Promising Strategy of mPTP Modulation in Cancer Therapy: An Emerging Progress and Future Insight
Abstract
1. Introduction
2. Cancer: Global Burden
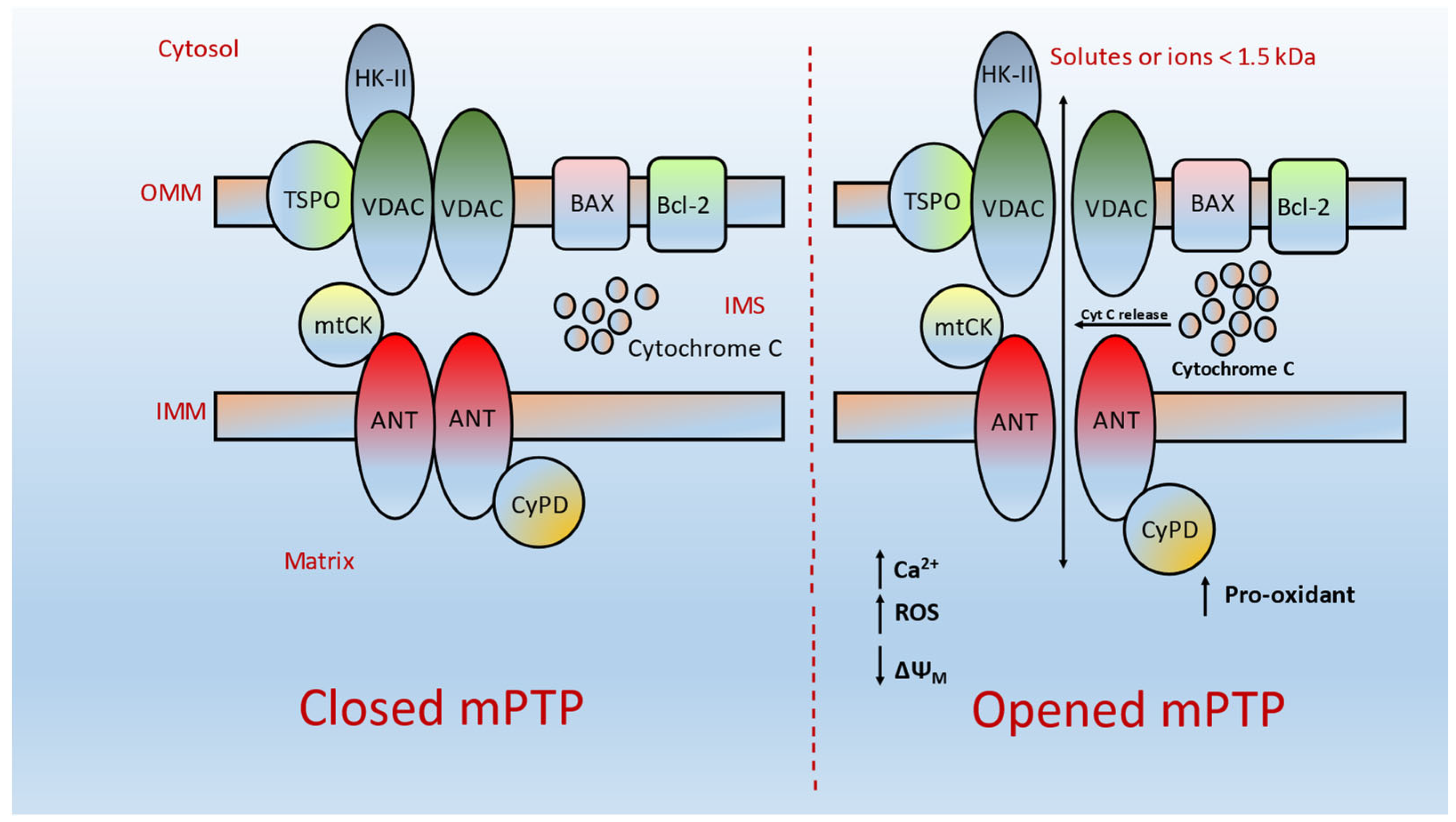
3. Possible Functional Implications of mPTP in Cancer
4. Mitochondrial Participation in Oxidative Stress and Cancer Development
5. Mitochondria as Model Organelle for Regulating Apoptosis Machinery
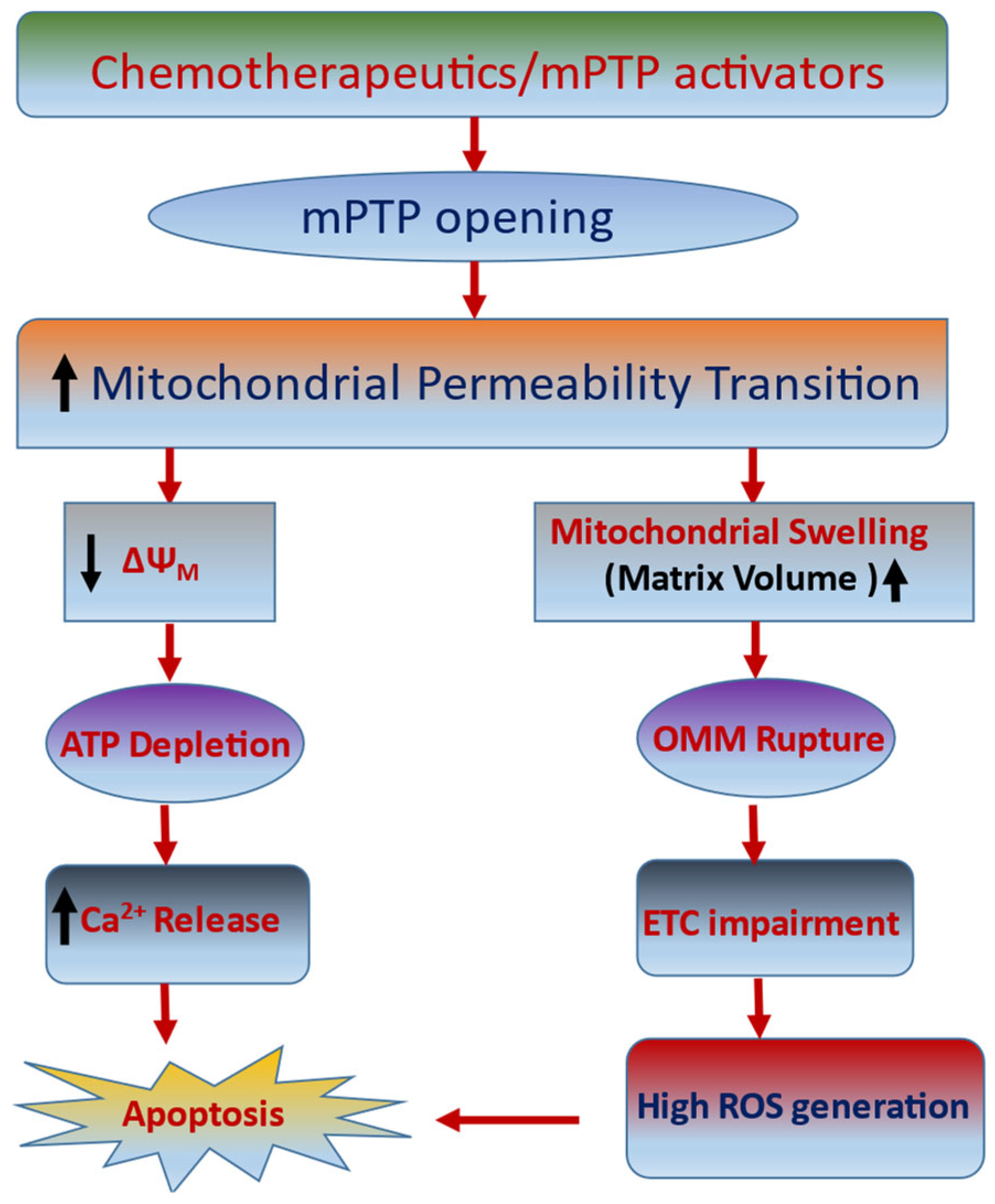
6. mPTP: A Gate for Apoptotic-Mediated Cancer Cell Death
7. Targeting Mitochondria as Cancer Therapeutic Strategy
8. Targeting mPTP Components as Novel Cancer Interventions
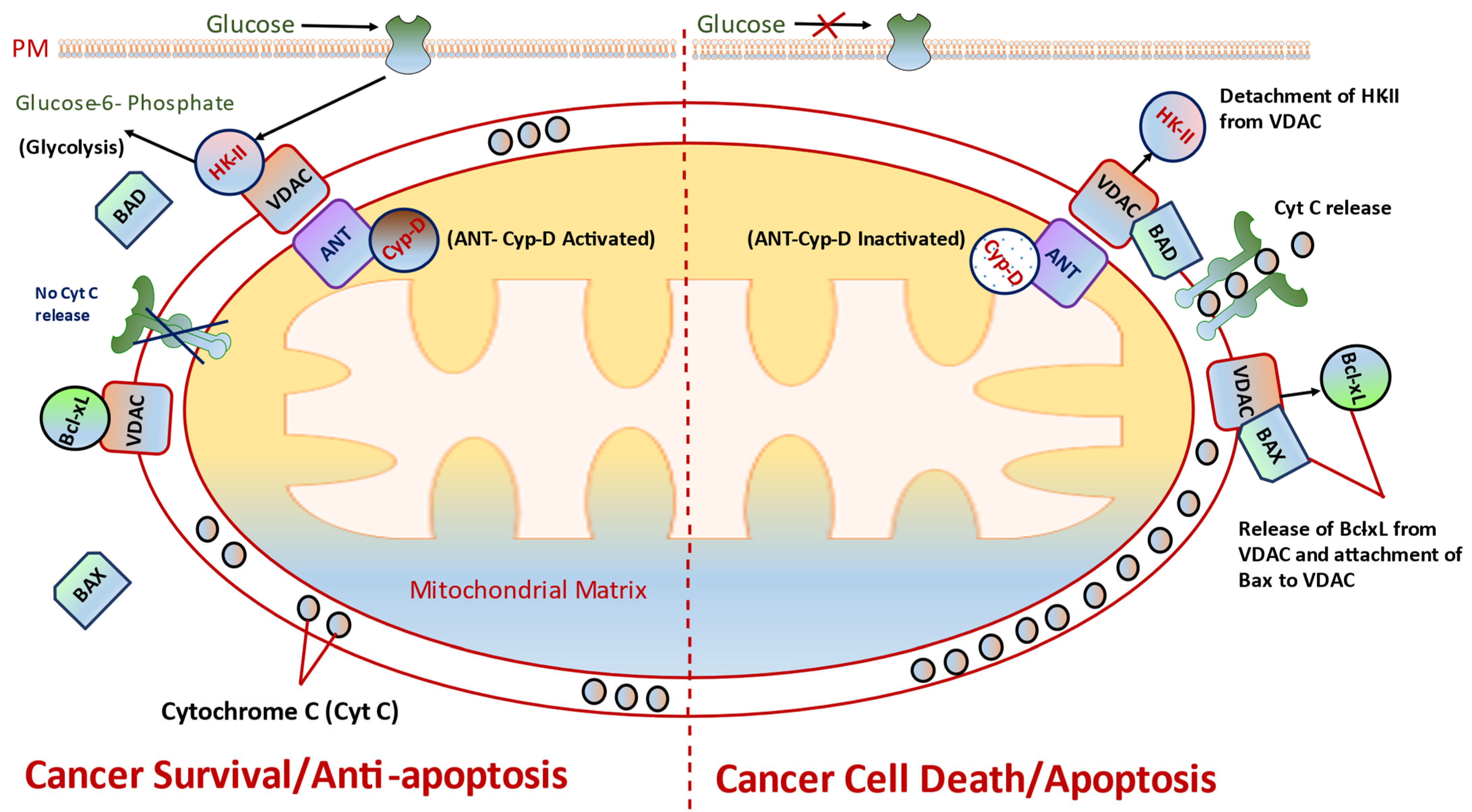
8.1. Targeting VDAC and HK-II
8.2. Targeting CyP-D, a Key Regulator of HKII Binding to VDAC
8.3. Targets Bcl-2 Family Proteins
9. Signaling Pathways Mediating mPTP Opening/Closing in Cancers
9.1. ERK Signaling
9.2. mTOR Signaling
10. Other Targeting Candidates for mPTP Opening
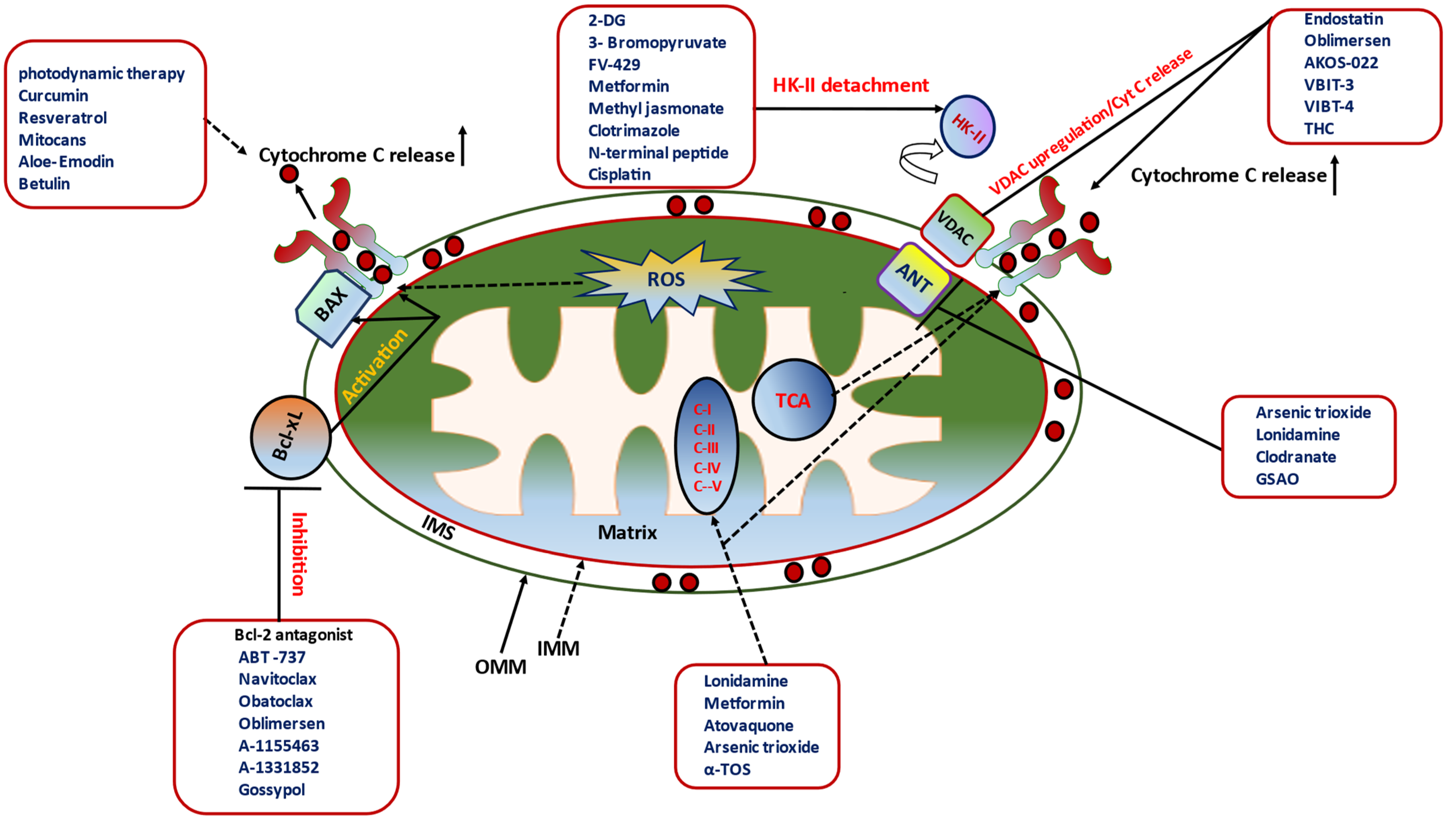
11. Pharmacological Switches of mPTP Opening/Closing in Cancer Therapeutics
11.1. Modulator/Antagonist of Bcl-2 Family Proteins
11.2. Pharmacological Disruptors of HKII–VDAC Interaction
11.3. Pharmacological Inhibition of ANT
11.4. Pharmacological Triggers of Cyt C Release in Cancer Cell Death
11.5. Pharmacological Modulation of ETC in Cancer Cell Death
11.6. Nutraceuticals (Natural Compounds): Excellent Armor for Apoptotic Cancer Cell Death
| Compounds/Inhibitors | Targets | Preclinical/Clinical Status Clinical Trial.gov Identifier/Reference | |
|---|---|---|---|
| Bcl-2 Modulators/Inhibitors | |||
| ABT737 | Bcl-2 family | Preclinical | [97,100,101] |
| ABT199 | Bcl-2 family | Phase II | NCT01889186 [112,113] |
| Gossypol | Bcl-2 family | Preclinical | [118,121] |
| Obatoclax | Bcl-2 family & Mcl-1 | Preclinical | [122] |
| AZD5991 | Mcl-1 | Phase I | NCT03218683 |
| AMG176 | Mcl-1 | Phase I | NCT02675452 |
| S63845 | Mcl-1 | Phase I | NCT02992483 |
| HKII -VDAC-Mediated Metabolic Inhibitors | |||
| 3-Bromopyruvate | HKII–VDAC interaction | Preclinical | [70] |
| 2-DG | HKII–DAC interaction | Preclinical Phase I/III | NCT00096707, [135,136] |
| FV-429 | HKII–AkT | Preclinical | [85] |
| Methyl Jasonate | HKII–VDAC interaction | Preclinical | [159,160] |
| Clotrimazole | HKII–VDAC interaction | Preclinical | [74,161] |
| Erastin | HKII–VDAC interaction | Preclinical | [164] |
| ANT Inhibitor | |||
| Arsenite | ANT proteoliposomes | Preclinical | [168] |
| Lonidamine | ANT | Preclinical | [171,172,173,174] |
| Clodranate | ANT | Preclinical | [169,175] |
| GSAO | ADP/ATP exchange via ANT | Preclinical | [176] |
| Cytochrome C Sensitizer | |||
| Photodynamic therapy | Cyt C | Phase III | NCT02064673, [180,181] |
| Resveratrol | Cyt C | Preclinical | [183,184] |
| Curcumin | Cyt C | Phase II | NCT02944578/NCT02782949 |
| Aloe-Emodin | Cyt C | Preclinical | [186] |
| Betulin | Cyt C | Preclinical | [187] |
| ETC Modulators | |||
| Metformin | Complex I | Phase III | NCT01101438, [192] |
| Lonidamine | Complex II | Phase II/III | NCT00237536/NCT00435448 |
| Atovaquone | Complex III | Phase I/Preclinical | NCT02628080, [195,196,197,198,199,200] |
| Arsenic Trioxide | Complex IV | Preclinical | [166,203,204,205,206] |
| Nutraceuticals on mPTP Modulation | |||
| Resveratrol (analogue, HS-1793) | Cyt C/Bcl-2 | Preclinical | [207,208,209] |
| Cernumidine | Bcl-2/BAX ratio | Preclinical | [210] |
| Lycorine | mitochondrial Ca2+ | Preclinical | [211] |
| Amorfrutin C | ETC | Preclinical | [44] |
| Isothiocyanate | Cyt C | Preclinical | [212] |
| α-conidendrin | Bcl-2 | Preclinical | [213] |
| Dehydrobruceine B | Cyt C | Preclinical | [214] |
| Frugoside | Cyt C | Preclinical | [215] |
| Methyl caffeate | Bcl-2 | Preclinical | [216] |
| Phloretin | ETC | Preclinical | [218] |
| Sesamol | ETC | Preclinical | [219] |
| Betulinic acid | Bcl-2 | Preclinical | [220] |
| Berberine | ANT | Preclinical | [221] |
| α-TOS | Complex II | Preclinical | [222,223] |
| Honokiol | CyP-D | Preclinical | [164,225] |
12. Preclinical and Clinical Models for mPTP-Mediated Cancer Cell Death
13. Concluding Remarks
Funding
Conflicts of Interest
References
- Bock, F.J.; Tait, S.W. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Wenner, C.E. Targeting mitochondria as a therapeutic target in cancer. J. Cell Physiol. 2012, 227, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Song, Y.S. Mitochondrial dynamics altered by oxidative stress in cancer. Free. Radic. Res. 2016, 50, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, T.; Das, S.; Mondal, M.; Roychoudhury, S.; Chakraborti, S. Oxidant, Mitochondria and Calcium: An Overview. Cell. Signal. 1999, 11, 77–85. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 639–647. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A. Calcium and Cell Death: The Mitochondrial Connection. In Calcium Signalling and Disease; Springer: Berlin/Heidelberg, Germany, 2007; Volume 45, pp. 481–506. [Google Scholar] [CrossRef]
- Wong, R.; Steenbergen, C.; Murphy, E. Mitochondrial Permeability Transition Pore and Calcium Handling. In Mitochondrial Bioenergetics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 810, pp. 235–242. [Google Scholar] [CrossRef]
- Haworth, R.A.; Hunter, D.R. Control of the mitochondrial permeability transition pore by high-affinity ADP binding at the ADP/ATP translocase in permeabilized mitochondria. J. Bioenerg. Biomembr. 2000, 32, 91–96. [Google Scholar] [CrossRef]
- Crompton, M. Mitochondria and aging: A role for the permeability transition? Aging Cell. 2004, 3, 3–6. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Rao, V.K.; Carlson, E.A.; Yan, S.S. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1267–1272. [Google Scholar] [CrossRef]
- You, J.S.; Jonas, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.-P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Luo, W.; Chen, S.; Lin, F.; Zhang, X.; Fan, S.; Shen, X.; Wang, Y.; Liang, G. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020, 10, 10290–10308. [Google Scholar] [CrossRef]
- Leanza, L.; Venturini, E.; Kadow, S.; Carpinteiro, A.; Gulbins, E.; Becker, K.A. Targeting a mitochondrial potassium channel to fight cancer. Cell Calcium 2014, 58, 131–138. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Nowinski, S.M.; Solmonson, A.; Rusin, S.F.; Maschek, J.A.; Bensard, C.L.; Fogarty, S.; Jeong, M.-Y.; Lettlova, S.; A Berg, J.; Morgan, J.T.; et al. Author response: Mitochondrial fatty acid synthesis coordinates oxidative metabolism in mammalian mitochondria. Elife 2020, 9, e58041. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of mitochondrial dysfunction in cancer progression. ExBiol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell. Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, S.; Yamashita, K.; Koshikawa, K.; Nakao, A.; Sidransky, D. Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin. Cancer Res. 2002, 8, 481–487. [Google Scholar] [PubMed]
- Keith, C.G.; Arnold, R.S.; Petros, J.A. Mitochondrial DNA mutations in prostate cancer bone metastases. J. Nat. Sci. 2015, 1, e147. [Google Scholar]
- Mazat, J.-P.; Devin, A.; Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Y.; Sheng, Y.; Wang, J.; Ruan, S.; Han, C. Mechanism of piperine in affecting apoptosis and proliferation of gastric cancer cells via ROS-mitochondria-associated signalling pathway. J. Cell Mol. Med. 2021, 25, 9513–9522. [Google Scholar] [CrossRef]
- Inghapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G.C. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 2013, 8, e52989. [Google Scholar]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Wang, X.; Ni, H.; Xu, W.; Wu, B.; Xie, T.; Zhang, C.; Cheng, J.; Li, Z.; Tao, L.; Zhang, Y. Difenoconazole induces oxidative DNA damage and mitochondria mediated apoptosis in SH-SY5Y cells. Chemosphere 2021, 283, 131160. [Google Scholar] [CrossRef]
- Ruiz-Ramos, R.; Lopez-Carrillo, L.; Rios-Perez, A.D.; De Vizcaya-Ruíz, A.; Cebrian, M.E. Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat. Res. 2009, 674, 109–115. [Google Scholar] [CrossRef]
- Strickertsson, J.A.B.; Desler, C.; Martin-Bertelsen, T.; Machado, A.M.D.; Wadstrøm, T.; Winther, O.; Rasmussen, L.J.; Friis-Hansen, L. Enterococcus faecalis Infection Causes Inflammation, Intracellular Oxphos-Independent ROS Production, and DNA Damage in Human Gastric Cancer Cells. PLoS ONE 2013, 8, e63147. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Waseem, M.; Sahu, U.; Salman, M.; Choudhury, A.; Kar, S.; Tabassum, H.; Parvez, S. Melatonin pre-treatment mitigates SHSY-5Y cells against oxaliplatin induced mitochondrial stress and apoptotic cell death. PLoS ONE 2017, 12, e0180953. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef]
- Llambi, F.; Green, D.R. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 2011, 21, 12–20. [Google Scholar] [CrossRef]
- Cidado, J.; Boiko, S.; Proia, T.; Ferguson, D.; Criscione, S.W.; Martin, M.S.; Pop-Damkov, P.; Su, N.; Franklin, V.N.R.; Chilamakuri, C.S.R.; et al. AZD4573 Is a Highly Selective CDK9 Inhibitor That Suppresses MCL-1 and Induces Apoptosis in Hematologic Cancer Cells. Clin. Cancer Res. 2020, 26, 922–934. [Google Scholar] [CrossRef]
- Ji, B.L.; Xia, L.P.; Zhou, F.X.; Mao, G.Z.; Xu, L.X. Aconitine induces cell apoptosis in human pancreatic cancer via NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4955–4964. [Google Scholar]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 300, 341–352. [Google Scholar]
- Scorrano, L.; Oakes, S.A.; Opferman, J.T.; Cheng, E.H.; Sorcinelli, M.D.; Pozzan, T.; Korsmeyer, S.J. BAX and BAK Regulation of Endoplasmic Reticulum Ca2+: A Control Point for Apoptosis. Science 2003, 300, 135–139. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wu, Y.L.; Gao, X.H.; Guo, F. Mitochondrial protein cyclophilin-D-mediated programmed necrosis attributes to berberine-induced cytotoxicity in cultured prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 450, 697–703. [Google Scholar] [CrossRef]
- Weidner, C.; Rousseau, M.; Micikas, R.J.; Fischer, C.; Plauth, A.; Wowro, S.J.; Siems, K.; Hetterling, G.; Kliem, M.; Schroeder, F.C.; et al. Amorfrutin C Induces Apoptosis and Inhibits Proliferation in Colon Cancer Cells through Targeting Mito-chondria. J. Nat. Prod. 2016, 79, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.B.; Ji, C.F.; Zhang, H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 2012, 17, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. Role of mitochondria and mitochondrial cytochrome c in tubeimoside I-mediated apoptosis of human cervical car-cinoma HeLa cell line. Cancer Chemother. Pharmacol. 2006, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, M.; Li, Z.; Gao, P.; Zhou, X.; Zhang, J. Bufalin induces apoptosis in the U-2OS human osteosarcoma cell line via triggering the mitochondrial pathway. Mol. Med. Rep. 2016, 13, 817–822. [Google Scholar] [CrossRef]
- Yao, L.C. Panax notoginseng Saponins Promote Cell Death and Chemosensitivity in Pancreatic Cancer through the Apoptosis and Autophagy Pathways. Anticancer Agents Med. Chem. 2021, 21, 1680–1688. [Google Scholar] [CrossRef]
- Kinnally, K.W.; Peixoto, P.M.; Ryu, S.-Y.; Dejean, L.M. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta 2011, 1813, 616–622. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Mizrachi, D. VDAC1: From structure to cancer therapy. Front. Oncol. 2012, 2, 164. [Google Scholar] [CrossRef]
- Bonora, M.; Pinton, P. The Mitochondrial Permeability Transition Pore and Cancer: Molecular Mechanisms Involved in Cell Death. Front. Oncol. 2014, 4, 302. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W., II; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Karch, J.; Kwong, J.Q.; Burr, A.R.; Sargent, M.A.; Elrod, J.W.; Peixoto, P.M.; Martinez-Caballero, S.; Osinska, H.; Cheng, E.H.-Y.; Robbins, J.; et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2013, 2, e00772. [Google Scholar] [CrossRef]
- Zunino, S.J.; Storms, D.H. Resveratrol-induced apoptosis is enhanced in acute lymphoblastic leukemia cells by modulation of the mitochondrial permeability transition pore. Cancer Lett. 2006, 240, 123–134. [Google Scholar] [CrossRef]
- Lemeshko, V.V. VDAC electronics: 1. VDAC-hexo(gluco)kinase generator of the mitochondrial outer membrane potential. Biochim. Biophys. Acta 2014, 1838, 1362–1371. [Google Scholar] [CrossRef]
- Chang, W.M. Mitochondrial calcium-mediated reactive oxygen species are essential for the rapid induction of the grp78 gene in 9L rat brain tumour cells. Cell Signal. 2003, 15, 57–64. [Google Scholar] [CrossRef]
- Szabo, I.; Zoratti, M.; Biasutto, L. Targeting mitochondrial ion channels for cancer therapy. Redox Biol. 2021, 42, 101846. [Google Scholar] [CrossRef]
- Messina, A.; Reina, S.; Guarino, F.; De Pinto, V. VDAC isoforms in mammals. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1466–1476. [Google Scholar] [CrossRef]
- De Stefani, D.; Bononi, A.; Romagnoli, A.; Messina, A.; De Pinto, V.; Pinton, P.; Rizzuto, R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012, 19, 267–273. [Google Scholar] [CrossRef]
- Vander Heiden, M.G. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 2001, 276, 19414–19419. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Zakar, M.; Rosenthal, K.; Abu-Hamad, S. Key regions of VDAC1 functioning in apoptosis induction and regulation by hexokinase. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Prezma, T.; Shteinfer, A.; Admoni, L.; Raviv, Z.; Sela, I.; Levi, I.; Shoshan-Barmatz, V. VDAC1-based peptides: Novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia. Cell Death Dis. 2013, 4, e809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shteinfer-Kuzmine, A.; Amsalem, Z.; Arif, T.; Zooravlov, A.; Shoshan-Barmatz, V. Selective induction of cancer cell death by VDAC 1-based peptides and their potential use in cancer therapy. Mol. Oncol. 2018, 12, 1077–1103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Fu, Y.; Wang, X.; Shi, H.; Huang, Y.; Song, X.; Li, L.; Song, N.; Luo, Y. Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J. 2008, 22, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Shteinfer-Kuzmine, A.; Verma, A. VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases. Biomolecules 2020, 10, 1485. [Google Scholar] [CrossRef]
- Abbaszadeh, Z.; Çeşmeli, S.; Avcı, B. Crucial players in glycolysis: Cancer progress. Gene 2020, 726, 144158. [Google Scholar] [CrossRef]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L.; et al. Hexokinase 2 Is Required for Tumor Initiation and Maintenance and Its Systemic Deletion Is Therapeutic in Mouse Models of Cancer. Cancer Cell 2013, 24, 213–228. [Google Scholar] [CrossRef]
- Krasnov, G.S.; Dmitriev, A.A.; Lakunina, V.A.; A Kirpiy, A.; Kudryavtseva, A.V. Targeting VDAC-bound hexokinase II: A promising approach for concomitant anti-cancer therapy. Expert Opin. Ther. Targets 2013, 17, 1221–1233. [Google Scholar] [CrossRef]
- Lin, D.T.; Lechleiter, J.D. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J. Biol. Chem. 2002, 277, 31134–31141. [Google Scholar] [CrossRef]
- Schubert, A.; Grimm, S. Cyclophilin D, a component of the permeability transition-pore, is an apoptosis repressor. Cancer Res. 2004, 64, 85–93. [Google Scholar] [CrossRef]
- Machida, K.; Ohta, Y.; Osada, H. Suppression of Apoptosis by Cyclophilin D via Stabilization of Hexokinase II Mitochondrial Binding in Cancer Cells. J. Biol. Chem. 2006, 281, 14314–14320. [Google Scholar] [CrossRef]
- Chiara, F.; Castellaro, D.; Marin, O.; Petronilli, V.; Brusilow, W.S.; Juhaszova, M.; Sollott, S.J.; Forte, M.; Bernardi, P.; Rasola, A. Hexokinase II Detachment from Mitochondria Triggers Apoptosis through the Permeability Transition Pore Independent of Voltage-Dependent Anion Channels. PLoS ONE 2008, 3, e1852. [Google Scholar] [CrossRef]
- Choudhary, D.; Goykar, H.; Karanwad, T.; Kannaujia, S.; Gadekar, V.; Misra, M. An understanding of mitochondria and its role in targeting nanocarriers for diagnosis and treatment of cancer. Asian J. Pharm. Sci. 2021, 16, 397–418. [Google Scholar] [CrossRef]
- Folda, A.; Citta, A.; Scalcon, V.; Calì, T.; Zonta, F.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Mitochondrial Thioredoxin System as a Modulator of Cyclophilin D Redox State. Sci. Rep. 2016, 6, 23071. [Google Scholar] [CrossRef]
- Chevrollier, A.; Loiseau, D.; Reynier, P.; Stepien, G. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 562–567. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.-K.; Kim, H.S.; Chung, H.H.; Song, Y.S. Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Front. Oncol. 2013, 3, 41. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria as targets for cancer chemotherapy. Semin. Cancer Biol. 2009, 19, 57–66. [Google Scholar] [CrossRef]
- Liu, Z.; Wild, C.; Ding, Y.; Ye, N.; Chen, H.; Wold, E.A.; Zhou, J. BH4 domain of Bcl-2 as a novel target for cancer therapy. Drug Discov. Today 2016, 21, 989–996. [Google Scholar] [CrossRef]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef]
- Danese, A.; Patergnani, S.; Bonora, M.; Wieckowski, M.R.; Previati, M.; Giorgi, C.; Pinton, P. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim. Biophys. Acta Bioenerg. 2017, 1858, 615–627. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. Inhibition of bcl-2 as cancer therapy. Ann Oncol. 2002, 13, 501–502. [Google Scholar] [CrossRef]
- Papa, A.; Pandolfi, P.P. The PTEN–PI3K Axis in Cancer. Biomolecules 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, N.; Qiao, C.; Ni, T.; Li, Z.; Yu, B.; Guo, Q.; Wei, L. FV-429 induces apoptosis and inhibits glycolysis by inhibiting Akt-mediated phosphorylation of hexokinase II in MDA-MB-231 cells. Mol. Carcinog. 2016, 55, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Billadeau, D.D. Glycogen synthase kinase-3β: A novel therapeutic target for pancreatic cancer. Expert Opin. Ther. Targets 2020, 24, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019, 10, 26 . [Google Scholar] [CrossRef]
- Liu, F.; Wu, X.; Jiang, X.; Qian, Y.; Gao, J. Prolonged inhibition of class I PI3K promotes liver cancer stem cell expansion by augmenting SGK3/GSK-3β/β-catenin signalling. J. Exp. Clin. Cancer Res. 2018, 37, 122. [Google Scholar] [CrossRef]
- Rasola, A.; Sciacovelli, M.; Chiara, F.; Pantic, B.; Brusilow, W.S.; Bernardi, P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc. Natl. Acad. Sci. USA 2010, 107, 726–731. [Google Scholar] [CrossRef]
- Monick, M.M.; Powers, L.S.; Barrett, C.W.; Hinde, S.; Ashare, A.; Groskreutz, D.J.; Nyunoya, T.; Coleman, M.; Spitz, D.R.; Hunninghake, G.W. Constitutive ERK MAPK Activity Regulates Macrophage ATP Production and Mitochondrial Integrity. J. Immunol. 2008, 180, 7485–7496. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, W.; Ding, Q.; Zhang, J.; Wu, X.; Tang, P.; Zhou, H.; Wan, B.; Yin, G. ERK inhibition sensitizes CZ415-induced anti-osteosarcoma activity in vitro and in vivo. Oncotarget 2017, 8, 82027–82036. [Google Scholar]
- An, J.; Li, L.; Zhang, X. Curcusone C induces apoptosis in endometrial cancer cells via mitochondria-dependent apoptotic and ERK pathway. Biotechnol. Lett. 2021, 43, 329–338. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Yao, Y.; Li, J.; Wang, W.; Wu, X. Equol Induces Mitochondria-Dependent Apoptosis in Human Gastric Cancer Cells via the Sustained Activation of ERK1/2 Pathway. Mol. Cells 2016, 39, 742–749. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Hoek, J.B. Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 2008, 40, 171–182. [Google Scholar] [CrossRef]
- Ahmad, A.; Sakr, W.A.; Rahman, K.W. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front. Biosci. 2012, 4, 410–425. [Google Scholar] [CrossRef]
- Nemec, K.N.; Khaled, A.R. Therapeutic modulation of apoptosis: Targeting the BCL-2 family at the interface of the mitochon-drial membrane. Yonsei Med. J. 2008, 49, 689–697. [Google Scholar] [CrossRef]
- Mark, F.V.D.; Wei, A.H.; Mason, K.D.; Vandenberg, C.J.; Chen, L.; Czabotar, P.E.; Willis, S.N.; Scott, C.L.; Day, C.L.; Cory, S.; et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006, 10, 389–399. [Google Scholar]
- Bray, K.; Chen, H.-Y.; Karp, C.M.; May, M.; Ganesan, S.; Karantza-Wadsworth, V.; DiPaola, R.S.; White, E. Bcl-2 Modulation to Activate Apoptosis in Prostate Cancer. Mol. Cancer Res. 2009, 7, 1487–1496. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Wang, J.-H. Bag3 promotes resistance to apoptosis through Bcl-2 family members in non-small cell lung cancer. Oncol. Rep. 2012, 27, 109–113. [Google Scholar] [CrossRef]
- Zinn, R.L.; E Gardner, E.; Dobromilskaya, I.; Murphy, S.; Marchionni, L.; Hann, C.L.; Rudin, C.M. Combination treatment with ABT-737 and chloroquine in preclinical models of small cell lung cancer. Mol. Cancer 2013, 12, 16. [Google Scholar] [CrossRef]
- Kutuk, O.; Letai, A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008, 68, 7985–7994. [Google Scholar] [CrossRef]
- Zall, H.; Weber, A.; Besch, R.; Zantl, N.; Häcker, G. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol. Cancer 2010, 9, 164. [Google Scholar] [CrossRef]
- Du, Y.; Wu, J.; Luo, L. Secreted Heat Shock Protein 90α Attenuated the Effect of Anticancer Drugs in Small-Cell Lung Cancer Cells Through AKT/GSK3β/β-Catenin Signaling. Cancer Control. 2018, 25, 1073274818804489. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Jung, J.Y.; Kim, A.; Chang, Y.S.; Kim, S.K. ABT-737 Synergizes with Cisplatin Bypassing Aberration of Apoptotic Pathway in Non-small Cell Lung Cancer. Neoplasia 2017, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Zhang, B.; Pan, S.-H.; Zhao, H.-M.; Zhang, Y.; Feng, W.-H.; Li, Y.-Y.; Cao, X.-C. Bcl-2 inhibitor ABT-737 enhances the cisplatin-induced apoptosis in breast cancer T47D cells. Zhonghua Zhong Liu Za Zhi 2011, 33, 891–895. [Google Scholar] [PubMed]
- Lee, E.Y.; Gong, E.-Y.; Shin, J.-S.; Moon, J.-H.; Shim, H.J.; Kim, S.-M.; Lee, S.; Jeong, J.; Gong, J.H.; Kim, M.J.; et al. Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin. Toxicol. In Vitro 2018, 46, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Stamelos, V.A. Navitoclax augments the activity of carboplatin and paclitaxel combinations in ovarian cancer cells. Gynecol Oncol. 2013, 128, 377–382. [Google Scholar] [CrossRef]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin Sensitizes Colon Cancer Cells to Antitumor Activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef]
- Wang, H.; Hong, B.; Li, X.; Deng, K.; Li, H.; Lui, V.W.Y.; Lin, W. JQ1 synergizes with the Bcl-2 inhibitor ABT-263 against MYCN-amplified small cell lung cancer. Oncotarget 2017, 8, 86312–86324. [Google Scholar] [CrossRef][Green Version]
- Gardner, E.E.; Connis, N.; Poirier, J.T.; Cope, L.; Dobromilskaya, I.; Gallia, G.L.; Rudin, C.M.; Hann, C.L. Rapamycin rescues ABT-737 efficacy in small cell lung cancer. Cancer Res. 2014, 74, 2846–2856. [Google Scholar] [CrossRef]
- Ackler, S.; Mitten, M.J.; Foster, K.; Oleksijew, A.; Refici, M.; Tahir, S.K.; Xiao, Y.; Tse, C.; Frost, D.J.; Fesik, S.W.; et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother. Pharmacol. 2010, 66, 869–880. [Google Scholar] [CrossRef]
- Scheffold, A.; Jebaraj, B.M.C.; Stilgenbauer, S. Venetoclax: Targeting BCL2 in Hematological Cancers. In Small Molecules in Hematology. Recent Results in Cancer Research; Martens, U., Ed.; Springer: Cham, Switzerland, 2018; Volume 212. [Google Scholar]
- Jakubowska, M.A.; Kerkhofs, M.; Martines, C.; Efremov, D.; Gerasimenko, J.; Gerasimenko, O.; Petersen, O.; Bultynck, G.; Vervliet, T.; Ferdek, P. ABT-199 (Venetoclax), a BH3-mimetic Bcl-2 inhibitor, does not cause Ca2+ -signalling dysregulation or toxicity in pancreatic acinar cells. Br. J. Pharmacol. 2019, 176, 4402–4415. [Google Scholar] [CrossRef]
- Drozd-Sokolowska, J.; Mądry, K.; Siewiorek, K.; Feliksbrot-Bratosiewicz, M.; Stokłosa, T.; Gierej, B.; Stefaniak, A.; Paszkowska-Kowalewska, M.; Sokołowski, J.; Sankowski, B.; et al. The Clinical Tumor Lysis Syndrome in a Patient with Mixed Phenotype Acute Leukemia Under-going Induction with Venetoclax and Azacitidine: A Case Report. Chemotherapy 2022, 67, 173–177. [Google Scholar] [CrossRef]
- Waggoner, M.; Katsetos, P.-C.J.; Thomas, M.E.; Galinsky, B.I.; Fox, P.-C.H. Practical Management of the Venetoclax-Treated Patient in Chronic Lymphocytic Leukemia and Acute Myeloid Leukemia. J. Adv. Pr. Oncol. 2022, 13, 400–415. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland, S.A., Jr.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results from a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef]
- Meng, Y.; Tang, W.; Dai, Y.; Wu, X.; Liu, M.; Ji, Q.; Ji, M.; Pienta, K.; Lawrence, T.; Xu, L. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol. Cancer Ther. 2008, 7, 2192–2202. [Google Scholar] [CrossRef]
- Oliver, C.L.; Miranda, M.B.; Shangary, S.; Land, S.; Wang, S.; E Johnson, D. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol. Cancer Ther. 2005, 4, 23–31. [Google Scholar] [CrossRef]
- Ye, W.; Chang, H.-L.; Wang, L.-S.; Huang, Y.-W.; Shu, S.; Sugimoto, Y.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. Induction of apoptosis by (-)-gossypol-enriched cottonseed oil in human breast cancer cells. Int. J. Mol. Med. 2010, 26, 113–119. [Google Scholar]
- Warnsmann, V.; Meyer, N.; Hamann, A.; Kögel, D.; Osiewacz, H.D. A novel role of the mitochondrial permeability transition pore in (−)-gossypol-induced mitochondrial dysfunction. Mech. Ageing Dev. 2018, 170, 45–58. [Google Scholar] [CrossRef]
- Azmi, A.S.; Mohammad, R.M. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J. Cell Physiol. 2009, 218, 13–21. [Google Scholar] [CrossRef]
- O'Brien, S.M.; Claxton, D.F.; Crump, M.; Faderl, S.; Kipps, T.; Keating, M.J.; Viallet, J.; Cheson, B.D. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 2009, 113, g299–g305. [Google Scholar] [CrossRef]
- Nguyen, M.; Cencic, R.; Ertel, F.; Bernier, C.; Pelletier, J.; Roulston, A.; Silvius, J.R.; Shore, G.C. Obatoclax is a direct and potent antagonist of membrane-restricted Mcl-1 and is synthetic lethal with treatment that induces Bim. BMC Cancer 2015, 15, 568. [Google Scholar] [CrossRef] [PubMed]
- Manero, F.; Gautier, F.; Gallenne, T.; Cauquil, N.; Greé, D.; Cartron, P.-F.; Geneste, O.; Greé, R.; Vallette, F.M.; Juin, P. The Small Organic Compound HA14-1 Prevents Bcl-2 Interaction with Bax to Sensitize Malignant Glioma Cells to Induction of Cell Death. Cancer Res. 2006, 66, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Liu, D.; Zhang, Z.-J.; Shan, S.; Han, X.; Srinivasula, S.M.; Croce, C.M.; Alnemri, E.S.; Huang, Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Arisan, E.D.; Kutuk, O.; Tezil, T.; Bodur, C.; Telci, D.; Basaga, H. Small inhibitor of Bcl-2, HA14-1, selectively enhanced the apoptotic effect of cisplatin by modulating Bcl-2 family members in MDA-MB-231 breast cancer cells. Breast Cancer Res. Treat. 2010, 119, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Kataoka, K.; Sora, S.; Hara, T.; Omura-Minamisawa, M.; Funayama, T.; Sakashita, T.; Nakano, T.; Kobayashi, Y. The small-molecule Bcl-2 inhibitor HA14-1 sensitizes cervical cancer cells, but not normal fibroblasts, to heavy-ion radiation. Radiother. Oncol. 2008, 89, 227–230. [Google Scholar] [CrossRef]
- Klasa, R.J.; Gillum, A.M.; Klem, R.E.; Frankel, S.R. Oblimersen Bcl-2 antisense: Facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002, 12, 193–213. [Google Scholar] [CrossRef]
- Moreira, J.N. Bcl-2-targeted antisense therapy (Oblimersen sodium): Towards clinical reality. Rev. Recent Clin. Trials 2006, 1, 217–235. [Google Scholar] [CrossRef]
- Tan, W.; Loke, Y.-H.; Stein, C.; Miller, P.; Colombini, M. Phosphorothioate Oligonucleotides Block the VDAC Channel. Biophys. J. 2007, 93, 1184–1191. [Google Scholar] [CrossRef][Green Version]
- Liu, T.; Lam, V.; Thieme, E.; Sun, D.; Wang, X.; Xu, F.; Wang, L.; Danilova, O.V.; Xia, Z.; Tyner, J.W.; et al. Pharmacologic Targeting of Mcl-1 Induces Mitochondrial Dysfunction and Apoptosis in B-Cell Lymphoma Cells in a TP53- and BAX-Dependent Manner. Clin. Cancer Res. 2021, 27, 4910–4922. [Google Scholar] [CrossRef]
- De Ridder, I.; Kerkhofs, M.; Veettil, S.P.; Dehaen, W.; Bultynck, G. Cancer cell death strategies by targeting Bcl-2’s BH4 domain. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118983. [Google Scholar] [CrossRef]
- Ho, N.; Morrison, J.; Silva, A.; Coomber, B.L. The effect of 3-bromopyruvate on human colorectal cancer cells is dependent on glucose concentration but not hexokinase II expression. Biosci. Rep. 2016, 36, e00299. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Roberts, D.J.; Tan-Sah, V.P.; Ding, E.; Smith, J.M.; Miyamoto, S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol. Cell 2014, 53, 521–533. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, W.-N.; Xue, Y.-N.; Zhang, L.-C.; Zhang, J.-J.; Lu, S.-Y.; Yan, X.-Y.; Yu, H.-M.; Su, J.; Sun, L.-K. SIRT3 aggravates metformin-induced energy stress and apoptosis in ovarian cancer cells. Exp. Cell Res. 2018, 367, 137–149. [Google Scholar] [CrossRef]
- Cheng, G.; Zielonka, J.; McAllister, D.L.; Tsai, S.; Dwinell, M.B.; Kalyanaraman, B. Profiling and targeting of cellular bioenergetics: Inhibition of pancreatic cancer cell proliferation. Br. J. Cancer 2014, 111, 85–93. [Google Scholar] [CrossRef]
- Bizjak, M.; Malavašič, P.; Dolinar, K.; Pohar, J.; Pirkmajer, S.; Pavlin, M. Combined treatment with Metformin and 2-deoxy glucose induces detachment of viable MDA-MB-231 breast cancer cells in vitro. Sci. Rep. 2017, 7, 1761. [Google Scholar] [CrossRef]
- Xi, H.; Kurtoglu, M.; Liu, H.; Wangpaichitr, M.; You, M.; Liu, X.; Savaraj, N.; Lampidis, T.J. 2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother. Pharmacol. 2011, 67, 899–910. [Google Scholar] [CrossRef]
- DiPaola, R.S.; Dvorzhinski, D.; Thalasila, A.; Garikapaty, V.; Doram, D.; May, M.; Bray, K.; Mathew, R.; Beaudoin, B.; Karp, C.; et al. Therapeutic starvation and autophagy in prostate cancer: A new paradigm for targeting metabolism in cancer therapy. Prostate 2008, 68, 1743–1752. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, H.; Liu, D.X.; Niu, T.-K.; Ren, X.; Patel, R.; Hait, W.N.; Yang, J.-M. Silencing of Elongation Factor-2 Kinase Potentiates the Effect of 2-Deoxy-d-Glucose against Human Glioma Cells through Blunting of Autophagy. Cancer Res. 2009, 69, 2453–2460. [Google Scholar] [CrossRef]
- Zhang, L.; Su, J.; Xie, Q.; Zeng, L.; Wang, Y.; Yi, D.; Yu, Y.; Liu, S.; Li, S.; Xu, Y. 2-Deoxy-d-Glucose Sensitizes Human Ovarian Cancer Cells to Cisplatin by Increasing ER Stress and Decreasing ATP Stores in Acidic Vesicles. J. Biochem. Mol. Toxicol. 2015, 29, 572–578. [Google Scholar] [CrossRef]
- Jalota, A.; Kumar, M.; Das, B.C.; Yadav, A.K.; Chosdol, K.; Sinha, S. Synergistic increase in efficacy of a combination of 2-deoxy-d-glucose and cisplatin in normoxia and hypoxia: Switch from autophagy to apoptosis. Tumor. Biol. 2016, 37, 12347–12358. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, E.H.; Mahmoud, H.T.; Al-Hudhud, M.Y.; Abdalla, M.Y.; Ahmad, I.M.; Yasin, S.R.; Elkarmi, A.Z.; Tahtamouni, L.H. 2-deoxy-D-Glucose Synergizes with Doxorubicin or L-Buthionine Sulfoximine to Reduce Adhesion and Migration of Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Wei, Y.-H.; Shieh, D.-B.; Lin, L.-L.; Cheng, S.-P.; Wang, P.-W.; Chuang, J.-H. 2-Deoxy-d-Glucose Can Complement Doxorubicin and Sorafenib to Suppress the Growth of Papillary Thyroid Carcinoma Cells. PLoS ONE 2015, 10, e0130959. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Banerji, A.K.; Tripathi, R.P.; Gupta, J.P.; Mathew, T.L.; Ravindranath, T.; Jain, V. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005, 181, 507–514. [Google Scholar] [CrossRef]
- Venkataramanaa, N.K.; Venkatesh, P.; Dwarakanath, B.; Vani, S. Protective effect on normal brain tissue during a combin\ational therapy of 2-deoxy-d-glucose and hypofractionated irradiation in malignant gliomas. Asian J. Neurosurg. 2013, 8, 9–14. [Google Scholar]
- Cheng, G.; Zielonka, J.; Dranka, B.P.; McAllister, D.; Mackinnon, A.C., Jr.; Joseph, J.; Kalyanaraman, B. Mitochondria-Targeted Drugs Synergize with 2-Deoxyglucose to Trigger Breast Cancer Cell Death. Cancer Res. 2012, 72, 2634–2644. [Google Scholar] [CrossRef]
- Kim, W.; Yoon, J.-H.; Jeong, J.-M.; Cheon, G.-J.; Lee, T.-S.; Yang, J.-I.; Park, S.-C.; Lee, H.-S. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol. Cancer Ther. 2007, 6, 2554–2562. [Google Scholar] [CrossRef]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.-M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 446. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Hu, M.; Zhang, Y.; Cui, P.; Li, X.; Li, J.; Vestin, E.; Brännström, M.; Shao, L.; et al. Differential Expression Patterns of Glycolytic Enzymes and Mitochondria-Dependent Apoptosis in PCOS Pa-tients with Endometrial Hyperplasia, an Early Hallmark of Endometrial Cancer, In Vivo and the Impact of Metformin In Vitro. Int. J. Biol. Sci. 2019, 15, 714–725. [Google Scholar] [CrossRef]
- Han, C.Y.; Patten, D.A.; Lee, S.G.; Parks, R.J.; Chan, D.W.; Harper, M.-E.; Tsang, B.K. p53 Promotes chemoresponsiveness by regulating hexokinase II gene transcription and metabolic repro-gramming in epithelial ovarian cancer. Mol. Carcinog. 2019, 58, 2161–2174. [Google Scholar] [CrossRef]
- Kang, Y.-T.; Hsu, W.-C.; Wu, C.-H.; Hsin, I.-L.; Wu, P.-R.; Yeh, K.-T.; Ko, J.-L. Metformin alleviates nickel-induced autophagy and apoptosis via inhibition of hexokinase-2, activating lipocalin-2, in human bronchial epithelial cells. Oncotarget 2017, 8, 105536–105552. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Webster, L.; Mackey, J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 2008, 99, 989–994. [Google Scholar] [CrossRef]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxid. Med. Cell Longev. 2019, 2019, 8201079. [Google Scholar] [CrossRef]
- Bao, F.; Yang, K.; Wu, C.; Gao, S.; Wang, P.; Chen, L.; Li, H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018, 125, 123–129. [Google Scholar] [CrossRef]
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl jasmonate binds to and detaches mitochondria-bound hexokinase. Oncogene 2008, 27, 4636–4643. [Google Scholar] [CrossRef]
- Uludağ, D.; Bay, S.; Sucu, B.O.; İpek, Ö.Ş.; Mohr, T.; Güzel, M.; Karakaş, N. Potential of Novel Methyl Jasmonate Analogs as Anticancer Agents to Metabolically Target HK-2 Activity in Glioblastoma Cells. Front. Pharmacol. 2022, 13, 828400. [Google Scholar]
- Pastorino, J.G.; Shulga, N.; Hoek, J.B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002, 277, 7610–7618. [Google Scholar] [CrossRef]
- Neary, C.L. Akt inhibition promotes hexokinase 2 redistribution and glucose uptake in cancer cells. J. Cell Physiol. 2013, 228, 1943–1948. [Google Scholar] [CrossRef]
- Reina, S.; De Pinto, V. Anti-Cancer Compounds Targeted to VDAC: Potential and Perspectives. Curr. Med. Chem. 2017, 24, 4447–4469. [Google Scholar] [CrossRef]
- Ben-Hail, D.; Begas-Shvartz, R.; Shalev, M.; Shteinfer-Kuzmine, A.; Gruzman, A.; Reina, S.; De Pinto, V.; Shoshan-Barmatz, V. Novel Compounds Targeting the Mitochondrial Protein VDAC1 Inhibit Apoptosis and Protect against Mitochondrial Dysfunction. J. Biol. Chem. 2016, 291, 24986–25003. [Google Scholar] [CrossRef]
- Huo, H.; Zhou, Z.; Qin, J.; Liu, W.; Wang, B.; Gu, Y. Erastin Disrupts Mitochondrial Permeability Transition Pore (mPTP) and Induces Apoptotic Death of Colorectal Cancer Cells. PLoS ONE 2016, 11, e0154605. [Google Scholar] [CrossRef] [PubMed]
- Shulga, N.; Wilson-Smith, R.; Pastorino, J.G. Hexokinase II detachment from the mitochondria potentiates cisplatin induced cytotoxicity through a caspase-2 dependent mechanism. Cell Cycle 2009, 8, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.L.; Moser, B.; Stock, W.; Gallagher, R.E.; Willman, C.L.; Stone, R.M.; Rowe, J.M.; Coutre, S.; Feusner, J.H.; Gregory, J.; et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 2010, 116, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Tabassum, H.; Parvez, S. Melatonin modulates permeability transition pore and 5-hydroxydecanoate induced KATP channel inhibition in isolated brain mitochondria. Mitochondrion 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.F.; Guo, X. Arsenite-induced apoptosis is prevented by selenite in A375 cell line. Biol. Trace Elem. Res. 2011, 140, 7–17. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef]
- Barbosa, I.A. Mitochondrial remodeling in cancer metabolism and survival: Potential for new therapies. Biochim. Biophys. Acta 2012, 1826, 238–254. [Google Scholar] [CrossRef]
- Mansi, J.L.; De Graeff, A.; Newell, D.R.; Glaholm, J.; Button, D.; Leach, M.; Payne, G.; Smith, I.E. A phase II clinical and pharmacokinetic study of Lonidamine in patients with advanced breast cancer. Br. J. Cancer 1991, 64, 593–597. [Google Scholar] [CrossRef]
- De Lena, M.; Lorusso, V.; Latorre, A.; Fanizza, G.; Gargano, G.; Caporusso, L.; Guida, M.; Catino, A.; Crucitta, E.; Sambiasi, D.; et al. Paclitaxel, cisplatin and lonidamine in advanced ovarian cancer. A phase II study. Eur. J. Cancer 2001, 37, 364–368. [Google Scholar] [CrossRef]
- Cervantes-Madrid, D.; Romero, Y.; Dueñas-González, A. Reviving Lonidamine and 6-Diazo-5-oxo-L-norleucine to Be Used in Combination for Metabolic Cancer Therapy. BioMed Res. Int. 2015, 2015, 690492. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Q.; Pan, J.; Lee, Y.; Ouari, O.; Hardy, M.; Zielonka, M.; Myers, C.R.; Zielonka, J.; Weh, K.; et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat. Commun. 2019, 10, 2205. [Google Scholar] [CrossRef]
- Diel, I.J.; Jaschke, A.; Solomayer, E.F.; Gollan, C.; Bastert, G.; Sohn, C.; Schuetz, F. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: A long-term follow-up. Ann. Oncol. 2008, 19, 2007–2011. [Google Scholar] [CrossRef]
- Don, A.S. A peptide trivalent arsenical inhibits tumor angiogenesis by perturbing mitochondrial function in angiogenic en-dothelial cells. Cancer Cell. 2003, 3, 497–509. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Bepari, A.; Assiri, R.A.; Niazi, S.K.; Nayaka, S.; Rudrappa, M.; Nagaraja, S.K.; Bhat, M.P. Saussurea lappa Exhibits Anti-Oncogenic Effect in Hepatocellular Carcinoma, HepG2 Cancer Cell Line by Bcl-2 Mediated Apoptotic Pathway and Mitochondrial Cytochrome C Release. Curr. Issues Mol. Biol. 2021, 43, 1114–1132. [Google Scholar] [CrossRef]
- Shin, M.; Lee, B.-M.; Kim, O.; Tran, H.N.K.; Lee, S.; Hwangbo, C.; Min, B.-S.; Lee, J.-H. Triterpenoids from Ziziphus jujuba induce apoptotic cell death in human cancer cells through mitochondrial reactive oxygen species production. Food Funct. 2018, 9, 3895–3905. [Google Scholar] [CrossRef]
- Qi, S.; Guo, L.; Yan, S.; Lee, R.J.; Yu, S.; Chen, S. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm. Sin. B 2019, 9, 279–293. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Ordaz, J.D.; Low, P.S. Targeting of a Photosensitizer to the Mitochondrion Enhances the Potency of Photodynamic Therapy. ACS Omega 2018, 3, 6066–6074. [Google Scholar] [CrossRef]
- Panzarini, E.; Tenuzzo, B.; Dini, L. Photodynamic therapy-induced apoptosis of HeLa cells. Ann. N. Y. Acad. Sci. 2009; 1171, 617–626. [Google Scholar]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Sareen, D.; Darjatmoko, S.R.; Albert, D.M.; Polans, A.S. Mitochondria, Calcium, and Calpain are Key Mediators of Resveratrol-Induced Apoptosis in Breast Cancer. Mol. Pharmacol. 2007, 72, 1466–1475. [Google Scholar] [CrossRef]
- Ma, X. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol. Cell Biochem. 2007, 302, 99–109. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Geng, J.X.; Hu, X.Y. Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac. J. Cancer Prev. 2013, 14, 4599–4602. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lin, K.-Y.; Chang, C.-C.; Fang, C.-L.; Lin, C.-P. Aloe-emodin-induced apoptosis in human gastric carcinoma cells. Food Chem. Toxicol. 2007, 45, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.-H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.K.; Uzunhisarcıklı, E.; Yerer, M.B.; Bishayee, A. The golden spice curcumin in cancer: A perspective on finalized clinical trials during the last 10 years. J. Cancer Res. Ther. 2022, 18, 19–26. [Google Scholar]
- Ralph, S.J.; Neuzil, J. Mitochondria as targets for cancer therapy. Mol. Nutr. Food Res. 2009, 53, 9–28. [Google Scholar] [CrossRef]
- Neuzil, J.; Dong, L.-F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.; Vale, C.; Langley, R. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of Mitochondrial Complex II by the Anticancer Agent Lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O'Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef]
- Birth, D.; Kao, W.-C.; Hunte, C. Structural analysis of atovaquone-inhibited cytochrome bc1 complex reveals the molecular basis of antimalarial drug action. Nat. Commun. 2014, 5, 4029. [Google Scholar] [CrossRef]
- Dixon, R.; Pozniak, A.L.; Watt, H.M.; Rolan, P.; Posner, J. Single-dose and steady-state pharmacokinetics of a novel microfluidized suspension of atovaquone in human immunodeficiency virus-seropositive patients. Antimicrob. Agents Chemother. 1996, 40, 556–560. [Google Scholar] [CrossRef]
- Fiorillo, M.; Lamb, R.; Tanowitz, H.B.; Mutti, L.; Krstic-Demonacos, M.; Cappello, A.R.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Repurposing atovaquone: Targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget 2016, 7, 34084–34099. [Google Scholar] [CrossRef]
- Falloon, J.; Sargent, S.; Piscitelli, S.C.; Bechtel, C.; Lafon, S.W.; Sadler, B.; Walker, R.E.; Kovacs, J.A.; Polis, M.A.; Davey, R.T.; et al. Atovaquone Suspension in HIV-Infected Volunteers: Pharmacokinetics, Pharmacodynamics, and TMP-SMX Interaction Study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1999, 19, 1050–1056. [Google Scholar] [CrossRef]
- Anderson, N.M. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell. 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Larochette, N.; Decaudinab, D.; Jacotota, E.; Brennerac, C.; Marzo, I.; Susin, S.A.; Zamzamia, N.; Xied, Z.; Reedd, J.; Kroemer, G. Arsenite Induces Apoptosis via a Direct Effect on the Mitochondrial Permeability Transition Pore. ExCell Res. 1999, 249, 413–421. [Google Scholar] [CrossRef]
- Tian, C.; Gao, P.; Zheng, Y.; Yue, W.; Wang, X.; Jin, H.; Chen, Q. Redox status of thioredoxin-1 (TRX1) determines the sensitivity of human liver carcinoma cells (HepG2) to arsenic trioxide-induced cell death. Cell Res. 2008, 18, 458–471. [Google Scholar] [CrossRef]
- Stevens, J.J.; Graham, B.; Dugo, E.; Sumner, B.B.; Ndebele, K.; Tchounwou, P.B. Arsenic Trioxide Induces Apoptosis via Specific Signaling Pathways in HT-29 Colon Cancer Cells. J. Cancer Sci. Ther. 2017, 9, 298–306. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Yang, M.-D.; Hsia, T.-C.; Chang, W.-S.; Hsu, C.-M.; Hsieh, Y.-H.; Chung, J.-G.; Bau, D.-T. Dithiothreitol enhanced arsenic-trioxide-induced cell apoptosis in cultured oral cancer cells via mitochondrial dysfunction and endoplasmic reticulum stress. Environ. Toxicol. 2017, 32, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Shen, J.; Cai, W.J.; Hong, C.; Zheng, M.H. The alteration of mitochondria is an early event of arsenic trioxide induced apoptosis in esophageal carcinoma cells. Int. J. Mol. Med. 2000, 5, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Yang, K.-M.; Park, Y.-S.; Choi, Y.-J.; Yun, J.-H.; Son, C.-H.; Suh, H.-S.; Jeong, M.-H.; Jo, W.-S. The novel resveratrol analogue HS-1793 induces apoptosis via the mitochondrial pathway in murine breast cancer cells. Int. J. Oncol. 2012, 41, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H. A novel resveratrol derivative, HS1793, overcomes the resistance conferred by Bcl-2 in human leukemic U937 cells. Biochem. Pharmacol. 2009, 77, 1337–1347. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar]
- Miranda, M.A.; Mondal, A.; Sachdeva, M.; Cabral, H.; Neto, Y.A.A.H.; Khan, I.; Groppo, M.; McChesney, J.D.; Bastos, J.K. Chemosensitizing Effect of Cernumidine Extracted from Solanum cernuum on Bladder Cancer Cells in Vitro. Chem. Biodivers. 2019, 16, e1900334. [Google Scholar] [CrossRef]
- Liu, W.Y. Lycorine Induces Mitochondria-Dependent Apoptosis in Hepatoblastoma HepG2 Cells Through ROCK1 Activation. Front. Pharmacol. 2019, 10, 651. [Google Scholar] [CrossRef]
- Bo, P.; Lien, J.-C.; Chen, Y.-Y.; Yu, F.-S.; Lu, H.-F.; Yu, C.-S.; Chou, Y.-C.; Yu, C.-C.; Chung, J.-G. Allyl Isothiocyanate Induces Cell Toxicity by Multiple Pathways in Human Breast Cancer Cells. Am. J. Chin. Med. 2016, 44, 415–437. [Google Scholar] [CrossRef]
- Hafezi, K.; Hemmati, A.A.; Abbaszadeh, H.; Valizadeh, A.; Makvandi, M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother. Res. 2020, 34, 1397–1408. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, Q.; Yang, G.; Huang, Z.; Shen, T.; Li, H.; Ren, D. Apoptosis induction of dehydrobruceine B on two kinds of human lung cancer cell lines through mitochondri-al-dependent pathway. Phytomedicine 2016, 23, 114–122. [Google Scholar] [CrossRef]
- Song, I.-S.; Jeong, Y.J.; Kim, J.E.; Shin, J.; Jang, S.-W. Frugoside Induces Mitochondria-Mediated Apoptotic Cell Death through Inhibition of Sulfiredoxin Expression in Melanoma Cells. Cancers 2019, 11, 854. [Google Scholar] [CrossRef]
- Balachandran, C.; Emi, N.; Arun, Y.; Yamamoto, Y.; Ahilan, B.; Sangeetha, B.; Duraipandiyan, V.; Inaguma, Y.; Okamoto, A.; Ignacimuthu, S.; et al. In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. Fruit Chem. Interactions 2015, 242, 81–90. [Google Scholar] [CrossRef]
- Han, X.; Deng, S.; Wang, N.; Liu, Y.; Yang, X. Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr. Res. 2016, 60, 30616. [Google Scholar] [CrossRef]
- Duan, H.; Wang, R.; Yan, X.; Liu, H.; Zhang, Y.; Mu, D.; Han, J.; Li, X. Phloretin induces apoptosis of human esophageal cancer via a mitochondria-dependent pathway. Oncol. Lett. 2017, 14, 6763–6768. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, B.; Wang, Y.; Zou, C.; Qiao, Q.; Diao, Z.; Mi, Y.; Zhu, D.; Liu, X. Sesamol Induces Human Hepatocellular Carcinoma Cells Apoptosis by Impairing Mitochondrial Function and Suppressing Autophagy. Sci. Rep. 2017, 7, srep45728. [Google Scholar] [CrossRef]
- Selzer, E.; Thallinger, C.; Hoeller, C.; Oberkleiner, P.; Wacheck, V.; Pehamberger, H.; Jansen, B. Betulinic acid-induced Mcl-1 expression in human melanoma--mode of action and functional significance. Mol. Med. 2002, 8, 877–884. [Google Scholar] [CrossRef]
- Pereira, C.V.; Machado, N.G.; Oliveira, P.J. Mechanisms of berberine (natural yellow 18)-induced mitochondrial dysfunction: Interaction with the ad-enine nucleotide translocator. Toxicol. Sci. 2008, 105, 408–417. [Google Scholar] [CrossRef]
- Dong, L.F.; Low, P.; Dyason, J.C.; Wang, X.-F.; Prochazka, L.; Witting, P.K.; Freeman, R.; Swettenham, E.; Valis, K.; Liu, J.; et al. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respir-atory complex II. Oncogene 2008, 27, 4324–4335. [Google Scholar] [CrossRef]
- Neuzil, J.; Wang, X.-F.; Dong, L.; Low, P.; Ralph, S. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006, 580, 5125–5129. [Google Scholar] [CrossRef]
- Prochazka, L.; Dong, L.-F.; Valis, K.; Freeman, R.; Ralph, S.J.; Turánek, J.; Neuzil, J. α-Tocopheryl succinate causes mitochondrial permeabilization by preferential formation of Bak channels. Apoptosis 2010, 15, 782–794. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Li, L.; Han, W.; Gu, Y.; Qiu, S.; Lu, Q.; Jin, J.; Luo, J.; Hu, X. Honokiol Induces a Necrotic Cell Death through the Mitochondrial Permeability Transition Pore. Cancer Res. 2007, 67, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Mazure, N.M. VDAC in cancer. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, L.-Y.; Tian, Y.; Zhang, Z.-Q.; Dong, W.-L.; Wang, X.-F.; Zhang, X.-Y.; Cao, C. C6 ceramide dramatically enhances docetaxel-induced growth inhibition and apoptosis in cultured breast cancer cells: A mechanism study. ExCell Res. 2015, 332, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, G.; Zhang, Q.; Tang, Q.; Huang, J.; Hu, C.; Liu, Y.; Wang, Q.; Liu, W.; Gao, N.; et al. Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 2018, 9, 598. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Chen, M.; Zeng, M. Hexavalent chromium-induced apoptosis in Hep3B cells is accompanied by calcium overload, mitochondrial damage, and AIF translocation. Ecotoxicol. Environ. Saf. 2021, 208, 111391. [Google Scholar] [CrossRef]
- Porter, G.A., Jr.; Beutner, G. Cyclophilin D, Somehow a Master Regulator of Mitochondrial Function. Biomolecules 2018, 8, 176. [Google Scholar] [CrossRef]
- Pan, H.; Wang, B.-H.; Lv, W.; Jiang, Y.; He, L. Esculetin induces apoptosis in human gastric cancer cells through a cyclophilin D-mediated mitochondrial permeability transition pore associated with ROS. Chem. Interactions 2015, 242, 51–60. [Google Scholar] [CrossRef]
- Yu, T.; Chen, C.; Sun, Y.; Sun, H.; Li, T.-H.; Meng, J.; Shi, X. ABT-737 sensitizes curcumin-induced anti-melanoma cell activity through facilitating mPTP death pathway. Biochem. Biophys. Res. Commun. 2015, 464, 286–291. [Google Scholar] [CrossRef]
- Xue, P.; Chen, Q.; Ren, X.; Liu, D.; Yang, X. A novel protoapigenone analog RY10-4 induces apoptosis of breast cancer cells by exacerbating mitochondrial Ca2+ influx through mitochondrial calcium uniporter. Toxicol. Appl. Pharmacol. 2021, 433, 115776. [Google Scholar] [CrossRef]
- Patenaude, A.; Deschesnes, R.G.; Rousseau, J.L.; Petitclerc, E.; Lacroix, J.; Côté, M.-F.C.; Gaudreault, R. New Soft Alkylating Agents with Enhanced Cytotoxicity against Cancer Cells Resistant to Chemotherapeutics and Hypoxia. Cancer Res. 2007, 67, 2306–2316. [Google Scholar] [CrossRef]
- Gainutdinov, T.; Molkentin, J.D.; Siemen, D.; Ziemer, M.; Debska-Vielhaber, G.; Vielhaber, S.; Gizatullina, Z.; Orynbayeva, Z.; Gellerich, F.N. Knockout of cyclophilin D in Ppif−/− mice increases stability of brain mitochondria against Ca2+ stress. Arch. Biochem. Biophys. 2015, 579, 40–46. [Google Scholar] [CrossRef]
- Chin, H.S.; Li, M.X.; Tan, I.K.L.; Ninnis, R.L.; Reljic, B.; Scicluna, K.; Dagley, L.F.; Sandow, J.J.; Kelly, G.L.; Samson, A.L.; et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat. Commun. 2018, 9, 4976. [Google Scholar] [CrossRef]
- Shangguan, X.; He, J.; Ma, Z.; Zhang, W.; Ji, Y.; Shen, K.; Yue, Z.; Li, W.; Xin, Z.; Zheng, Q.; et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat. Commun. 2021, 12, 1812. [Google Scholar] [CrossRef]
- Wang, N.N.; Zhang, P.-Z.; Zhang, J.; Wang, H.-N.; Li, L.; Ren, F.; Dai, P.-F.; Li, H.; Lv, X.-F. Penfluridol triggers mitochondrial-mediated apoptosis and suppresses glycolysis in colorectal cancer cells through down-regulating hexokinase-2. Anat. Rec. 2021, 304, 520–530. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L. MicroRNA-143 suppresses oral squamous cell carcinoma cell growth, invasion and glucose metabolism through targeting hexokinase 2. Biosci. Rep. 2017, 37, BSR20160404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waseem, M.; Wang, B.-D. Promising Strategy of mPTP Modulation in Cancer Therapy: An Emerging Progress and Future Insight. Int. J. Mol. Sci. 2023, 24, 5564. https://doi.org/10.3390/ijms24065564
Waseem M, Wang B-D. Promising Strategy of mPTP Modulation in Cancer Therapy: An Emerging Progress and Future Insight. International Journal of Molecular Sciences. 2023; 24(6):5564. https://doi.org/10.3390/ijms24065564
Chicago/Turabian StyleWaseem, Mohammad, and Bi-Dar Wang. 2023. "Promising Strategy of mPTP Modulation in Cancer Therapy: An Emerging Progress and Future Insight" International Journal of Molecular Sciences 24, no. 6: 5564. https://doi.org/10.3390/ijms24065564
APA StyleWaseem, M., & Wang, B.-D. (2023). Promising Strategy of mPTP Modulation in Cancer Therapy: An Emerging Progress and Future Insight. International Journal of Molecular Sciences, 24(6), 5564. https://doi.org/10.3390/ijms24065564







