Hippocampal Noradrenaline Is a Positive Regulator of Spatial Working Memory and Neurogenesis in the Rat
Abstract
:1. Introduction
2. Results
2.1. General Observations
2.2. Behavioural Analyses
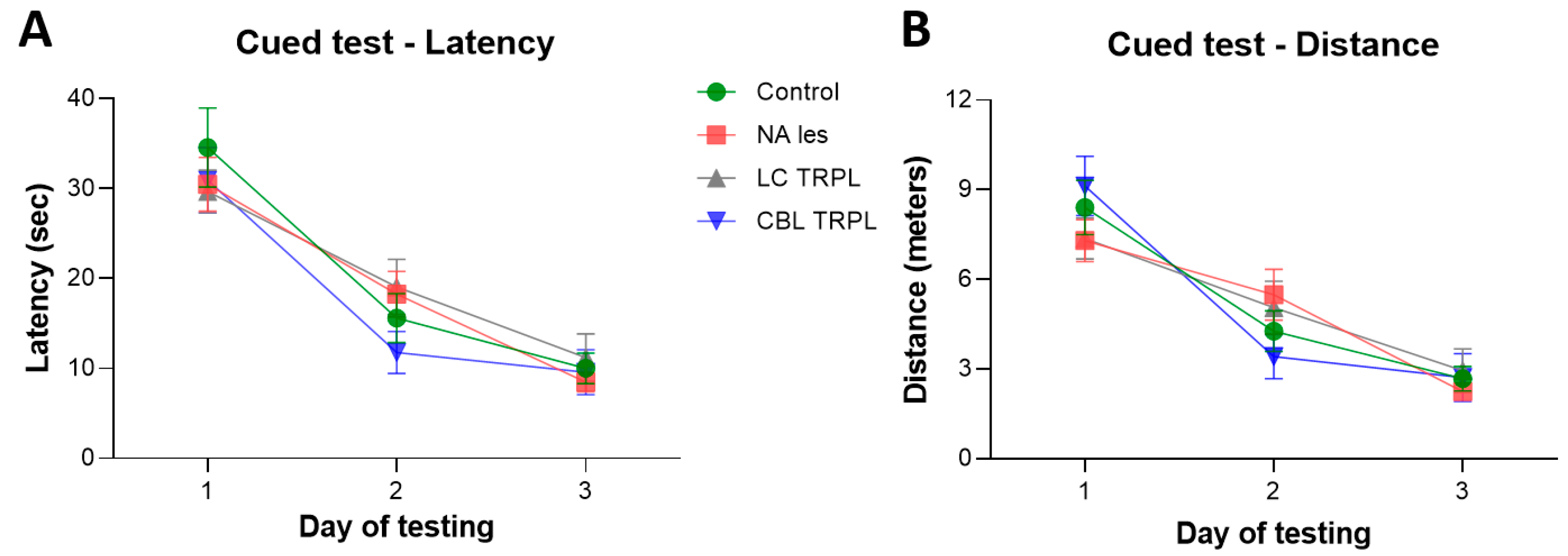
2.2.1. Morris Water Maze
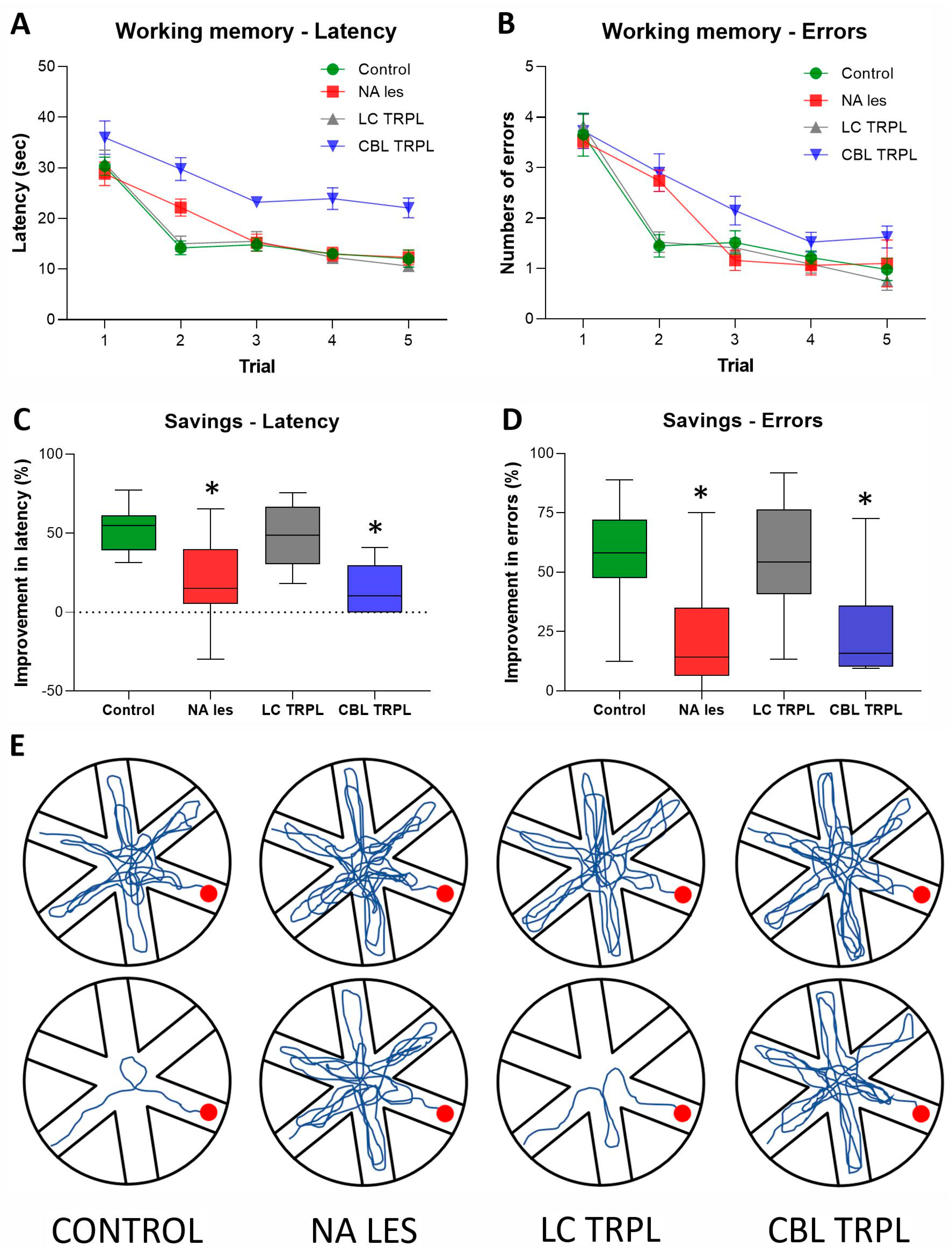
2.2.2. Radial Arm Water Maze
2.3. Morphological Analyses
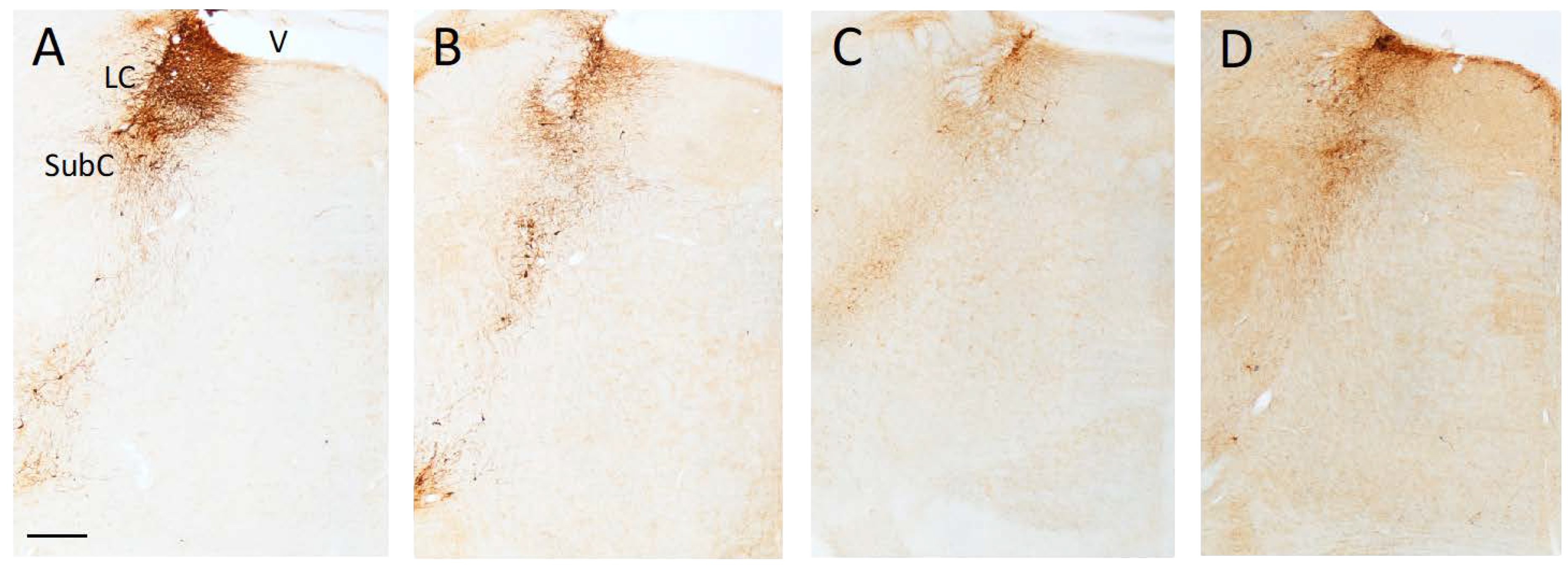
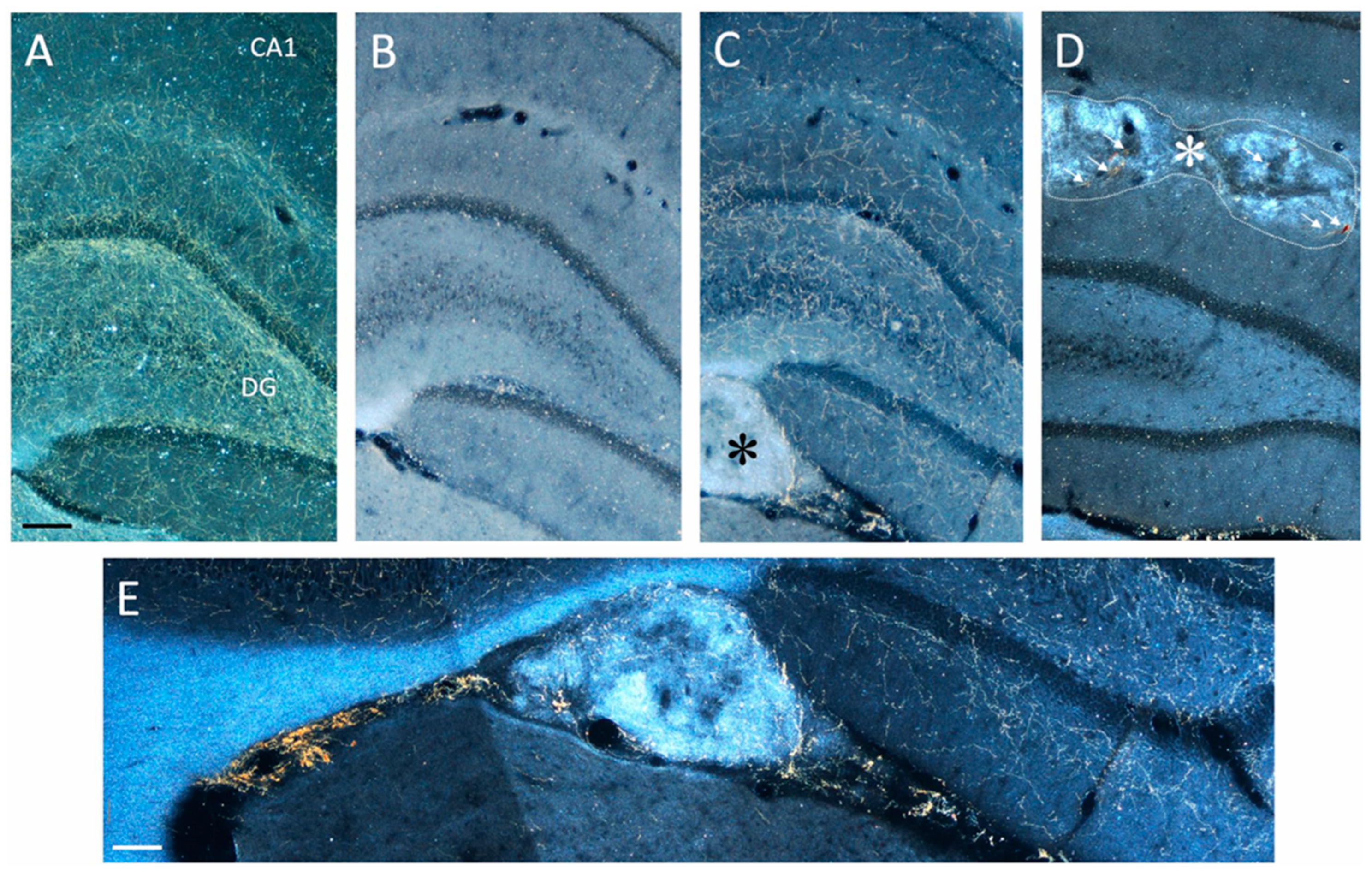
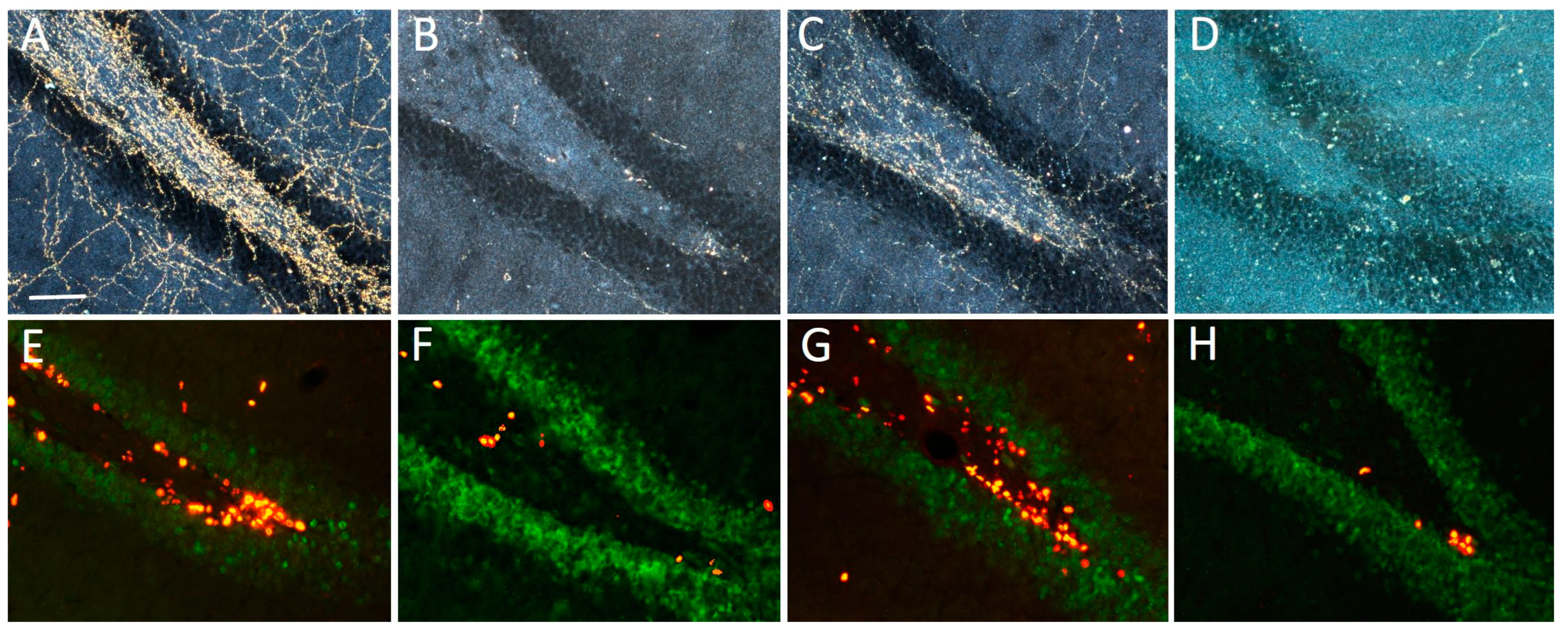
2.3.1. Lesion and Transplant Effects on DBH-Positive Neurons and Fibers
2.3.2. Effects on BrdU-Positive Progenitors in SGZ
3. Discussion
3.1. Effects of Lesion
3.2. Effects of Transplants
4. Materials and Methods
4.1. Subjects and Experimental Design
4.2. Lesion and Transplantation Surgery
4.3. Behavioural Tests
4.4. Morris Water Maze
4.5. Injections of 5-Bromo-2′Deoxyuridine and Post-Mortem Procedures
4.6. Microscopic Analysis and Quantitative Evaluation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaral, D.G.; Sinnamon, H.M. The locus coeruleus: Neurobiology of a central noradrenergic nucleus. Progr. Neurobiol. 1977, 9, 147–196. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. Locus coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 2015, 35, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Marien, M.R.; Colpaert, F.C.; Rosenquist, A.C. Noradrenergic mechanisms in neurodegenerative diseases: A theory. Brain Res. Rev. 2004, 45, 38–78. [Google Scholar] [CrossRef]
- Gannon, M.; Che, P.; Chen, Y.; Jiao, K.; Roberson, E.D.; Wang, Q. Noradrenergic dysfunction in Alzheimer’s disease. Front. Neurosci. 2015, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Slater, C.; Wang, Q. Alzheimer’s disease: An evolving understanding of noradrenergic involvement and the promising future of electroceutical therapies. Clin. Transl. Med. 2021, 11, e397. [Google Scholar] [CrossRef]
- Leanza, G.; Gulino, R.; Zorec, R. Noradrenergic hypothesis linking neurodegeneration-based cognitive decline and astroglia. Front. Mol. Neurosci. 2018, 11, 254. [Google Scholar] [CrossRef] [Green Version]
- German, D.C.; Manaye, K.F.; White, C.L.; Woodward, D.J.; McIntire, D.D.; Smith, W.K.; Kalaria, R.N.; Mann, D.M. Disease-specific patterns of locus coeruleus cell loss. Ann. Neurol. 1992, 32, 667–676. [Google Scholar] [CrossRef]
- Matthews, K.L.; Chen, C.P.L.H.; Esiri, M.M.; Keene, J.; Minger, S.L.; Francis, P.T. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol. Psychiatry 2002, 51, 407–416. [Google Scholar] [CrossRef]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003, 60, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Haglund, M.; Sjöbeck, M.; Englund, E. Locus ceruleus degeneration is ubiquitous in Alzheimer’s disease: Possible implications for diagnosis and treatment. Neuropathology 2006, 26, 528–532. [Google Scholar] [CrossRef]
- Grudzien, A.; Shaw, P.; Weintraub, S.; Bigio, E.; Mash, D.C.; Mesulam, M.M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging 2007, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr. Opin. Neurol. 2012, 25, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Stratman, K.; Heinsen, H.; Korf, H.-W.; Del Turco, D.; Ghebremedhin, E.; Seidel, K.; Bouzrou, M.; Grinberg, L.T.; Bohl, J.; Wharton, S.B.; et al. Precortical phase of Alzheimer’s disease (AD)-related Tau cytoskeletal pathology. Brain Pathol. 2016, 26, 371–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno- Jiménez, E.P.; Flor- García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouki, A.; Aashutosh, S.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 2019, 24, 974–982. [Google Scholar] [CrossRef]
- Choi, S.H.; Tanzi, R.E. Is Alzheimer’s disease a neurogenesis disorder? Cell Stem Cell 2019, 25, 7–9. [Google Scholar] [CrossRef]
- Kulkarni, V.A.; Jha, S.; Vaidya, V.A. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur. J. Neurosci. 2002, 16, 2008–2012. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, D.J.; Mackay, E.W.; Hamlin, A.S.; Marathe, S.V.; Nandam, L.S.; Vaidya, V.A.; Bartlett, P.F. Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. J. Neurosci. 2010, 30, 2795–2806. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, D.J.; Nanavaty, I.; Prosper, B.W.; Marathe, S.; Husain, B.F.A.; Kernie, S.G.; Bartlett, P.F.; Vaidya, V.A. Opposing effects of alpha2- and beta-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis. PLoS ONE 2014, 9, e98736. [Google Scholar] [CrossRef]
- Coradazzi, M.; Gulino, R.; Fieramosca, F.; Verga Falzacappa, L.; Riggi, M.; Leanza, G. Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol. Aging 2016, 48, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Weselek, G.; Keiner, S.; Fauser, M.; Wagenführ, L.; Müller, J.; Kaltschmidt, B.; Brandt, M.D.; Gerlach, M.; Redecker, C.; Hermann, A.; et al. Norepinephrine is a negative regulator of the adult periventricular neural stem cell niche. Stem Cells 2020, 38, 1188–1201. [Google Scholar] [CrossRef]
- Gulino, R.; Kostenko, A.; de Leo, G.; Emmi, S.A.; Nunziata, D.; Leanza, G. Hippocampal noradrenaline regulates spatial working memory in the rat. In Noradrenaline Signaling and Astroglia, 1st ed.; Vardjan, N., Zorec, R., Eds.; Academic Press: Oxford, UK, 2017; pp. 201–220. [Google Scholar] [CrossRef]
- Pintus, R.; Riggi, M.; Cannarozzo, C.; Valeri, A.; de Leo, G.; Romano, M.; Gulino, R.; Leanza, G. Essential role of hippocampal noradrenaline in the regulation of spatial working memory and TDP-43 tissue pathology. J. Comp. Neurol. 2018, 526, 1131–1147. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, S.E.; Foote, S.L.; Grzanna, R. Efferent projections of nucleus locus coeruleus: Morphologic subpopulations have different efferent targets. Neuroscience 1986, 18, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Leslie, F.M.; Loughlin, S.E.; Sternberg, D.B.; McGaugh, J.L.; Young, L.E.; Zornetzer, S.F. Noradrenergic changes and memory loss in aged mice. Brain Res. 1985, 359, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.L.; Stone, W.S.; Ingram, D.K.; Reynolds, J.; Gold, P.E.; Conti, L.H.; Pontecorvo, M.J.; Wenk, G.L.; Olton, D.S. Individual differences in aging: Behavioral and neurobiological correlates. Neurobiol. Aging 1989, 10, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Khakpour-Taleghani, B.; Lashgari, R.; Motamedi, F.; Naghdi, N. Effect of reversible inactivation of locus ceruleus on spatial reference and working memory. Neuroscience 2009, 158, 1284–1291. [Google Scholar] [CrossRef]
- Mair, R.D.; Zhang, Y.; Bailey, K.R.; Toupin, M.M.; Mair, R.G. Effects of clonidine in the locus coeruleus on prefrontal- and hippocampal-dependent measures of attention and memory in the rat. Psychopharmacology 2005, 181, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sontag, T.A.; Hauser, J.; Kaunzinger, I.; Gerlach, M.; Tucha, O.; Lange, K.W. Effects of the noradrenergic neurotoxin DSP4 on spatial memory in the rat. J. Neural Transm. 2008, 115, 299–303. [Google Scholar] [CrossRef]
- Wenk, G.; Hughey, D.; Boundy, V.; Kim, A.; Walker, L.; Olton, D. Neurotransmitters and memory: Role of cholinergic, serotonergic, and noradrenergic systems. Behav. Neurosci. 1987, 101, 325–332. [Google Scholar] [CrossRef]
- Ohno, M.; Yamamoto, T.; Kobayashi, M.; Watanabe, S. Impairment of working memory induced by scopolamine in rats with noradrenergic DSP-4 lesions. Eur. J. Pharmacol. 1993, 238, 117–120. [Google Scholar] [CrossRef]
- Compton, D.M.; Dietrich, K.L.; Smith, J.S.; Davis, B.K. Spatial and non-spatial learning in the rat following lesions to the nucleus locus coeruleus. Neuroreport 1995, 7, 177–182. [Google Scholar] [CrossRef]
- Steckler, T.; Keith, A.B.; Wiley, R.G.; Sahgal, A. Cholinergic lesions by 192 IgG-saporin and short-term recognition memory: Role of the septohippocampal projection. Neuroscience 1995, 66, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.W.; McGaugh, J.L. Effects of concurrent manipulations of cholinergic and noradrenergic function on learning and retention in mice. Brain Res. 1989, 477, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.W.; Gallagher, M. Scopolamine-disruption of radial arm maze performance: Modification by noradrenergic depletion. Brain Res. 1987, 417, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Kobayashi, M.; Kishi, A.; Watanabe, S. Working memory failure by combined blockade of muscarinic and beta-adrenergic transmission in the rat hippocampus. Neuroreport 1997, 8, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Yoshimatsu, A.; Kobayashi, M.; Watanabe, S. Noradrenergic DSP-4 lesions aggravate impairment of working memory produced by hippocampal muscarinic blockade in rats. Pharmacol. Biochem. Behav. 1997, 57, 257–261. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Ikonomovic, M.D.; Styren, S.D.; Beckett, L.; Wisniewski, S.; Bennett, D.A.; Cochran, E.J.; Kordower, J.H.; Mufson, E.J. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. 2002, 51, 145–155. [Google Scholar] [CrossRef]
- Jackisch, R.; Gansser, S.; Cassel, J.C. Noradrenergic denervation facilitates the release of acetylcholine and serotonin in the hippocampus: Towards a mechanism underlying upregulations described in MCI patients? Exp. Neurol. 2008, 213, 345–353. [Google Scholar] [CrossRef]
- Vizi, E.S. Modulation of cortical release of acetylcholine by noradrenaline released from nerves arising from the rat locus coeruleus. Neuroscience 1980, 5, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- de Leo, G.; Gulino, R.; Coradazzi, M.; Leanza, G. Acetylcholine and noradrenaline differentially regulate hippocampus-dependent spatial learning and memory. Brain Commun. 2022, 5, fcac338. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Segal, M.; Stenevi, U. Functional reinnervation of rat hippocampus by locus coeruleus implants. Brain Res. 1979, 170, 409–426. [Google Scholar] [CrossRef]
- Björklund, A.; Nornes, H.; Gage, F.H. Cell suspension grafts of noradrenergic locus coeruleus neurons in rat hippocampus and spinal cord: Reinnervation and transmitter turnover. Neuroscience 1986, 18, 685–698. [Google Scholar] [CrossRef]
- Kalén, P.; Cenci, M.A.; Lindvall, O.; Björklund, A. Host brain regulation of fetal locus coeruleus neurons grafted to the hippocampus in 6-hydroxydopamine-treated rats. An intracerebral microdialysis study. Eur. J. Neurosci. 1991, 3, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Leanza, G.; Martìnez-Serrano, A.; Björklund, A. Amelioration of spatial navigation and short-term memory deficits by grafts of foetal basal forebrain tissue placed into the hippocampus and cortex of rats with selective cholinergic lesions. Eur. J. Neurosci. 1998, 10, 2353–2370. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef] [Green Version]
- Yanpallewar, S.U.; Fernandes, K.; Marathe, S.V.; Vadodaria, K.C.; Jhaveri, D.; Rommelfanger, K.; Ladiwala, U.; Jha, S.; Muthig, V.; Hein, L.; et al. Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J. Neurosci. 2010, 30, 1096–1109. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Nakagawa, S.; Boku, S.; Nishikawa, H.; Takamura, N.; Kato, A.; Inoue, T.; Koyama, T. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through beta2 receptor. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 44–51. [Google Scholar] [CrossRef]
- Brezun, J.M.; Daszuta, A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 1999, 89, 999–1002. [Google Scholar] [CrossRef]
- Alenina, N.; Klempin, F. The role of serotonin in adult hippocampal neurogenesis. Behav. Brain Res. 2015, 277, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezun, J.M.; Daszuta, A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur. J. Neurosci. 2000, 12, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Aztiria, E.; Capodieci, G.; Arancio, L.; Leanza, G. Extensive training in a maze task reduces neurogenesis in the adult rat dentate gyrus probably as a result of stress. Neurosci. Lett. 2007, 416, 133–137. [Google Scholar] [CrossRef]
- Cooper-Kuhn, C.M.; Winkler, J.; Kuhn, H.G. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J. Neurosci. Res. 2004, 77, 155–165. [Google Scholar] [CrossRef]
- Mohapel, P.; Leanza, G.; Kokaia, M.; Lindvall, O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 2005, 26, 939–946. [Google Scholar] [CrossRef]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef] [Green Version]
- Leanza, G.; Cataudella, T.; Dimauro, R.; Monaco, S.; Stanzani, S. Release properties and functional integration of noradrenergic-rich tissue grafted to the denervated spinal cord of the adult rat. Eur. J. Neurosci. 1999, 11, 1789–1799. [Google Scholar] [CrossRef]
- Coradazzi, M.; Gulino, R.; Garozzo, S.; Leanza, G. Selective lesion of the developing central noradrenergic system: Short- and long-term effects and reinnervation by noradrenergic-rich tissue grafts. J. Neurochem. 2010, 114, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Stenevi, U.; Schmidt, R.H.; Dunnett, S.B.; Gage, F.H. Intracerebral grafting of neuronal cell suspensions. I. Introduction and general methods of preparation. Acta Physiol. Scand. Suppl. 1983, 522, 1–7. [Google Scholar]
- Antonini, V.; Prezzavento, O.; Coradazzi, M.; Marrazzo, A.; Ronsisvalle, S.; Arena, E.; Leanza, G. Anti-amnesic properties of (±)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats. J. Neurochem. 2009, 109, 744–754. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Garrud, P.; Rawlins, J.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.M.; Park, C.R.; Heman, K.L.; Rose, G.M. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 1999, 9, 542–552. [Google Scholar] [CrossRef]
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef]
- Grzanna, R.; Molliver, M.E. The locus coeruleus in the rat: An immunohistochemical delineation. Neuroscience 1980, 5, 21–40. [Google Scholar] [CrossRef]
- Rasband, W.S.; Bright, D.S. NIH Image: A public domain image processing program for Macintosh. Microbeam Anal. Soc. J. 1995, 4, 137–149. [Google Scholar]

| Group | Equilibrium Time on Ramp (%) | Latency to Cross Ramp (s) | Latency to Reverse on Grids (s) | Number of Falls in Grids |
|---|---|---|---|---|
| Control (n = 12) | 96.9 ± 0.7 | 6.9 ± 0.4 | 6.2 ± 0.7 | 2.4 ± 0.5 |
| Lesioned (n = 16) | 97.8 ± 0.6 | 6.9 ± 0.3 | 6.6 ± 0.7 | 2.5 ± 0.5 |
| LC Transplant (n = 16) | 98.2 ± 0.5 | 7.0 ± 0.3 | 6.3 ± 0.5 | 2.4 ± 0.5 |
| CBL Transplant (n = 8) | 96.3 ± 1.3 | 7.1 ± 0.5 | 7.0 ± 0.9 | 2.4 ± 0.7 |
| Group | DBH-ir Neurons in LC/SubC | DBH-ir Fibers in CA1 | DBH-ir Fibers in CA3 | DBH-ir Fibers in DG |
|---|---|---|---|---|
| Control (n = 12) | 1782.4 ± 44.2 | 62.0 ± 3.6 | 61.6 ± 1.4 | 64.3 ± 2.5 |
| Lesioned (n = 16) | 395.6 ± 36.1 * | 18.9 ± 1.1 * | 21.0 ± 1.3 * | 20.3 ± 0.8 * |
| LC Transplant (n = 16) | 458.6 ± 48.8 * | 55.4 ± 1.8 | 57.3 ± 2.8 | 59.3 ± 3.5 |
| CBL Transplant (n = 8) | 451.7 ± 23.6 * | 23.6 ± 0.4 * | 21.4 ± 0.8 * | 21.5 ± 1.2 * |
| Group | BrdU-ir Cells in SGZ | BrdU-ir Cells in Hilus |
|---|---|---|
| Control (n = 12) | 8480.5 ± 607.9 | 2675.7 ± 741.0 |
| Lesioned (n = 16) | 3242.5 ± 310.6 * | 2018.1 ± 243.9 |
| LC Transplant (n = 16) | 9047.3 ± 575.3 | 2358.5 ± 481.2 |
| CBL Transplant (n = 8) | 3018.1 ± 233.8 * | 2838.6 ± 289.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulino, R.; Nunziata, D.; de Leo, G.; Kostenko, A.; Emmi, S.A.; Leanza, G. Hippocampal Noradrenaline Is a Positive Regulator of Spatial Working Memory and Neurogenesis in the Rat. Int. J. Mol. Sci. 2023, 24, 5613. https://doi.org/10.3390/ijms24065613
Gulino R, Nunziata D, de Leo G, Kostenko A, Emmi SA, Leanza G. Hippocampal Noradrenaline Is a Positive Regulator of Spatial Working Memory and Neurogenesis in the Rat. International Journal of Molecular Sciences. 2023; 24(6):5613. https://doi.org/10.3390/ijms24065613
Chicago/Turabian StyleGulino, Rosario, Domenico Nunziata, Gioacchino de Leo, Anna Kostenko, Serena Alexa Emmi, and Giampiero Leanza. 2023. "Hippocampal Noradrenaline Is a Positive Regulator of Spatial Working Memory and Neurogenesis in the Rat" International Journal of Molecular Sciences 24, no. 6: 5613. https://doi.org/10.3390/ijms24065613





