Evaluation of the Selected Mechanical and Aesthetic Properties of Experimental Resin Dental Composites Containing 1-phenyl-1,2 Propanedione or Phenylbis(2,4,6-trimethylbenzoyl)-phosphine Oxide as a Photoinitiator
Abstract
:1. Introduction
2. Results
- a.
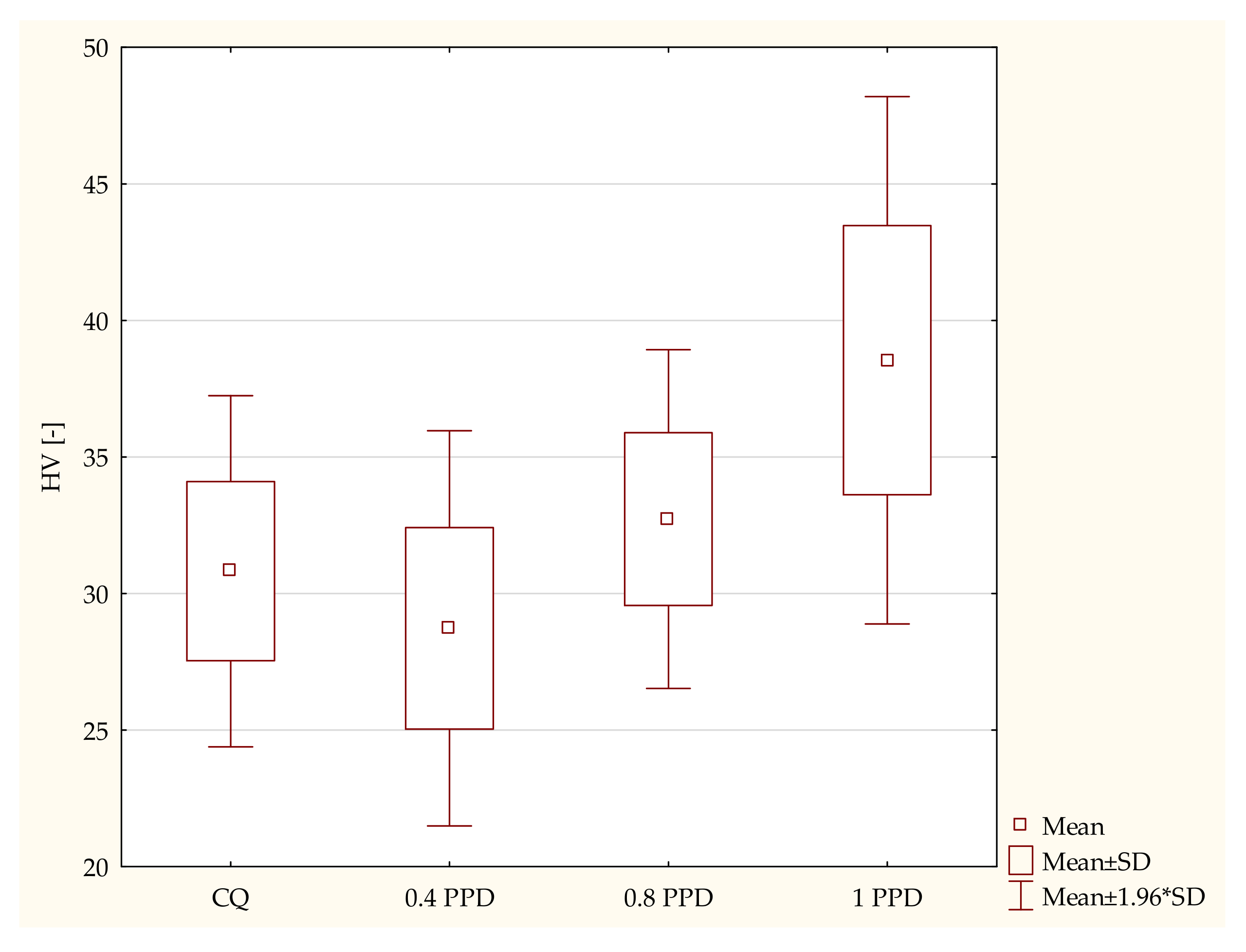
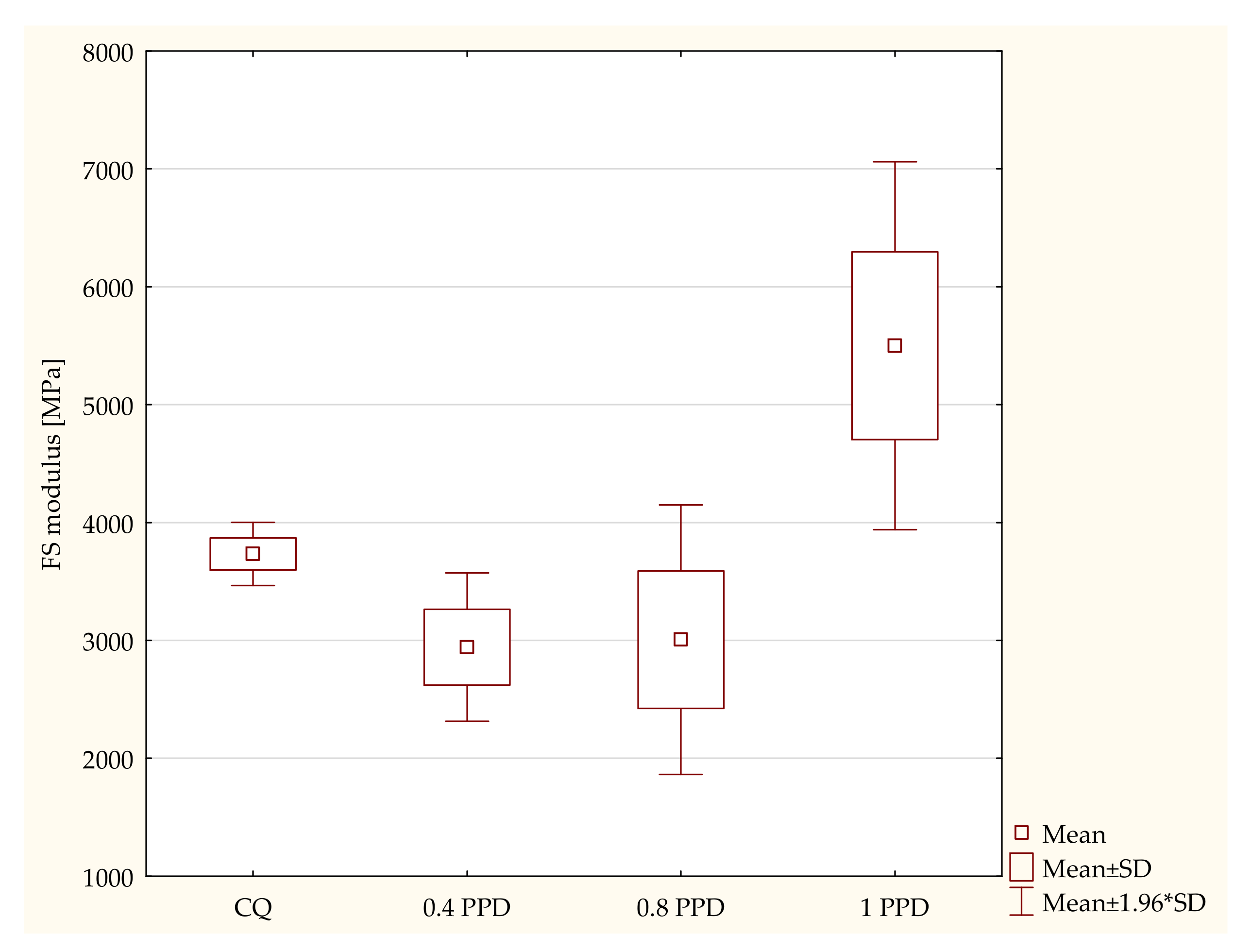
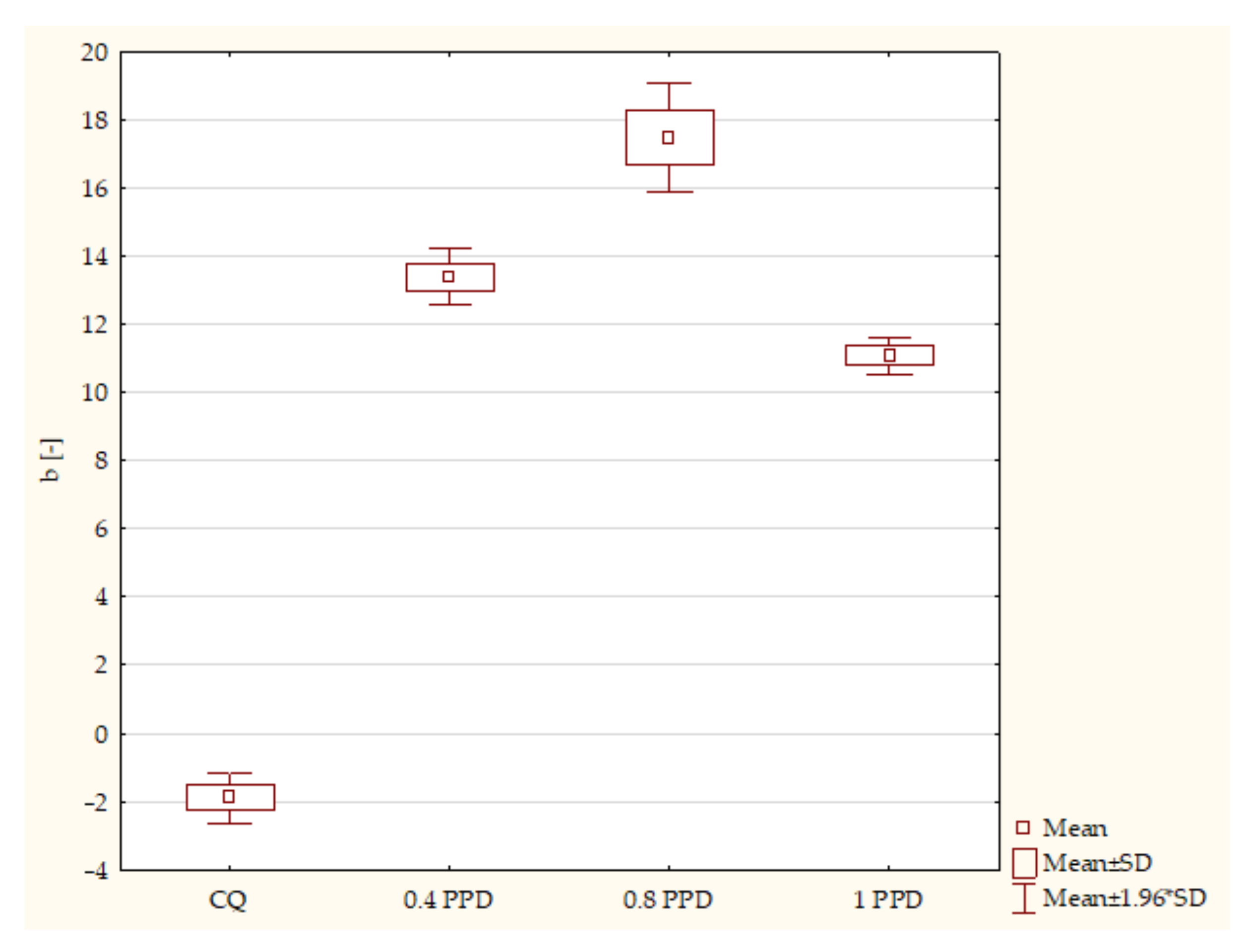
- The influence of the amounts of PPD and DMAEMA on the mechanical properties of the experimental composites
- Samples containing CQ and 1 wt. % of PPD (p-value = 0.00044), with higher values in the composite with 1 wt. % PPD.
- The dental composite including PPD 0.4 wt. % and PPD 1 wt. % (p-value = 0.00000), with higher values in the composite with 1 wt. % PPD.
- Specimens containing 0.8 wt. % PPD and 1wt. % PPD (p-value = 0.01090), with higher values in the dental resin with 1 wt. % PPD.
- Samples containing CQ and 0.8 wt. % PPD (p-value = 0.00379), with higher values in composites containing CQ;
- Experimental composites with 0.8 wt. % PPD and 1 wt. % PPD (p-value = 0.01334), with higher values in resin containing 1 wt. % PPD.
- Composites with CQ and 1 wt. % PPD (p-value = 0.00035), with higher values in dental resins with 1 wt. % PPD.
- Experimental dental resins with 0.4 wt. % and 1 wt. % PPD (p-value = 0.00000), with higher values in specimens with 1 wt. % PPD.
- Samples with 0.8 wt. % PPD and 1 wt. % PPD (p-value = 0.00000), with higher values in composites with 1 wt. % PPD.
- Composites with CQ and 0.4 wt. % PPD (p-value = 0.00001), with higher values in samples with 0.4 wt. % PPD;
- Samples containing CQ and 0.8 wt. % PPD (p-value = 0.00000), with higher values in composites with 0.8 wt. % PPD;
- Experimental resins containing CQ and 1 wt. % PPD (p-value = 0.00008), with higher values in specimens with 1 wt. % PPD;
- Specimens containing 0.4 wt. % PPD and 0.8 wt. % PPD (p-value = 0.00001), with higher values in dental resins with 0.8 wt. % PPD;
- Composites with 0.8 wt. % PPD and 1 wt. % PPD (p-value = 0.00000), with higher values in samples with 0.8 wt. % PPD.
- Composites with CQ and 0.4 wt. % PPD (p-value = 0.00000), with higher values in samples containing 0.4 wt. % PPD;
- Simples containing CQ and 0.8 wt. % PPD (p-value = 0.00000), with higher values in dental resins with 0.8 wt. % PPD;
- Experimental dental resin containing CQ and 1 wt. % PPD (p-value = 0.00000), with higher values in specimens with 1 wt. % PPD;
- Specimens with 0.4 wt. % PPD and 0.8 wt. % PPD (p-value = 0.00008), with higher values in dental resins with 0.8 wt. % PPD;
- Composites with 0.4 wt. % PPD and 1 wt. % PPD (p-value = 0.00445), with higher values in samples with 0.4 wt. % PPD;
- Dental resins 0.8 wt. % PPD and 1 wt. % PPD (p-value = 0.00000), with higher values in samples with 0.8 wt. % PPD.
- b.
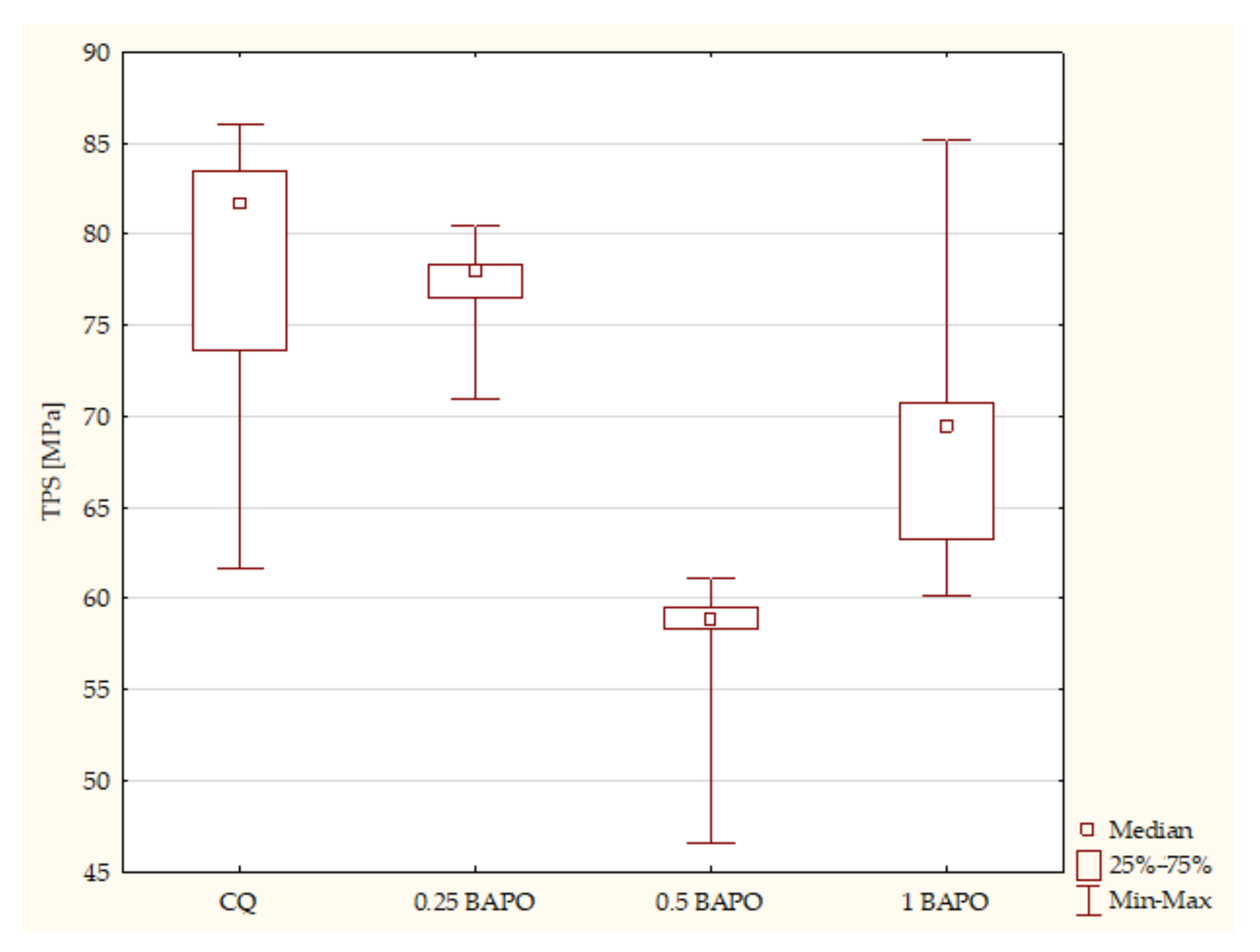
- The influence of the amount of BAPO on the mechanical properties of the experimental composites
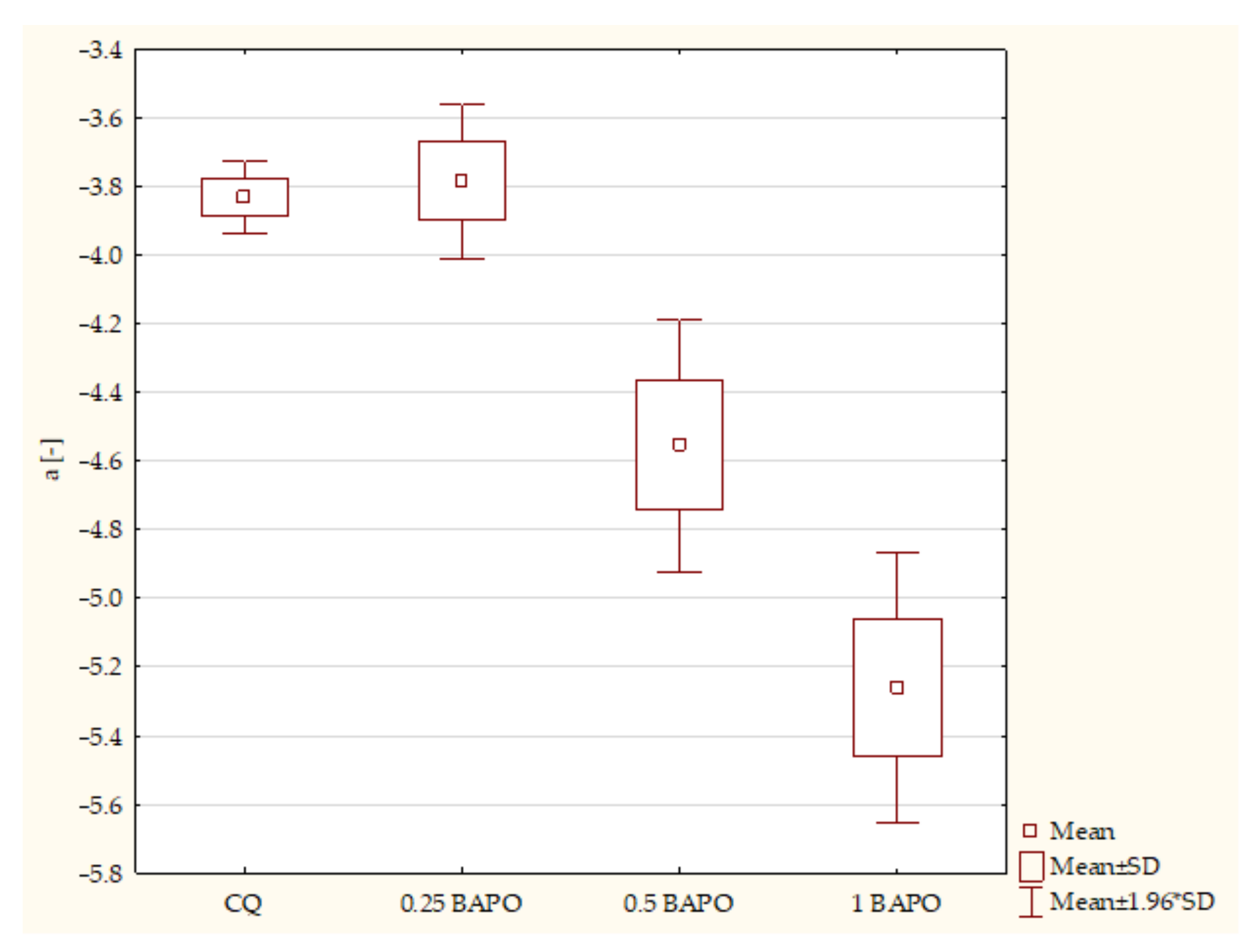
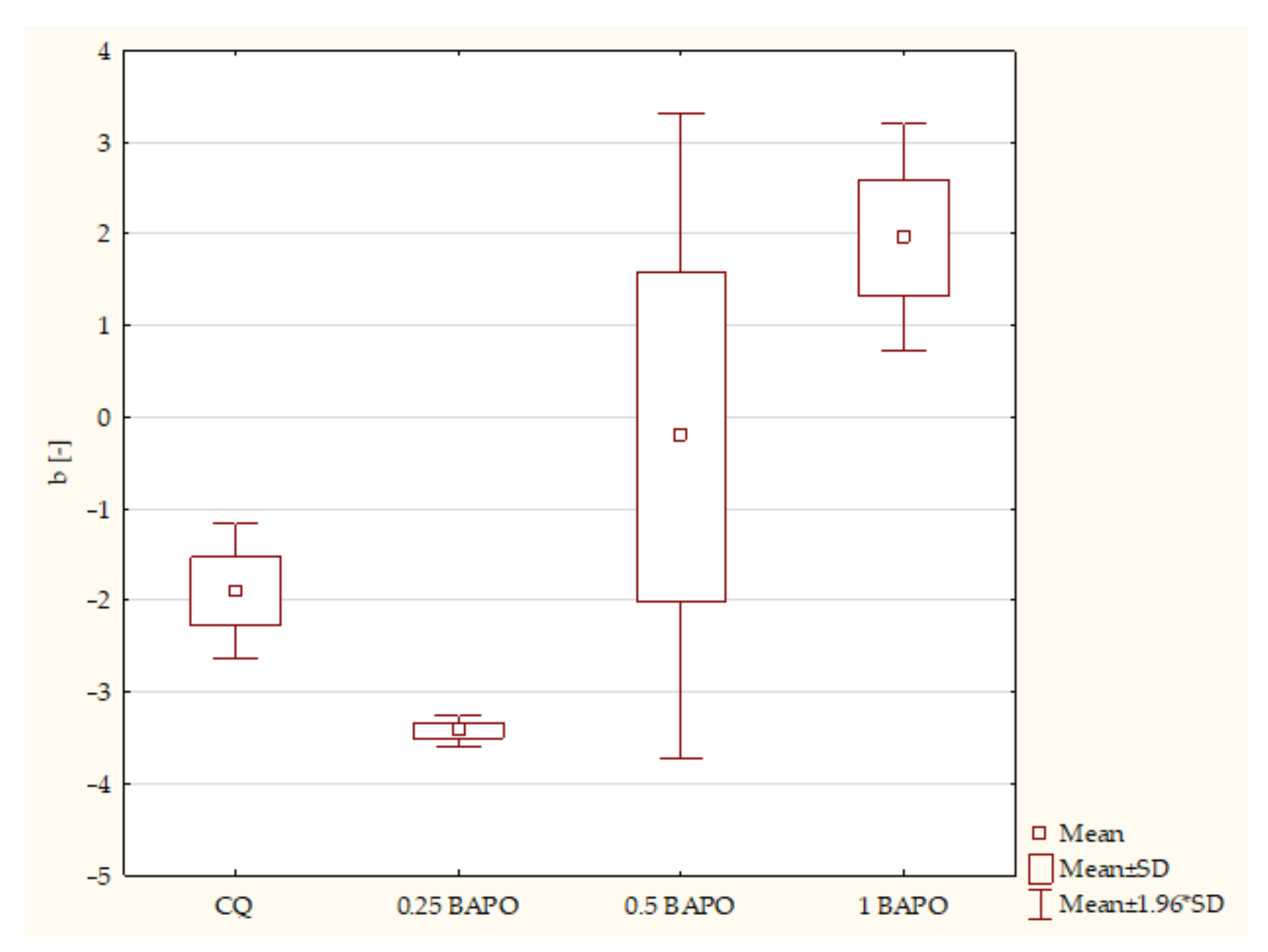
- Composites with CQ and 0.5 wt. % BAPO (p-value = 0.00001), with higher values in samples with 0.5 wt. % BAPO;
- Experimental dental resins with CQ and 1 wt. % BAPO (p-value = 0.00000), with higher values in the specimens with 1 wt. % BAPO;
- Samples with 0.25 wt. % BAPO and 1 wt. % BAPO (p-value = 0.00183), with higher values in composites with 1 wt. % BAPO.
- Composites with CQ and 0.5 wt. % BAPO (p-value = 0.01161), with higher values in the control group;
- Experimental resins with 0.25 wt. % BAPO and 1 wt. % BAPO (p-value = 0.02761), with higher values in composites with 0.25 wt. % BAPO.
- Composites with CQ and 0.5 wt. % BAPO (p-value = 0.00151), with higher values in samples containing CQ;
- Dental resins containing CQ and 1 wt. % BAPO (p-value = 0.00003), with higher values in specimens with CQ;
- Experimental composites with 0.25 wt. % BAPO and 0.5 wt. % BAPO (p-value = 0.00096), with higher values in composites with 0.25 wt. % BAPO;
- Specimens containing 0.25 wt. % BAPO and 1 wt. % BAPO (p-value = 0.00001), with higher values in samples with 0.25 wt. % BAPO;
- Samples containing 0.5 wt. % BAPO and 1 wt. % BAPO (p-value = 0.00184), with higher values in composites with 0.5 wt. % BAPO.
- Composites with CQ and 1 wt. % BAPO (p-value = 0.01428), with higher values in samples with 1 wt. % BAPO;
- Dental resins containing 0.25 wt. % BAPO and 0.5 wt. % BAPO (p-value = 0.02739), with higher values in specimens with 0.5 wt. % BAPO;
- Dental resins with 0.25 wt. % BAPO and 1 wt. % BAPO (p-value = 0.00167), with higher values in composites with 1 wt. % BAPO.
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Sunbul, H.; Silikas, N.; Watts, D.C. Polymerization shrinkage kinetics and shrinkage-stress in dental resin-composites. Dent. Mater. 2016, 32, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Camargo, F.M.; Della Bona, Á.; Moraes, R.R.; Coutinho de Souza, C.R.; Schneider, L.F. Influence of viscosity and amine content on C==C conversion and color stability of experimental composites. Dent. Mater. 2015, 31, e109–e115. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Sokołowski, J.; Szynkowska-Jó’zwik, M.I.; Gozdek, T.; Kopacz, K.; Bociong, K. Can TPO as Photoinitiator Replace “Golden Mean” Camphorquinone and Tertiary Amines in Dental Composites? Testing Experimental Composites Containing Different Concentration of Diphenyl(2,4,6-trimethylbenzoyl)phosphine Oxide. Int. J. Mol. Sci. 2022, 23, 11594. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Muncka, J.; Peumansa, M.; Yoshidac, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeeka, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Hadis, M.A.; Shortall, A.C.; Palin, W.M. Competitive light absorbers in photoactive dental resin-based materials. Dent. Mater. 2012, 28, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; King, N.M.; Suh, B.I.; Pashley, D.H. Effect of delayed activation of light-cured resin composites on bonding of all-in-one adhesives. J. Adhes Dent. 2001, 3, 11803709. [Google Scholar]
- Alvim, H.H.; Alecio, A.C.; Vasconcellos, W.A.; Furlan, M.; de Oliveira, J.E.; Saad, J.R.C. Analysis of camphorquinone in com-positeresins as a function of shade. Dent. Mater. 2006, 3, 1245–1249. [Google Scholar]
- Georg, H.; Canuto, S.; Coutinho, K. Solvent effects on the UV-visible absorption spectrum of benzophenone in water: A com-bined Monte Carlo quantum mechanics study including solute polarization. J. Chem. Phys. 2007, 126, 034507. [Google Scholar] [CrossRef] [PubMed]
- da Silva Alves Maciel, D.; Caires-Filho, A.B.; Fernandez-Garcia, M.; Anauate-Netto, C.; Alonso, R.C.B. Effect of Camphorqui-none Concentration in Physical-Mechanical Properties of Experimental Flowable Resin Composites. BioMed. Res. Int. 2018, 2018, 7921247. [Google Scholar]
- Allen, N.S. Photoinitiatorsfor UV and visible curing of coatings: Mechanism and properties. Jphotochem. Photobiol. A Chem. 1996, 100, 101–107. [Google Scholar] [CrossRef]
- Taira, M.; Urabe, H.; Hirose, T.; Wakasa, K.; Yamaki, M. Analysis of Photo-initiators in Visible-light-cured Dental CompositeRe-sins. J. Dent. Res. 1987, 67, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Kant, R.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Gianninic, M.; Arrais, C.A.G.; Price, R.B.T. Light curing in dentistry and clinical implications: A literature review Polymerization. Dent. Mater. 2017, 31, 64–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, H.; Takahashi, H.; Kanie, T.; Ban, S. Effect of various visible light photoinitiators on the polymerization and coloroflight-activated resins. Dent. Mater. J. 2009, 28, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verzola, K.C.; Dressano, D.; Saraceni, C.H.C.; Goncalves, L.S.; Hadis, M.; Watts, D.C.; Palin, W.M.; Fonseca Lima, A. Bis(4-methyl phenyl)iodonium as an alternative component to diphenyliodonium in camphorquinone-based ternary initiating systems. Dent. Mater. 2020, 6, 1282–1288. [Google Scholar] [CrossRef]
- Neumann, M.G.; Miranda, W.G.; Schmitt, C.C.; Rueggeberg, F.A.; Correa, I.C. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J. Dent. 2005, 33, 525–532. [Google Scholar] [CrossRef]
- Fonseca, A.; Salvador, M.V.O.; Dressano, D.; Saracenia, C.H.C.; Gonçalvesc, L.S.; Hadisd, M.; Palin, W.M. Increased rates of photopolymerisation by ternary type II photoinitiator systems in dental resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78. [Google Scholar]
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Fonseca Lima, A. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1,2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764. [Google Scholar] [CrossRef]
- Ikemura, K.; Endo, T. A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites. Dent. Mat. J. 2010, 29, 481–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, G.; Ganster, B.; Salz, U.; Moszner, N.; Liska, R. Photoinitiators With Functional Groups. IX. Hydrophilic Bisacylphosphine Oxides for Acidic Aqueous Formulations. InterScience 2005, 44, 1686–1700. [Google Scholar] [CrossRef]
- Vinicius, M.; Bertolo, L.; De Cássia, R.; Moraes, M. Influence of Photoinitiator System on Physical-Chemical Properties of Experimental Self-Adhesive Composites. Braz. Dent. J. 2017, 28, 35–39. [Google Scholar]
- Chiu, C.C. Liquid Bis-acylphosphine Oxide (BAPO) Photoinitiators. US Patent US20160168177A1, 16 June 2016. [Google Scholar]
- Moore, B.K.; Platt, J.A.; Borges, G.; Chu, T.M.; Katsilieri, I. Depth of cure of dental resin composites: ISO 4049 depth and microhardness of types of materials and shades. Oper Dent. 2008, 33, 408–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulkfill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorativeresins. Dent. Mater. 1985, 1, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Chae, K.; Rawls, H.R. Development of a new photoinitiation system for dental light-cure composite resins. Dent. Mater. 1999, 15, 120–127. [Google Scholar] [CrossRef]
- Brandt, W.C.; de Oliveira Tomaselli, L.; Correr-Sobrinho, L.; Sinhoreti, M.A.C. Can phenyl-propanedione influence Knoop hardness, rate of polymerization and bond strength of resin composite restorations? J. Dent. 2011, 39, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Cunha Brandt, W.; Schneider, L.F.J.; Frollini, E.; Correr-Sobrinho, L.; Sinhoreti, M.A.C. Effect of different photo-initiators and light curing units on degree of conversion of composites. Dent. Mater. 2010, 24, 263–270. [Google Scholar]
- de Resende, L.F.M.; Catelan, A.; Baroudi, K.; Palialol, A.R.M.; de Resende, A.M.; Andreucci, A.C.; Zanatta, R.F.; Liporoni, P.C.S. Mechanical Properties of Experimental Composites with Different Photoinitiator. Eur. J. Dent. 2022, 16, 167–172. [Google Scholar] [CrossRef]
- Almeida, S.M.; Meereis, C.T.W.; Leal, F.B.; Carvalho, R.V.; Boeira, P.O.; Chisini, L.A.; Cuevas-Suárez, C.E.; Lima, G.S.; Piva, E. Evaluation of alternative photoinitiator systems in two-step self-etch adhesive systems. Dent. Mater. 2020, 36, e29–e37. [Google Scholar] [CrossRef] [PubMed]
- Favarao, J.; Oliveira, D.C.R.S.; Zanini, M.M.; Rocha, M.G.; Correr-Sobrinho, L.; Sinhoreti, M.A.C. Effect of curing-light attenuation on color stability and physical and chemical properties of resin cements containing different photoinitiators. J. Mech. Behav. Biomed. Mater. 2021, 113, 104110. [Google Scholar] [CrossRef]
- Salgado, V.E.; Borba, M.M.; Cavalcante, L.M.; De Moraes, R.R.; Schneider, L.F. Effect of Photoinitiator Combinations on Hardness, Depth of Cure, and Color of Model Resin Composites. J. Esthet. Restor. Dent. 2015, 27, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Felix, C.A. Effect of delivering light in specific narrow bandwidths from 394 to 515nm on the micro-hardness of resin composites. Dent. Mater. 2009, 25, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.G.; de Oliveira, D.; Sinhoreti, M.; Roulet, J.F.; Correr, A.B. The Combination of CQ-amine and TPO Increases the Polymerization Shrinkage Stress and Does Not Improve the Depth of Cure of Bulk-fill Composites. Oper Dent. 2019, 44, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; D’Alpino, P.H.; Lopes, L.G.; Pereira, J.C. Mechanical properties of dental restorative materials: Relative contribution of laboratory tests. J. Appl. Oral. Sci. 2003, 11, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, A.; Sokolowski, J.; Gozdek, T.; Kopacz, K.; Bociong, K. The influence of various photoinitiators on the properties ofcommercial dental composite. Polymers 2021, 13, 3972. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displace-mentsensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- ISO 4049; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019; pp. 1–29.



| PPD Amount [wt. %] | ||||||
|---|---|---|---|---|---|---|
| Distance (µm) | 0.4 | 0.8 | 1 | |||
| Microhardness (MPa) | Reduced Modulus (GPa) | Microhardness (MPa) | Reduced Modulus (GPa) | Microhardness (MPa) | Reduced Modulus (GPa) | |
| 0 | 117.1 ± 10.1 | 2.5 ± 0.08 | 44.5 ± 4.1 | 1.44 ± 0.14 | 481.8 ± 18.1 | 6.27 ± 0.04 |
| 250 | 167.3 ± 14.1 | 2.9 ±0.11 | 234.6 ± 15.1 | 3.46 ± 0.06 | 563.6 ± 18.8 | 6.77 ± 0.07 |
| 500 | 186.3 ± 31.4 | 3.87 ± 0.56 | 247.3 ± 36.6 | 2.5 ± 0.23 | 874.5 ± 29.1 | 11.44 ± 0.12 |
| 750 | 76.4 ± 8.9 | 2.03 ± 0.12 | 203.0 ± 25.4 | 2.42 ± 0.34 | 699.9 ± 21.2 | 8.73 ± 0.09 |
| 1000 | 22.7 ± 2.3 | 0.86 ± 0.02 | 10.1 ± 3.0 | 0.46 ± 0.12 | 406.7 ± 20.0 | 5.77 ± 0.5 |
| BAPO Amount [wt. %] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Distance (µm) | CQ/DMAEMA | 0.25 | 0.5 | 1 | ||||
| Microhardness (MPa) | Reduced Modulus (GPa) | Microhardness (MPa) | Reduced Modulus (GPa) | Microhardness (MPa) | Reduced Modulus (GPa) | Microhardness (MPa) | Reduced Modulus (GPa) | |
| 0 | 67.52 ± 2.48 | 2.15 ± 0.05 | 35.74 ± 5.72 | 1.71 ± 0.14 | 236.93 ± 15.97 | 4.77 ± 0.19 | 573.38 ± 19.33 | 7.18 ±0.18 |
| 450 | 352.5 ± 55.43 | 5.38 ±0.38 | 39.9 ± 8.81 | 1.8 ± 0.13 | 605.37 ± 91.38 | 6.66 ± 0.69 | 615.18 ± 33.66 | 6.16 ± 0.31 |
| 900 | 671.43 ± 89.87 | 5.51 ±0.1 | 72.61 ± 11.07 | 3.31 ± 0.97 | 793.9 ± 62.65 | 8.6 ±0.72 | 674.62 ± 141.5 | 6.29 ± 0.16 |
| 1350 | 753.49 ± 2.86 | 8.34 ± 0.56 | 87.56 ± 8.07 | 2.74 ± 0.09 | 553.93 ± 0.98 | 7.42 ± 1.25 | 533.11 ± 128.7 | 7.76 ± 0.74 |
| 1800 | 12.2 ± 1.48 | 0.72 ± 0.02 | 71.2 ± 14.01 | 2.39 ± 0.19 | 432.59 ± 32.44 | 5.78 ± 0.15 | 482.64 ± 11.92 | 5.76 ± 0.11 |
| Group | Photoinitiator System | Manufacturer | Concentration of Photoinitiator |
|---|---|---|---|
| A. | CQ and DMAEMA | Sigma-Aldrich Inc., St. Louis, MO, USA | 0.4 wt. % and 0.8 wt. % |
| B. | BAPO | 0.25 wt. % | |
| C. | BAPO | 0.5 wt. % | |
| D. | BAPO | 1 wt. % | |
| E. | PPD and DMAEMA | 0.4 wt. % and 0.8 wt. % | |
| F. | PPD and DMAEMA | 0.8 wt. % and 1.6 wt. % | |
| G. | PPD and DMAEMA | 1 wt. % and 2 wt. % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska-Kuczyńska, A.; Sokołowski, J.; Szynkowska-Jóźwik, M.I.; Gozdek, T.; Klajn, K.; Kopacz, K.; Bociong, K. Evaluation of the Selected Mechanical and Aesthetic Properties of Experimental Resin Dental Composites Containing 1-phenyl-1,2 Propanedione or Phenylbis(2,4,6-trimethylbenzoyl)-phosphine Oxide as a Photoinitiator. Int. J. Mol. Sci. 2023, 24, 5573. https://doi.org/10.3390/ijms24065573
Kowalska-Kuczyńska A, Sokołowski J, Szynkowska-Jóźwik MI, Gozdek T, Klajn K, Kopacz K, Bociong K. Evaluation of the Selected Mechanical and Aesthetic Properties of Experimental Resin Dental Composites Containing 1-phenyl-1,2 Propanedione or Phenylbis(2,4,6-trimethylbenzoyl)-phosphine Oxide as a Photoinitiator. International Journal of Molecular Sciences. 2023; 24(6):5573. https://doi.org/10.3390/ijms24065573
Chicago/Turabian StyleKowalska-Kuczyńska, Andrea, Jerzy Sokołowski, Małgorzata Iwona Szynkowska-Jóźwik, Tomasz Gozdek, Katarzyna Klajn, Karolina Kopacz, and Kinga Bociong. 2023. "Evaluation of the Selected Mechanical and Aesthetic Properties of Experimental Resin Dental Composites Containing 1-phenyl-1,2 Propanedione or Phenylbis(2,4,6-trimethylbenzoyl)-phosphine Oxide as a Photoinitiator" International Journal of Molecular Sciences 24, no. 6: 5573. https://doi.org/10.3390/ijms24065573





