.G.; McShane, D.J.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Fries, J.F.; Leavitt, R.Y.; et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum. 1990, 33, 1068–1073. [Google Scholar] [CrossRef]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA—Associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Hoffman, G.S.; Merkel, P.A.; Min, Y.I.; Uhlfelder, M.L.; Hellmann, D.B.; Specks, U.; Allen, N.B.; Davis, J.C.; Spieraet, R.F.; et al. A disease-specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001, 44, 912–920. [Google Scholar] [CrossRef] [PubMed]

| Total n = 74 | GPA n = 43 | EGPA n = 21 | MP n = 10 | |
|---|---|---|---|---|
| Demographic Data | ||||
| Age at diagnosis (in years) med [Q1; Q3] | 64 [51; 73] | 64 [50; 75] | 59 [51; 64] | 79 [72; 82] |
| Men, n (%) | 44 (59.4) | 23 (53.5) | 14 (66.7) | 7 (70) |
| Women, n (%) | 30 (40.5) | 20 (46.5) | 7 (33.3) | 3 (30) |
| Clinical Overview | ||||
| General signs, n (%) | 59 (79.7) | 35 (81.4) | 15 (71.4) | 9 (79.7) |
| ENT involvement, n (%) | 57 (77) | 37 (86) | 19 (90.5) | 1 (10) |
| Pulmonary involvement, n (%) | 54 (73) | 28 (65.1) | 20 (95.2) | 6 (60) |
| Cardiac involvement n (%) | 12 (16.2) | 4 (9.3) | 6 (28.6) | 2 (20) |
| Renal involvement n (%) | 35 (47.3) | 26 (60.5) | 3 (14.3) | 6 (60) |
| Peripheral neurological disease, n (%) | 18 (24.3) | 10 (13.5) | 5 (6.7) | 3 (30) |
| Central neurological disease, n (%) | 7 (9.46) | 4 (9.3) | 3 (14.3) | 0 (0) |
| Digestive involvement, n (%) | 4 (5.41) | 3 (6.98) | 1 (4.76) | 0 (0) |
| BVAS med [Q1; Q3] | 10 (7; 14) | 9 (7; 13) | 10 (8; 18) | 8 (6; 17) |
| Diagnosis | ||||
| Histologic evidence, n (%) | 43 (58.9) | 32 (43.8) | 6 (28.6) | 5 (50) |
| ANCA, n (%) | 59 (79.45) | 40 (93.02) | 9 (40) | 10 (100) |
| IF | ||||
| c-ANCA, n (%) | 32 (43.2) | 31 (72.1) | 1 (4.76) | 0 (0) |
| p-ANCA, n (%) | 27 (36.5) | 9 (20.1) | 8 (38) | 10 (100) |
| Specificity (ELISA) | ||||
| PR3, n (%) | 30 (40.8) | 30 (70.7) | 0 (0) | 0 (0) |
| MPO, n (%) | 22 (30.9) | 6 (14.6) | 6 (30) | 10 (100) |
| Biological Data Med [Q1; Q3] | ||||
| Creatinine (µmol/L) | 76.6 [64; 100] n = 73 | 85 [65; 130] n = 43 | 73.1 [54; 78.9] n = 21 | 68 [64.4; 100] n = 9 |
| GFR (mL/min by MDRD) | 91 [68; 101.5] n = 48 | 88 [38; 102] n = 23 | 97 [91; 104] n = 16 | 82.3 [61.9; 87] n = 9 |
| CRP (mg/L) | 83 [19.7; 136] | 92.9 [31.7; 147] | 29.7 [11.9; 69.8] | 132.5 [91; 184] |
| Fb (g/L) | 5.5 [4.8; 7.2] n = 70 | 6.1 [5.2; 7.6] n = 39 | 4.8 [4.2; 5.7] n = 21 | 6.2 [5.2; 7.6] n = 10 |
| PCT (µg/L) | 0.1 [0.05; 0.3] | 0.14 [0.06; 0.39] | 0.06 [0.04; 0.14] | 0.11 [0.06; 0.32] |
| Markers | Univariate Analysis | Diagnostic Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Relapsing Group n = 72 | Infected Group n = 88 | p | AUC | Threshold | Se | Sp | PPV | NPV | |
| PCT med (µg/L) [Q1; Q3] | 0.09 [005; 0.2] | 0.2 [0.08; 0.93] | <0.001 | 0.66 | 0.2 | 53.4% | 73.6% | 71.2% | 56.4% |
| CRP med (mg/L) [Q1; Q3] | 31.5 [11; 120] | 64.7 [25; 131] | 0.001 | 0.61 | 5 | 94.2% | 11.3% | 56.3% | 61.5% |
| Fibrinogen (g/L) [Q1; Q3] | 5.5 [4.5; 7.1] | 5.5 [4.5; 6.8] | 0.98 | NE | 4 | 89.6% | 11.3% | 52.2% | 50.0% |
| WBC (G/L) [Q1; Q3] | 9.3 [6.6; 11.5] | 8.6 [6.7; 11.2] | 0.61 | NE | 10 | 36.1% | 57.1% | 50.0% | 43.0% |
| Neutrophils (G/L) [Q1; Q3] | 7.4 [4.7; 9.6] | 6.8 [4.8; 9.6] | 0.70 | NE | 7 | 44.7% | 46.3% | 48.6% | 42.5% |
| T° ≥ 38° n (%) | 12 (16.6) | 45 (51.1) | <0.001 | NE | NE | 51.1% | 83.1% | 78.9% | 57.8% |
| Markers | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| RR [Q1; Q3] | p | RR [Q1; Q3] | p | |
| PCT ≥ 0.2 µg/L | 1.63 [1.24; 2.15] | 0.001 | 2.14 [1.02; 4.50] | 0.04 |
| CRP ≥ 5 mg/L | 1.46 [0.72; 2.95] | 0.29 | 0.96 [0.28; 3.24] | 0.94 |
| Temperature ≥ 38 °C | 1.80 [1.43; 2.43] | <0.001 | 4.16 [1.87; 9.24] | <0.001 |
| WBC ≥ 10,000 | 0.88 [0.64; 1.19] | 0.41 | 0.72 [0.35; 1.49] | 0.38 |
| Neutrophils ≥ 7000 | 0.84 [0.62; 1.15] | 0.29 | 0.65 [0.31; 1.34] | 0.24 |
| Fibrinogen ≥ 4 | 1.04 [0.60; 1.81] | 0.88 | 0.80 [0.22; 2.91] | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poirot-Seynaeve, X.; Smets, P.; Pereira, B.; Olagne, L.; Stievenart, J.; Sapin, V.; Aumaitre, O.; Andre, M.; Trefond, L. Interest of Procalcitonin in ANCA Vasculitides for Differentiation between Flare and Infections. Int. J. Mol. Sci. 2023, 24, 5557. https://doi.org/10.3390/ijms24065557
Poirot-Seynaeve X, Smets P, Pereira B, Olagne L, Stievenart J, Sapin V, Aumaitre O, Andre M, Trefond L. Interest of Procalcitonin in ANCA Vasculitides for Differentiation between Flare and Infections. International Journal of Molecular Sciences. 2023; 24(6):5557. https://doi.org/10.3390/ijms24065557
Chicago/Turabian StylePoirot-Seynaeve, Xavier, Perrine Smets, Bruno Pereira, Louis Olagne, Julien Stievenart, Vincent Sapin, Olivier Aumaitre, Marc Andre, and Ludovic Trefond. 2023. "Interest of Procalcitonin in ANCA Vasculitides for Differentiation between Flare and Infections" International Journal of Molecular Sciences 24, no. 6: 5557. https://doi.org/10.3390/ijms24065557
APA StylePoirot-Seynaeve, X., Smets, P., Pereira, B., Olagne, L., Stievenart, J., Sapin, V., Aumaitre, O., Andre, M., & Trefond, L. (2023). Interest of Procalcitonin in ANCA Vasculitides for Differentiation between Flare and Infections. International Journal of Molecular Sciences, 24(6), 5557. https://doi.org/10.3390/ijms24065557







