Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra Bailey)
Abstract
:1. Introduction
2. Results
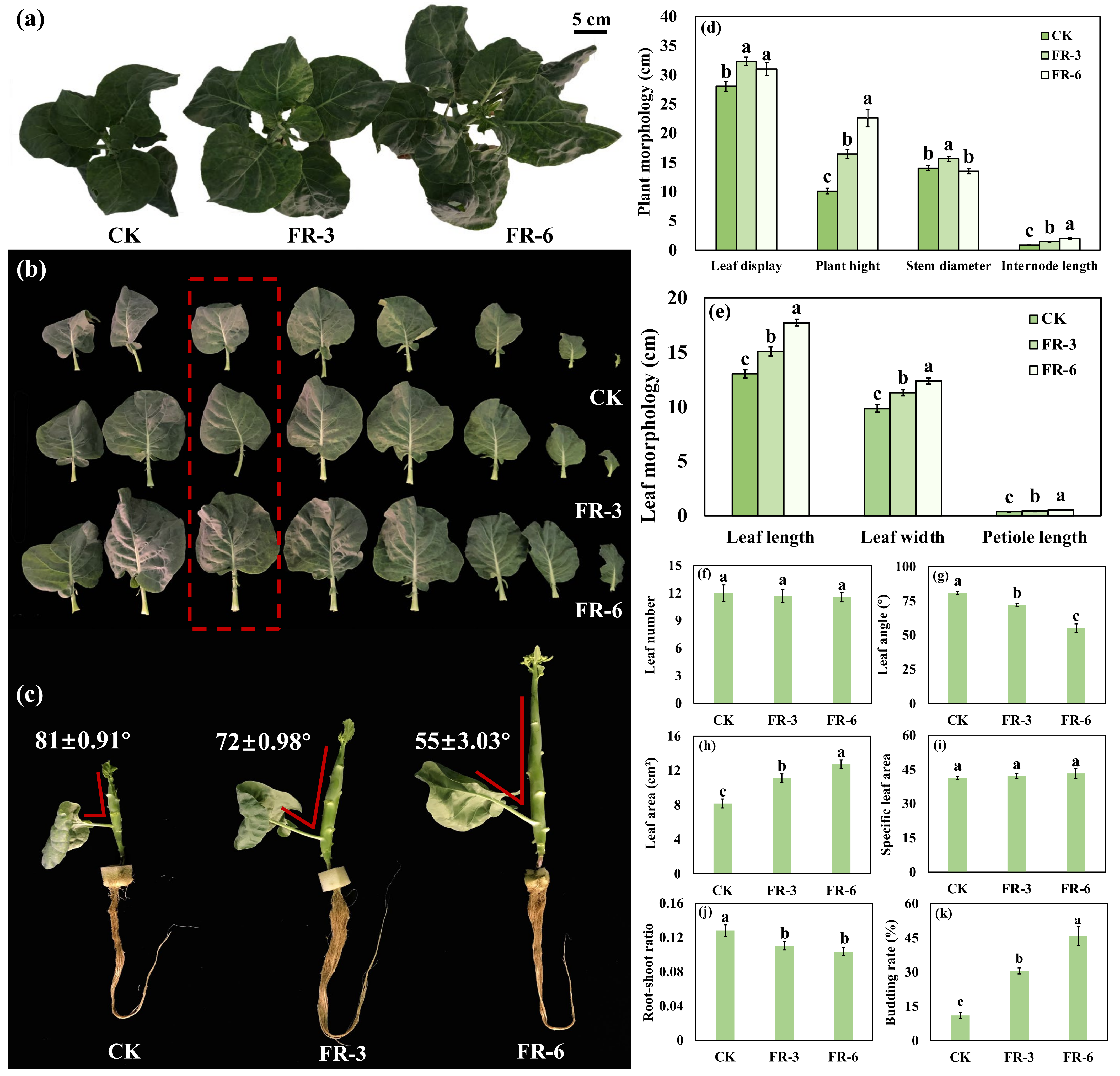
2.1. Plant Biomass and Morphological Indices Indicate an Elevated Growth of Chinese Kale under Supplementary Far-Red Light
2.2. Effects of Supplementary Far-Red Light on Photosynthetic Traits
2.3. Effects of Supplementary Far-Red Light on Mineral Profiles
2.4. Identification of Differentially Expressed Genes in Response to Supplementary Far-Red Light
2.5. Expression Patterns of DEGs of Functionally Enriched Pathways under Supplementary Far-Red Light
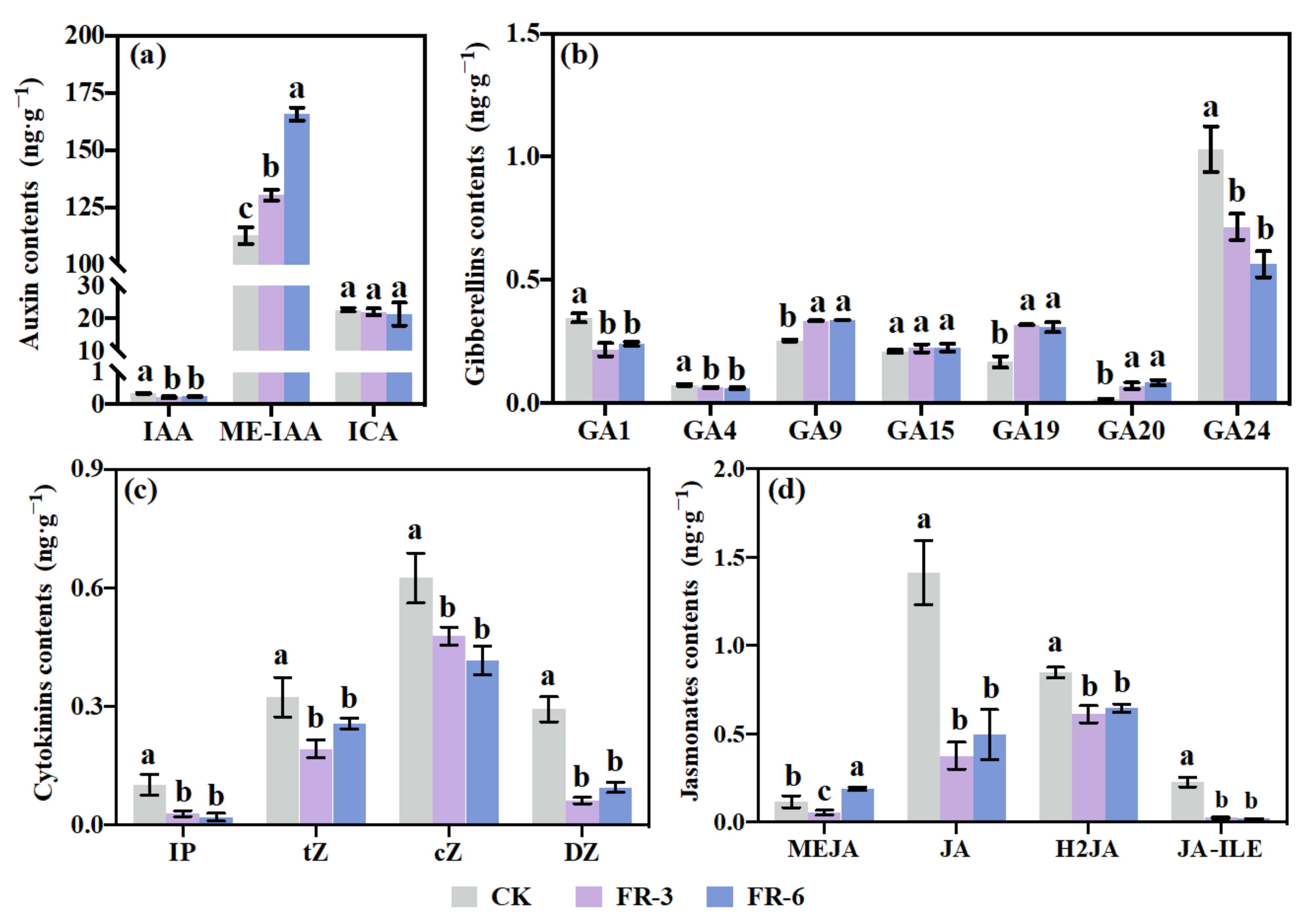
2.6. Endogenous Phytohormone Accumulation in Response to Supplementary Far-Red Light
2.7. Correlation Analysis of Phytohormones and Morphological Traits
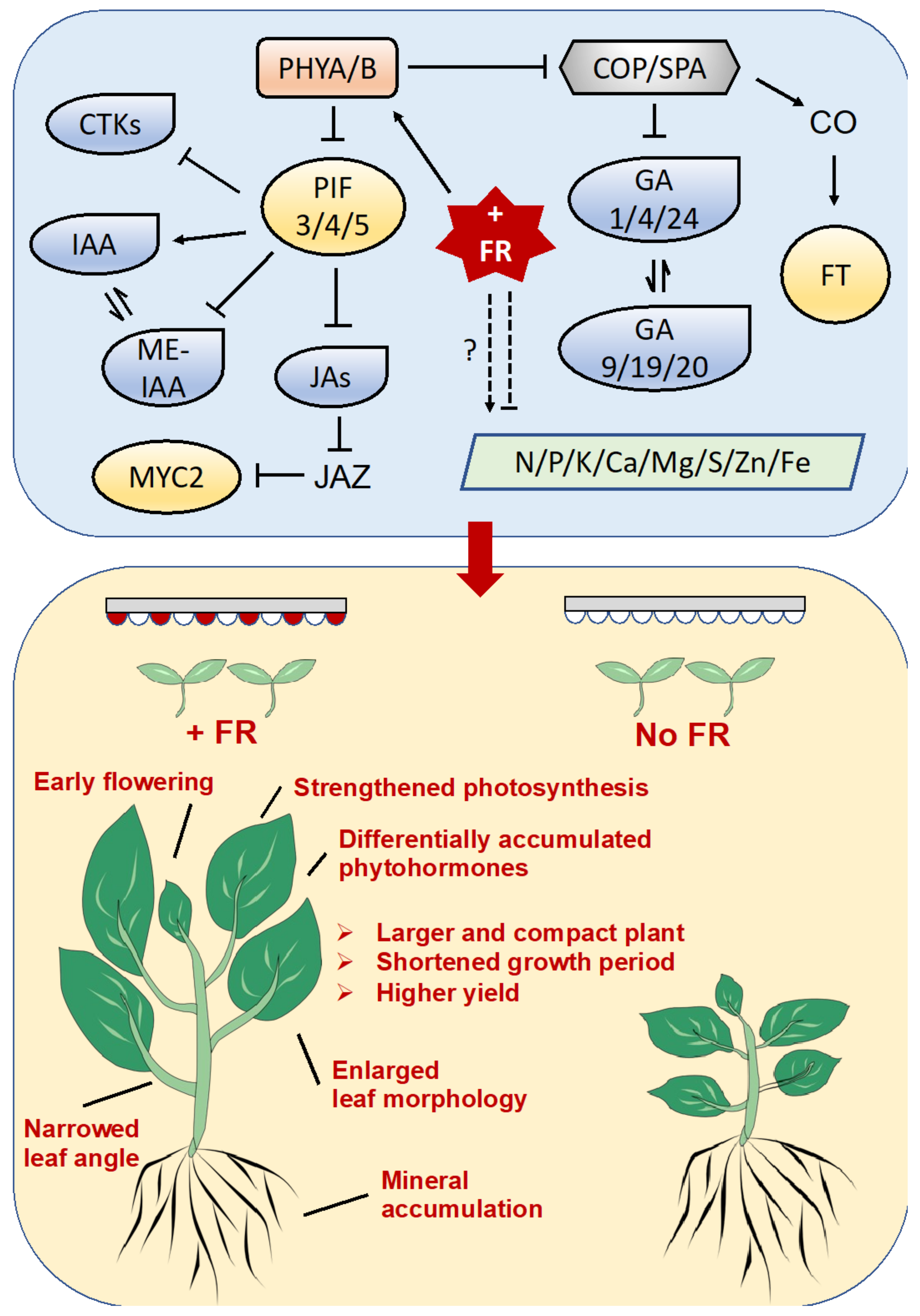
3. Discussion
3.1. The Elongation Growth of Chinese Kale under Supplementary Far-Red Light
3.2. The Biomass of Chinese Kale under Supplementary Far-Red Light
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Light Treatments and Sample Preparation
4.3. Measurements of Plant Mass and Morphology
4.4. Photosynthetic Trait Determination
4.5. Mineral Element Determination
4.6. Quantification of Phytohormones and Gibberellins
4.7. RNA Extraction, Sequencing, and De Novo Assembly
4.8. Transcriptome Data Analysis
4.9. Validation of RNA-seq Data Using qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casal, J.J. Shade Avoidance. Arab. Book Am. Soc. Plant Biol. 2012, 10, e0157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Liu, Y.; Wang, H.; Ma, X.; Wang, B.; Wu, G.; Wang, H. Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat. Commun. 2017, 8, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, S.; van Iersel, M.W.; Bugbee, B. Photosynthesis in sun and shade: The surprising importance of far-red photons. New Phytol. 2022, 236, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Yoneda, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K.; Shimizu, H. Impact of blue, red, and far-red light treatments on gene expression and steviol glycoside accumulation in Stevia rebaudiana. Phytochemistry 2017, 137, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.L.; Kubota, C. End-of-day far-red light quality and dose requirements for tomato rootstock hypocotyl elongation. HortScience 2010, 45, 1501–1506. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Tarkowská, D.; Clarke, J.L.; Blystad, D.R.; Gislerød, H.R.; Torre, S.; Olsen, J.E. Impact of end-of-day red and far-red light on plant morphology and hormone physiology of poinsettia. Sci. Hortic. 2014, 174, 77–86. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.C.; Staal, M.; Elzenga, J.T.M.; Yokoyama, R.; Nishitani, K.; Voesenek, L.A.C.J.; Pierik, R. Light quality-mediated petiole elongation in arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol. 2010, 154, 978–990. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Pierik, R. Cell wall modification involving XTHs controls phytochrome-mediated petiole elongation in Arabidopsis thaliana. Plant Signal. Behav. 2010, 5, 20–22. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ljung, K.; Breton, G.; Schmitz, R.J.; Pruneda-Paz, J.; Cowing-Zitron, C.; Cole, B.J.; Ivans, L.J.; Pedmale, U.V.; Jung, H.S.; et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012, 26, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Emery, R.J.N.; Pharis, R.P.; Reid, D.M. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: Putative roles for plant hormones in leaf and internode growth. J. Exp. Bot. 2007, 58, 2145–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courbier, S.; Snoek, B.L.; Kajala, K.; Li, L.; Van Wees, S.C.M.; Pierik, R. Mechanisms of far-red light-mediated dampening of defense against Botrytis cinerea in tomato leaves. Plant Physiol. 2021, 187, 1250–1266. [Google Scholar] [CrossRef] [PubMed]
- Michaud, O.; Krahmer, J.; Galbier, F.; Lagier, M.; Galvão, V.C.; Ince, Y.Ç.; Trevisan, M.; Knerova, J.; Dickinson, P.; Hibberd, J.M.; et al. Abscisic acid modulates neighbor proximity-induced leaf hyponasty in Arabidopsis. Plant Physiol. 2022, 191, 542–557. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Li, Y.; Ou, S.; Gao, M.; Zhang, Y.; Song, S.; Liu, H. Regulation of growth and main health-promoting compounds of Chinese kale baby-leaf by UV-A and FR light. Front. Plant Sci. 2021, 12, 799376. [Google Scholar] [CrossRef]
- Li, Y.; Shi, R.; Jiang, H.; Wu, L.; Zhang, Y.; Song, S.; Su, W.; Liu, H. End-of-day LED lightings Influence the leaf color, growth and phytochemicals in two cultivars of lettuce. Agronomy 2020, 10, 1475. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2020, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, X.Z.; Guo, W.Z.; Wang, L.C.; Qiao, X.J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Nawkar, G.M.; Kang, C.H.; Maibam, P.; Park, J.H.; Jung, Y.J.; Chae, H.B.; Chi, Y.H.; Jung, I.J.; Kim, W.Y.; Yun, D.J.; et al. HY5, a positive regulator of light signaling, negatively controls the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 2084–2089. [Google Scholar] [CrossRef] [Green Version]
- Sakuraba, Y.; Yanagisawa, S. Light signalling-induced regulation of nutrient acquisition and utilisation in plants. Semin. Cell Dev. Biol. 2018, 83, 123–132. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Chen, Y.; Liu, C.; Yang, Y. Light regulation of potassium in plants. Plant Physiol. Biochem. 2022, 170, 316–324. [Google Scholar] [CrossRef]
- Cerny, T.A.; Faust, J.E.; Layne, D.R.; Rajapakse, N.C. Influence of photoselective films and growing season on stem growth and flowering of six plant species. J. Am. Soc. Hortic. Sci. 2003, 128, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Libenson, S.; Rodríguez, V.; López Pereira, M.; Sánchez, R.A.; Casal, J.J. Erratum: Low red to far-red ratios reaching the stem reduce grain yield in sunflower. Crop Sci. 2002, 42, 1761. [Google Scholar] [CrossRef]
- Liscum, E.; Reed, J.W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-Acetic acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roosjen, M.; Paque, S.; Weijers, D. Auxin response factors: Output control in auxin biology. J. Exp. Bot. 2018, 69, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courbier, S.; Pierik, R. Canopy light quality modulates stress responses in plants. iScience 2019, 22, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zhang, F.; Friml, J.; Ding, Z. Auxin signaling: Research advances over the past 30 years. J. Integr. Plant Biol. 2022, 64, 371–392. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hou, X.; Tsuge, T.; Ding, M.; Aoyama, T.; Oka, A.; Gu, H.; Zhao, Y.; Qu, L.J. The possible action mechanisms of indole-3-acetic acid methyl ester in Arabidopsis. Plant Cell Rep. 2008, 27, 575–584. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinig, C.; Johnston, J.; German, Z.M.; Demink, L.M. Local and global costs of adaptive plasticity to density in Arabidopsis thaliana. Am. Nat. 2006, 167, 826–836. [Google Scholar] [CrossRef]
- Schittenhelm, S.; Menge-Hartmann, U.; Oldenburg, E. Photosynthesis, carbohydrate metabolism, and yield of phytochrome-b- overexpressing potatoes under different light regimes. Crop Sci. 2004, 44, 131–143. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kim, H.J.; Yang, T.; Choi, S.; Wang, Y.J.; Lin, M.Y.; Liceaga, A.M. Supplemental intracanopy far-red radiation to red LED light improves fruit quality attributes of greenhouse tomatoes. Sci. Hortic. 2020, 261, 108985. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zheng, D.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Effects of supplementary blue and UV-A LED lights on morphology and phytochemicals of Brassicaceae baby-leaves. Molecules 2020, 25, 5678. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Biomass Indices | Light Treatments | ||
|---|---|---|---|
| CK | FR-3 | FR-6 | |
| Fresh weight (g) | |||
| Plant | 55.94 ± 4.31 b | 78.22 ± 2.92 a | 82.29 ± 4.11 a |
| Shoot | 50.23 ± 3.87 b | 71.32 ± 2.83 a | 76.69 ± 3.92 a |
| Root | 5.72 ± 0.51 b | 6.90 ± 0.36 a | 5.60 ± 0.23 b |
| Dry weight (g) | |||
| Plant | 3.59 ± 0.34 b | 4.91 ± 0.25 a | 4.75 ± 0.18 a |
| Shoot | 3.18 ± 0.30 b | 4.43 ± 0.23 a | 4.30 ± 0.16 a |
| Root | 0.41 ± 0.05 a | 0.49 ± 0.03 a | 0.44 ± 0.03 a |
| Moisture content (%) | |||
| Plant | 93.62 ± 0.16 a | 93.73 ± 0.16 a | 94.18 ± 0.23 a |
| Shoot | 93.71 ± 0.13 b | 93.81 ± 0.14 b | 94.34 ± 0.21 a |
| Root | 92.80 ± 0.50 a | 92.86 ± 0.46 a | 91.97 ± 0.60 a |
| Photosynthetic Traits | Light Treatments | ||
|---|---|---|---|
| CK | FR-3 | FR-6 | |
| Photosynthetic pigments (mg·g−1) | |||
| Chlorophyll a | 0.55 ± 0.01 c | 0.95 ± 0.06 a | 0.75 ± 0.03 b |
| Chlorophyll b | 0.20 ± 0.00 c | 0.35 ± 0.03 a | 0.27 ± 0.01 b |
| Total chlorophylls | 0.76 ± 0.01 c | 1.31 ± 0.09 a | 1.03 ± 0.04 b |
| Carotenoids | 0.11 ± 0.00 c | 0.16 ± 0.00 a | 0.15 ± 0.00 b |
| Chlorophyll fluorescence parameters | |||
| Fv/Fm | 0.74 ± 0.01 b | 0.76 ± 0.01 a | 0.76 ± 0.01 a |
| Y(II) | 0.33 ± 0.01 c | 0.41 ± 0.02 a | 0.37 ± 0.01 b |
| ETR | 39.39 ± 1.05 c | 43.90 ± 0.70 b | 48.11 ± 0.73 a |
| Y(NO) | 0.44 ± 0.01 a | 0.44 ± 0.01 a | 0.44 ± 0.01 a |
| Y(NPQ) | 0.26 ± 0.02 a | 0.21 ± 0.01 b | 0.21 ± 0.01 b |
| qN | 0.51 ± 0.00 a | 0.46 ± 0.01 b | 0.41 ± 0.01 c |
| NPQ | 0.56 ± 0.00 b | 0.60 ± 0.02 a | 0.60 ± 0.01 a |
| qL | 0.33 ± 0.01 b | 0.36 ± 0.01 a | 0.36 ± 0.01 a |
| qP | 0.50 ± 0.01 b | 0.60 ± 0.01 a | 0.59 ± 0.00 a |
| Mineral Elements | Light Treatments | ||
|---|---|---|---|
| CK | FR-3 | FR-6 | |
| Major element concentration (g·kg−1) | |||
| N | 47.74 ± 0.35 a | 46.15 ± 0.68 ab | 44.78 ± 0.67 b |
| P | 5.51 ± 0.04 a | 5.08 ± 0.06 b | 4.56 ± 0.11 c |
| K | 61.70 ± 0.67 ab | 62.14 ± 0.73 a | 59.36 ± 0.92 b |
| Ca | 34.07 ± 0.44 b | 35.62 ± 0.48 b | 38.34 ± 0.79 a |
| Mg | 6.75 ± 0.10 a | 6.60 ± 0.13 a | 6.90 ± 0.08 a |
| S | 19.81 ± 0.21 a | 18.33 ± 0.43 a | 19.76 ± 0.83 a |
| Minor element concentration (mg·kg−1) | |||
| Zn | 33.85 ± 0.46 a | 30.41 ± 0.85 b | 30.41 ± 0.63 b |
| Fe | 40.75 ± 6.95 a | 21.72 ± 1.55 b | 19.74 ± 1.65 b |
| Major element accumulation (g per plant) | |||
| N | 131.80 ± 17.11 b | 191.00 ± 21.03 a | 201.20 ± 16.49 a |
| P | 15.20 ± 1.84 b | 21.00 ± 1.99 a | 20.50 ± 1.84 a |
| K | 170.50 ± 23.09 b | 257.20 ± 26.42 a | 266.40 ± 16.87 a |
| Ca | 94.10 ± 13.02 c | 147.60 ± 18.07 b | 172.40 ± 17.62 a |
| Mg | 18.60 ± 2.30 b | 27.30 ± 3.12 a | 31.00 ± 2.42 a |
| S | 54.70 ± 6.89 b | 75.80 ± 8.14 a | 88.80 ± 11.78 a |
| Minor element accumulation (mg per plant) | |||
| Zn | 0.09 ± 0.01 b | 0.13 ± 0.01 a | 0.14 ± 0.01 a |
| Fe | 0.12 ± 0.06 a | 0.09 ± 0.02 a | 0.09 ± 0.02 a |
| Parameters | Light Treatments | ||
|---|---|---|---|
| CK | FR-3 | FR-6 | |
| Single-band photon flux density (μmol·m−2·s−1) | |||
| UV-A (350–400 nm) | 0.27 | 0.35 | 0.13 |
| B (400–500 nm) | 31.41 | 29.66 | 29.93 |
| G (500–600 nm) | 47.92 | 46.51 | 47.00 |
| R (600–700 nm) | 170.73 | 168.96 | 167.06 |
| FR (700–800 nm) | 4.30 | 13.24 | 22.91 |
| Integrated photon flux density (μmol·m−2·s−1) | |||
| PPFD | 250.06 | 245.13 | 243.99 |
| YPFD | 223.73 | 219.76 | 218.61 |
| TPFD | 224.71 | 222.07 | 222.20 |
| Radiation ratio | |||
| R/B | 5.44 | 5.70 | 5.58 |
| R/G | 3.56 | 3.63 | 3.55 |
| R/FR | 39.68 | 12.76 | 7.29 |
| Daily light integral (mol·m−2·d) | |||
| 10 h | 9.00 | 8.82 | 8.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, H.; Gao, M.; He, R.; Liu, X.; Su, W.; Liu, H. Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra Bailey). Int. J. Mol. Sci. 2023, 24, 5563. https://doi.org/10.3390/ijms24065563
Li Y, Jiang H, Gao M, He R, Liu X, Su W, Liu H. Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra Bailey). International Journal of Molecular Sciences. 2023; 24(6):5563. https://doi.org/10.3390/ijms24065563
Chicago/Turabian StyleLi, Yamin, Haozhao Jiang, Meifang Gao, Rui He, Xiaojuan Liu, Wei Su, and Houcheng Liu. 2023. "Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra Bailey)" International Journal of Molecular Sciences 24, no. 6: 5563. https://doi.org/10.3390/ijms24065563
APA StyleLi, Y., Jiang, H., Gao, M., He, R., Liu, X., Su, W., & Liu, H. (2023). Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra Bailey). International Journal of Molecular Sciences, 24(6), 5563. https://doi.org/10.3390/ijms24065563










