Pharmacogenetics of the Primary and Metastatic Osteosarcoma: Gene Expression Profile Associated with Outcome
Abstract
1. Introduction
2. Results
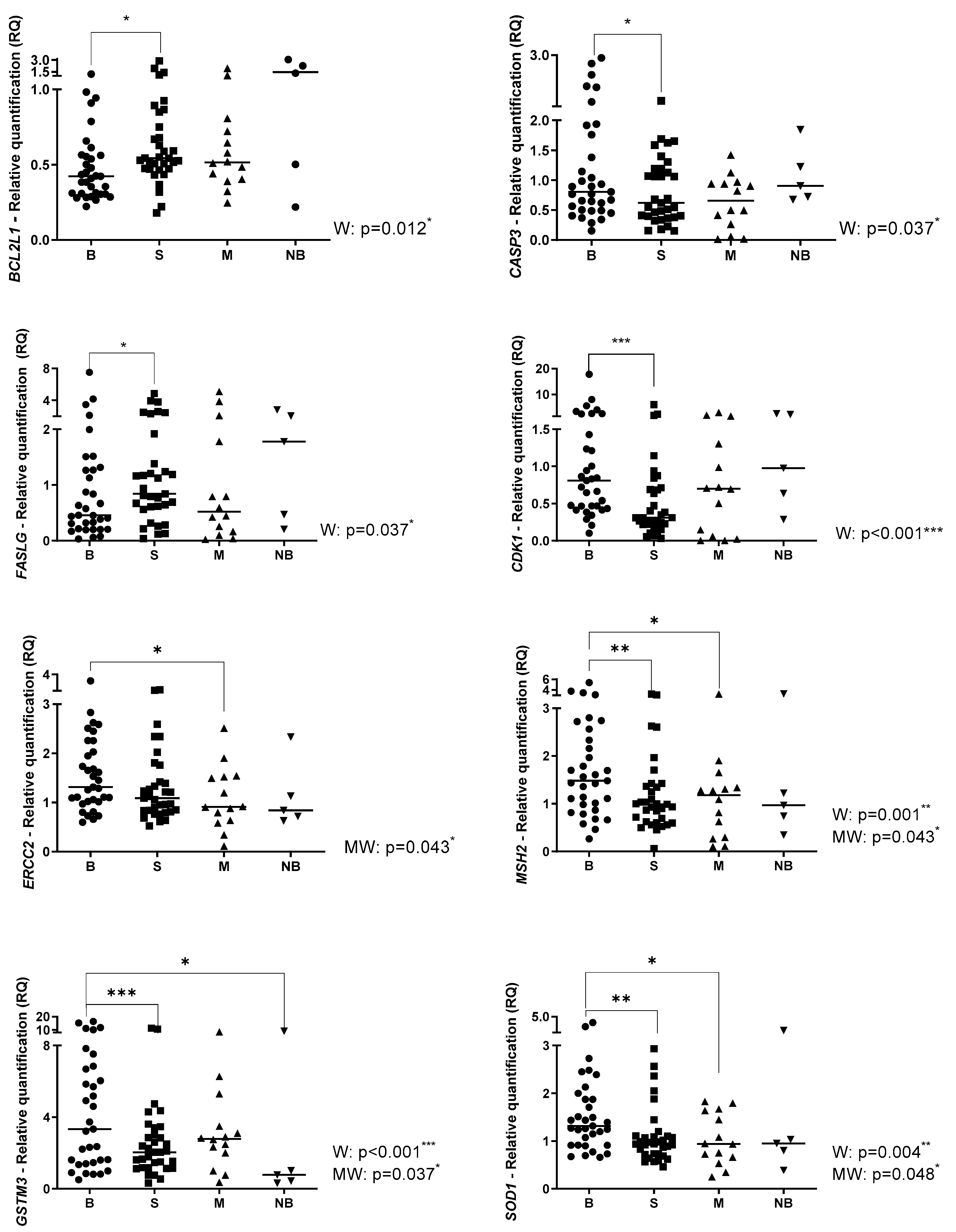
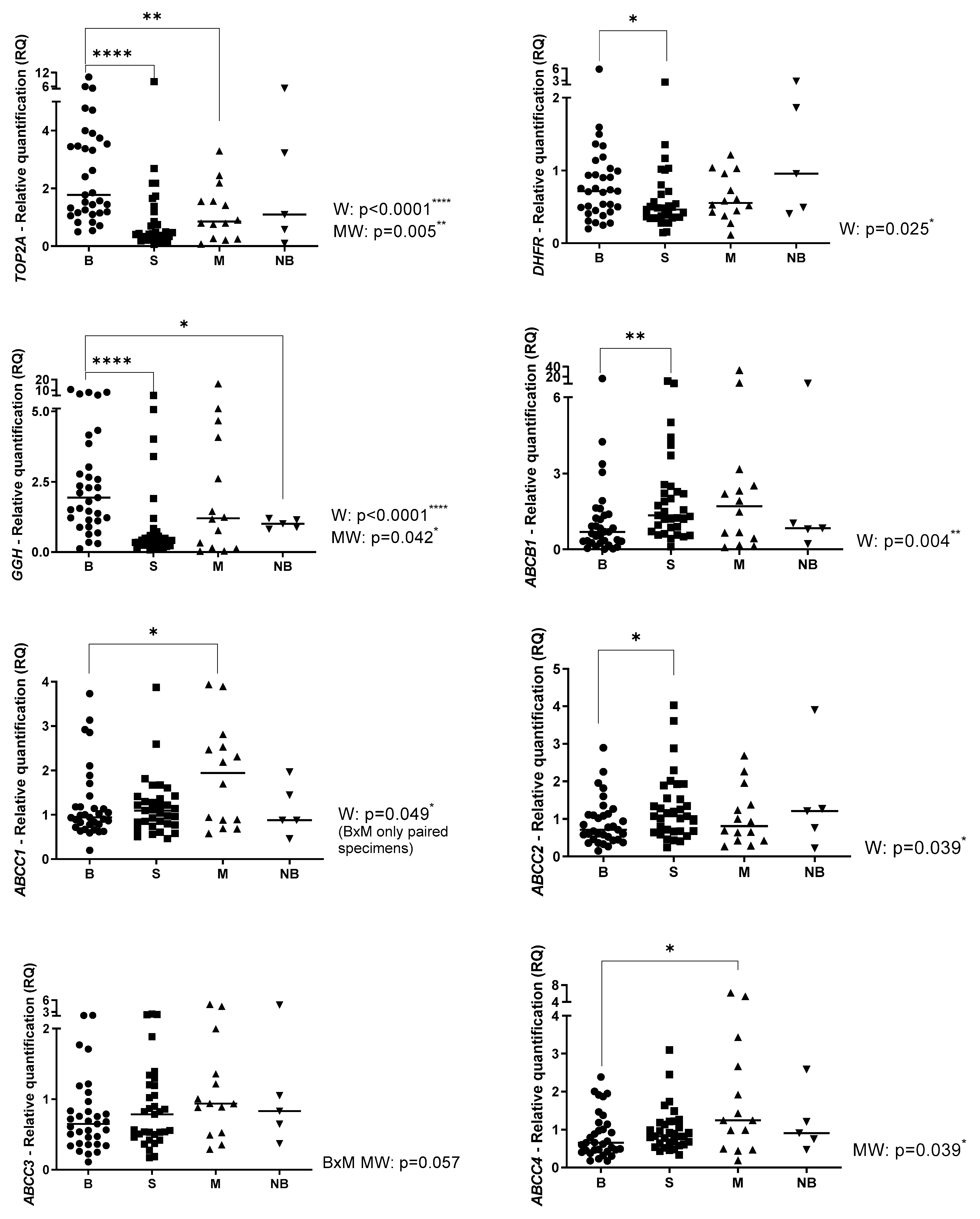
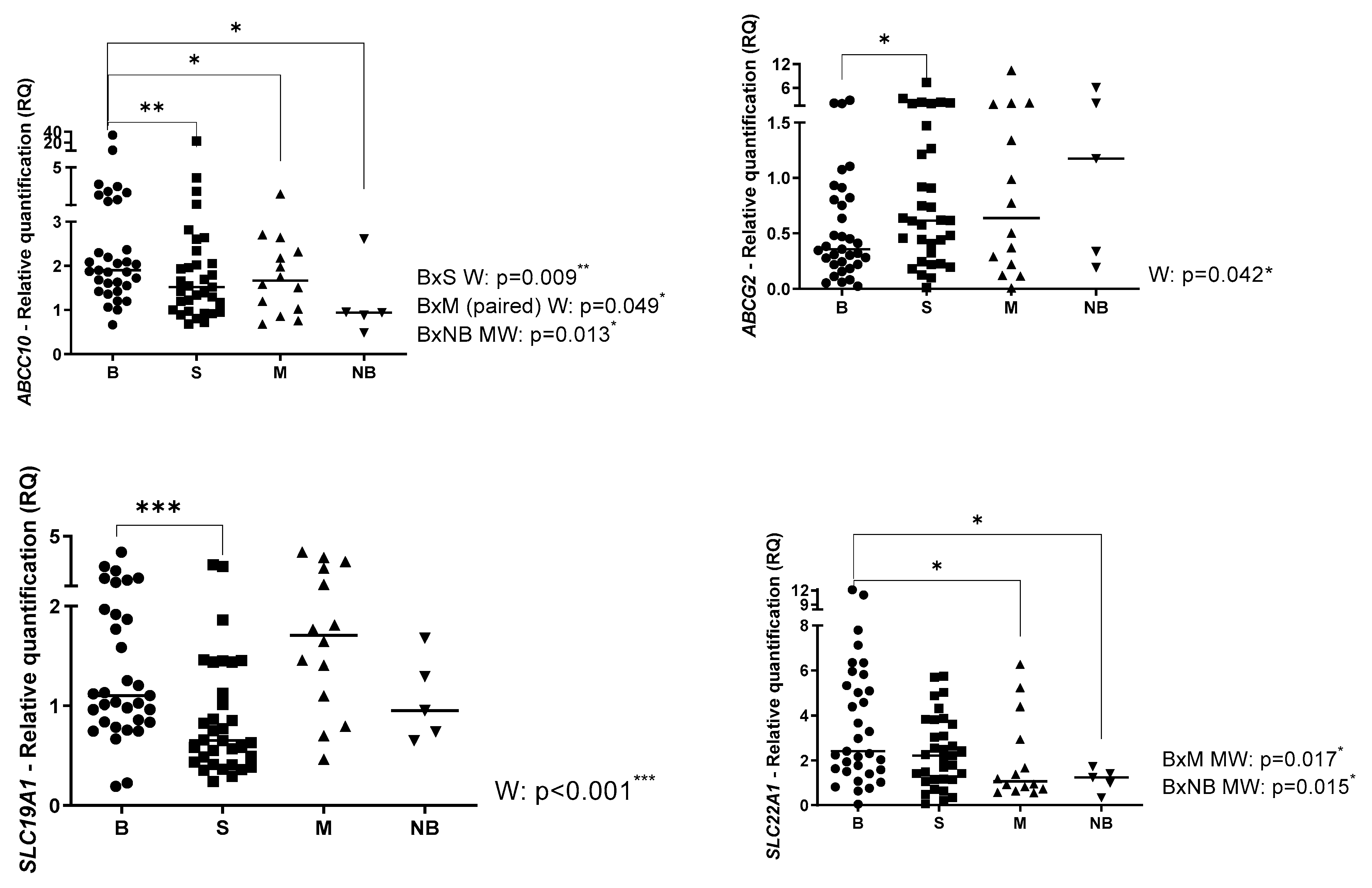
2.1. Gene Expression Profile in Primary OS, Metastatic OS and Normal Bone
2.2. Gene Expression Profile Associated with Clinical Parameters
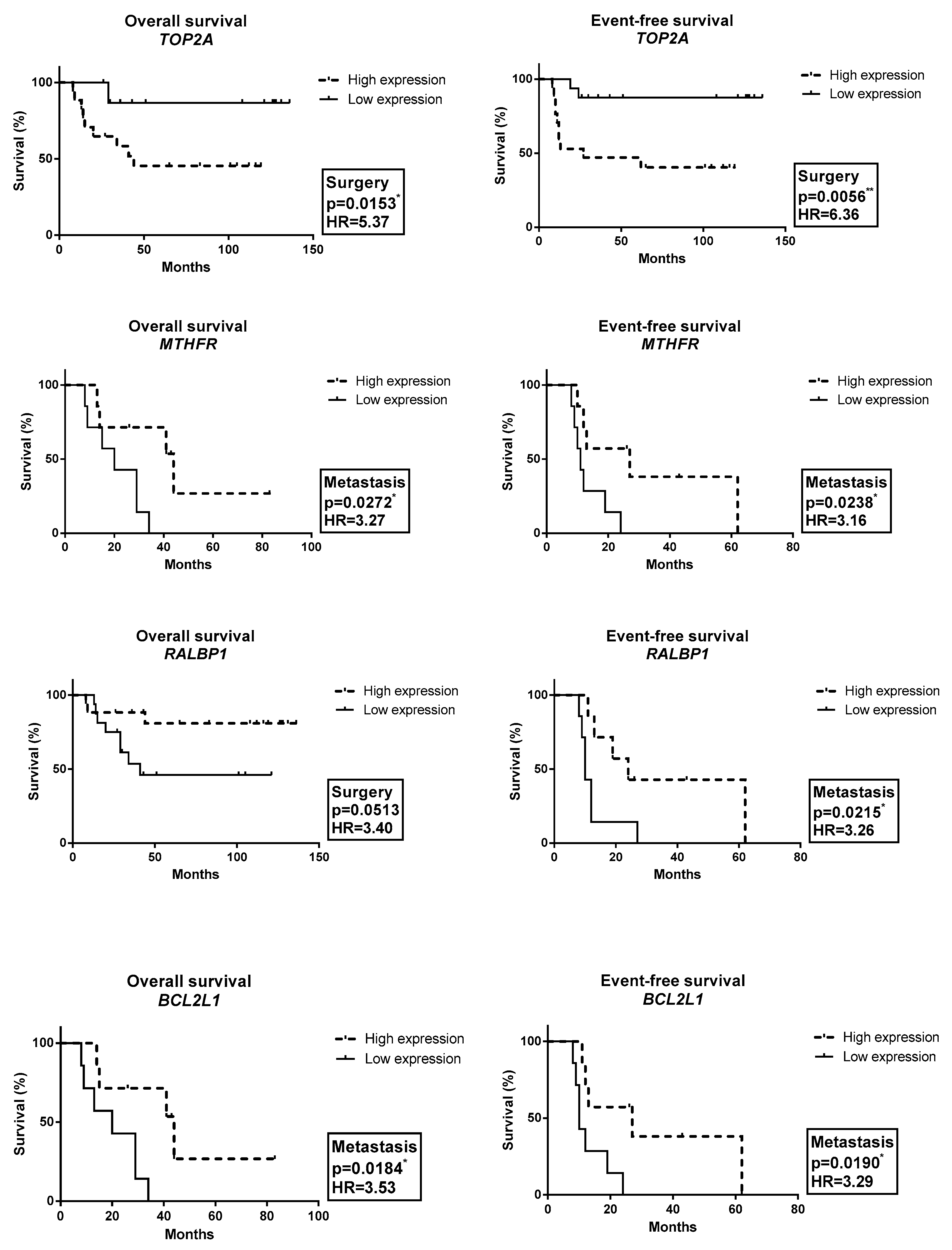
2.3. Gene Expression Profile Associated with OAS and EFS
3. Discussion
4. Materials and Methods
4.1. Patients and Specimens
4.2. Gene Expression (qRT-PCR)
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valery, P.C.; Laversanne, M.; Bray, F. Bone cancer incidence by morphological subtype: A global assessment. Cancer Causes Control 2015, 26, 1127–1139. [Google Scholar] [CrossRef]
- Kager, L.; Tamamyan, G.; Bielack, S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017, 13, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.S.; Bielack, S.S.; Marina, N.; Smeland, S.; Jovic, G.; Hook, J.M.; Krailo, M.; Anninga, J.; Butterfass-Bahloul, T.; Böhling, T.; et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann. Oncol. 2015, 26, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Deenen, M.J.; Cats, A.; Beijnen, J.H.; Schellens, J.H. Part 2: Pharmacogenetic variability in drug transport and phase I anticancer drug metabolism. Oncologist 2011, 16, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Deenen, M.J.; Cats, A.; Beijnen, J.H.; Schellens, J.H. Part 1: Background, methodology, and clinical adoption of pharmacogenetics. Oncologist 2011, 16, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Hattinger, C.M. The pharmacogenomics of osteosarcoma. Pharm. J. 2017, 17, 11–20. [Google Scholar] [CrossRef]
- Vos, H.I.; Coenen, M.J.; Guchelaar, H.J.; Te Loo, D.M. The role of pharmacogenetics in the treatment of osteosarcoma. Drug Discov. Today 2016, 21, 1775–1786. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Biason, P.; Iacoboni, E.; Gagno, S.; Fanelli, M.; Tavanti, E.; Serena, V.; Stefano, F.; Andrea, R.; Rossana, R.; et al. Candidate germline polymorphisms of genes belonging to the pathways of four drugs used in osteosarcoma standard chemotherapy associated with risk, survival and toxicity in non-metastatic high-grade osteosarcoma. Oncotarget 2016, 7, 61970–61987. [Google Scholar] [CrossRef]
- Hagleitner, M.M.; Coenen, M.J.; Gelderblom, H.; Makkinje, R.R.; Vos, H.I.; de Bont, E.S.; van der Graaf, W.T.; Schreuder, H.; Flucke, U.; van Leeuwen, F.; et al. A First Step toward Personalized Medicine in Osteosarcoma: Pharmacogenetics as Predictive Marker of Outcome after Chemotherapy-Based Treatment. Clin. Cancer Res. 2015, 21, 3436–3441. [Google Scholar] [CrossRef]
- Whelan, J.S.; Davis, L.E. Osteosarcoma, chondrosarcoma, and chordoma. J. Clin. Oncol. 2018, 36, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/gene/7153 (accessed on 10 December 2018).
- Ren, L.; Liu, J.; Gou, K.; Xing, C. Copy number variation and high expression of DNA topoisomerase II alpha predict worse prognosis of cancer: A meta-analysis. J. Cancer 2018, 9, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Lasthaus, C.; Guerin, E.; Marcellin, L.; Pencreach, E.; Gaub, M.P.; Guenot, D.; Entz-Werle, N. Role of Topoisomerases in Pediatric High Grade Osteosarcomas: TOP2A Gene Is One of the Unique Molecular Biomarkers of Chemoresponse. Cancers 2013, 5, 662–675. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Tavanti, E.; Fanelli, M.; Vella, S.; Picci, P.; Serra, M. Pharmacogenomics of genes involved in antifolate drug response and toxicity in osteosarcoma. Expert Opin. Drug Metab. Toxicol. 2017, 13, 245–257. [Google Scholar] [CrossRef]
- Hegyi, M.; Arany, A.; Semsei, A.F.; Csordas, K.; Eipel, O.; Gezsi, A.; Kutszegi, N.; Csoka, M.; Muller, J.; Erdelyi, D.; et al. Pharmacogenetic analysis of high-dose methotrexate treatment in children with osteosarcoma. Oncotarget 2017, 8, 9388–9398. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/gene/598 (accessed on 10 December 2018).
- Shkreta, L.; Froehlich, U.; Paquet, E.R.; Toutant, J.; Elela, S.A.; Chabot, B. Anticancer drugs affect the alternative splicing of Bcl-x and other human apoptotic genes. Mol. Cancer Ther. 2008, 7, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, H.; He, M.; Wu, H.; Zhu, Y.; Su, Z. The association of glutathione S-transferase polymorphisms in patients with osteosarcoma: Evidence from a meta-analysis. Eur. J. Cancer Care (Engl.) 2015, 24, 417–424. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Dong, W. GSTM3 A/B polymorphism and risk for head and neck cancer: A meta-analysis. PLoS ONE 2014, 9, e83851. [Google Scholar] [CrossRef]
- Tao, Y.; Ou, Y.; Yin, H.; Chen, Y.; Zhong, S.; Gao, Y.; Zhao, Z.; He, B.; Huang, Q.; Deng, Q. Establishment and characterization of human osteosarcoma cells resistant to pyropheophorbide-α methyl ester-mediated photodynamic therapy. Int. J. Oncol. 2017, 51, 1427–1438. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Michelacci, F.; Sella, F.; Magagnoli, G.; Benini, S.; Gambarotti, M.; Palmerini, E.; Picci, P.; Serra, M.; Ferrari, S. Excision repair cross-complementation group 1 protein expression predicts survival in patients with high-grade, non-metastatic osteosarcoma treated with neoadjuvant chemotherapy. Histopathology 2015, 67, 338–347. [Google Scholar] [CrossRef]
- Chen, X.J.; Tong, Z.C.; Kang, X.; Wang, Z.C.; Huang, G.L.; Yang, T.M.; Dong, L. ERCC polymorphisms and risk of osteosarcoma: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6658–6666. [Google Scholar] [PubMed]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.J.; Gorlick, R. Chemotherapy resistance in osteosarcoma: Current challenges and future directions. Expert Rev. Anticancer. Ther. 2006, 6, 1075–1085. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Pasello, M.; Ferrari, S.; Picci, P.; Serra, M. Emerging drugs for high-grade osteosarcoma. Expert Opin. Emerg. Drugs 2010, 15, 615–634. [Google Scholar] [CrossRef]
- Nakka, M.; Allen-Rhoades, W.; Li, Y.; Kelly, A.J.; Shen, J.; Taylor, A.M.; Barkauskas, D.; Yustein, J.; Andrulis, I.; Wunder, J.; et al. Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma. Oncotarget 2017, 8, 96738–96752. [Google Scholar] [CrossRef]
- Available online: http://www.cbapharma.com/cbt1/cbt1.html (accessed on 10 December 2018).
- Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/mdr-modulator-cbt-1 (accessed on 10 December 2018).
- Fanelli, M.; Hattinger, C.M.; Vella, S.; Tavanti, E.; Michelacci, F.; Gudeman, B.; Barnett, D.; Picci, P.; Serra, M. Targeting ABCB1 and ABCC1 with their Specific Inhibitor CBT-1® can Overcome Drug Resistance in Osteosarcoma. Curr. Cancer Drug Targets 2016, 16, 261–274. [Google Scholar] [CrossRef]

| Clinical Parameter | Gene | Expression | Specimen | p |
|---|---|---|---|---|
| Metastasis at diagnosis * vs. Non-metastasis at diagnosis | ABCB1 | ↑ | M | 0.039 |
| ABCC6 | ↑ | B | 0.048 | |
| ABCC10 | ↑ | B | 0.048 | |
| BCL2L1 | ↑ | S | 0.026 | |
| SLC19A1 | ↑ | S | 0.010 | |
| *** Poor responder * vs. Good responder | ABCC3 | ↓ | S | 0.031 |
| ERCC1 | ↑ | B | 0.021 | |
| FASLG | ↓ | S | 0.017 | |
| SLC22A1 | ↓ | B | 0.014 | |
| TOP2A | ↑ | S | 0.036 | |
| Large tumor (>12 cm) * vs. Small tumor | ABCG2 | ↓ | S | 0.027 |
| CASP3 | ↓ | B | 0.033 | |
| MSH2 | ↓ | B | 0.045 | |
| Amputation * vs. Conservative surgery | ABCC11 | ↑ | M | 0.042 |
| DHFR | ↑ | M | 0.007 | |
| ERCC1 | ↑ | M | 0.042 | |
| FASLG | ↓ | S | <0.0001 | |
| ↓ | M | 0.042 | ||
| GSTM3 | ↑ | S | 0.022 | |
| MTHFR | ↓ | S | 0.003 | |
| SLC19A1 | ↑ | B | 0.002 | |
| ↑ | S | 0.016 | ||
| SLC22A1 | ↓ | B | 0.024 | |
| TOP2A | ↑ | S | 0.010 | |
| Relapse * vs. Non-relapse | ABCC3 | ↓ | M | 0.026 |
| ABCC5 | ↓ | M | 0.051 ** | |
| FASLG | ↓ | S | 0.041 | |
| TOP2A | ↑ | S | 0.038 |
| Clinical Features | N (%) |
|---|---|
| Gender | |
| Male | 64% |
| Female | 36% |
| Age | |
| ≤13 | 42% |
| >13 | 58% |
| Metastasis at diagnosis | |
| Yes | 33 |
| No | 67 |
| Primary site | |
| Femur | 67 |
| Tibia | 24 |
| Humerus | 9 |
| Surgery | |
| Conservative | 85 |
| Amputation | 15 |
| Histology | |
| Mixed | 24 |
| Osteoblastic | 55 |
| Condroblastic | 9 |
| Fibroblastic | 3 |
| Telangiectatic | 3 |
| Not identified | 6 |
| Tumor size | |
| <12 cm | 55 |
| ≥12 cm | 42 |
| Not identified | 3 |
| Grade of tumor necrosis | |
| ≤90% | 49 |
| >90% | 45 |
| Not identified | 6 |
| Pulmonary metastasis | |
| Yes | 42 |
| No | 57 |
| Relapse | |
| Yes | 33 |
| No | 67 |
| Death | |
| Yes | 33 |
| No | 67 |
| Function | Gene | Assay |
|---|---|---|
| Apoptosis | BCL2L1 | Hs00236329_m1 |
| CASP3 | Hs00234387_m1 | |
| FASL | Hs00181226_g1 | |
| Cell cycle | CDK1 | Hs00938777_m1 |
| Damage recognition | HMGB1 | Hs01923466_g1 |
| DNA repair | ERCC1 | Hs01012158_m1 |
| ERCC2 | Hs00361161_m1 | |
| MSH2 | Hs00953527_m1 | |
| Detoxification | GSTM1 | Hs01683722_gH |
| GSTM3 | Hs00356079_m1 | |
| GSTP1 | Hs00943350_g1 | |
| GSTT1 | Hs02512069_s1 | |
| SOD1 | Hs00533490_m1 | |
| Doxorubicin pathway | TOP2A | Hs01032137_m1 |
| Endogenous | ACTB | Hs01060665_g1 |
| GAPDH | Hs02758991_g1 | |
| Folate pathway | DHFR | Hs00758822_s1 |
| GGH | Hs00914163_m1 | |
| MTHFR | Hs01114487_m1 | |
| Transport | ABCB1 | Hs00184500_m1 |
| ABCC1 | Hs01561502_m1 | |
| ABCC2 | Hs00166123_m1 | |
| ABCC3 | Hs00978452_m1 | |
| ABCC4 | Hs00988717_m1 | |
| ABCC5 | Hs00981089_m1 | |
| ABCC6 | Hs00184566_m1 | |
| ABCC10 | Hs01056200_m1 | |
| ABCC11 | Hs01090758_m1 | |
| ABCG2 | Hs01053790_m1 | |
| ATP7B | Hs00163739_m1 | |
| RALBP1 | Hs01034984_g1 | |
| SLC19A1 | Hs00953344_m1 | |
| SLC22A1 | Hs00427552_m1 | |
| SLC31A1 | Hs00977266_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo-Paolillo, A.; Tesser-Gamba, F.; Seixas Alves, M.T.; Filho, R.J.G.; Oliveira, R.; Petrilli, A.S.; Toledo, S.R.C. Pharmacogenetics of the Primary and Metastatic Osteosarcoma: Gene Expression Profile Associated with Outcome. Int. J. Mol. Sci. 2023, 24, 5607. https://doi.org/10.3390/ijms24065607
Trujillo-Paolillo A, Tesser-Gamba F, Seixas Alves MT, Filho RJG, Oliveira R, Petrilli AS, Toledo SRC. Pharmacogenetics of the Primary and Metastatic Osteosarcoma: Gene Expression Profile Associated with Outcome. International Journal of Molecular Sciences. 2023; 24(6):5607. https://doi.org/10.3390/ijms24065607
Chicago/Turabian StyleTrujillo-Paolillo, Alini, Francine Tesser-Gamba, Maria Teresa Seixas Alves, Reynaldo Jesus Garcia Filho, Renato Oliveira, Antonio Sergio Petrilli, and Silvia Regina Caminada Toledo. 2023. "Pharmacogenetics of the Primary and Metastatic Osteosarcoma: Gene Expression Profile Associated with Outcome" International Journal of Molecular Sciences 24, no. 6: 5607. https://doi.org/10.3390/ijms24065607
APA StyleTrujillo-Paolillo, A., Tesser-Gamba, F., Seixas Alves, M. T., Filho, R. J. G., Oliveira, R., Petrilli, A. S., & Toledo, S. R. C. (2023). Pharmacogenetics of the Primary and Metastatic Osteosarcoma: Gene Expression Profile Associated with Outcome. International Journal of Molecular Sciences, 24(6), 5607. https://doi.org/10.3390/ijms24065607





