B. abortus Infection Promotes an Imbalance in the Adipocyte–Osteoblast Crosstalk Favoring Bone Resorption
Abstract
:1. Introduction
2. Results
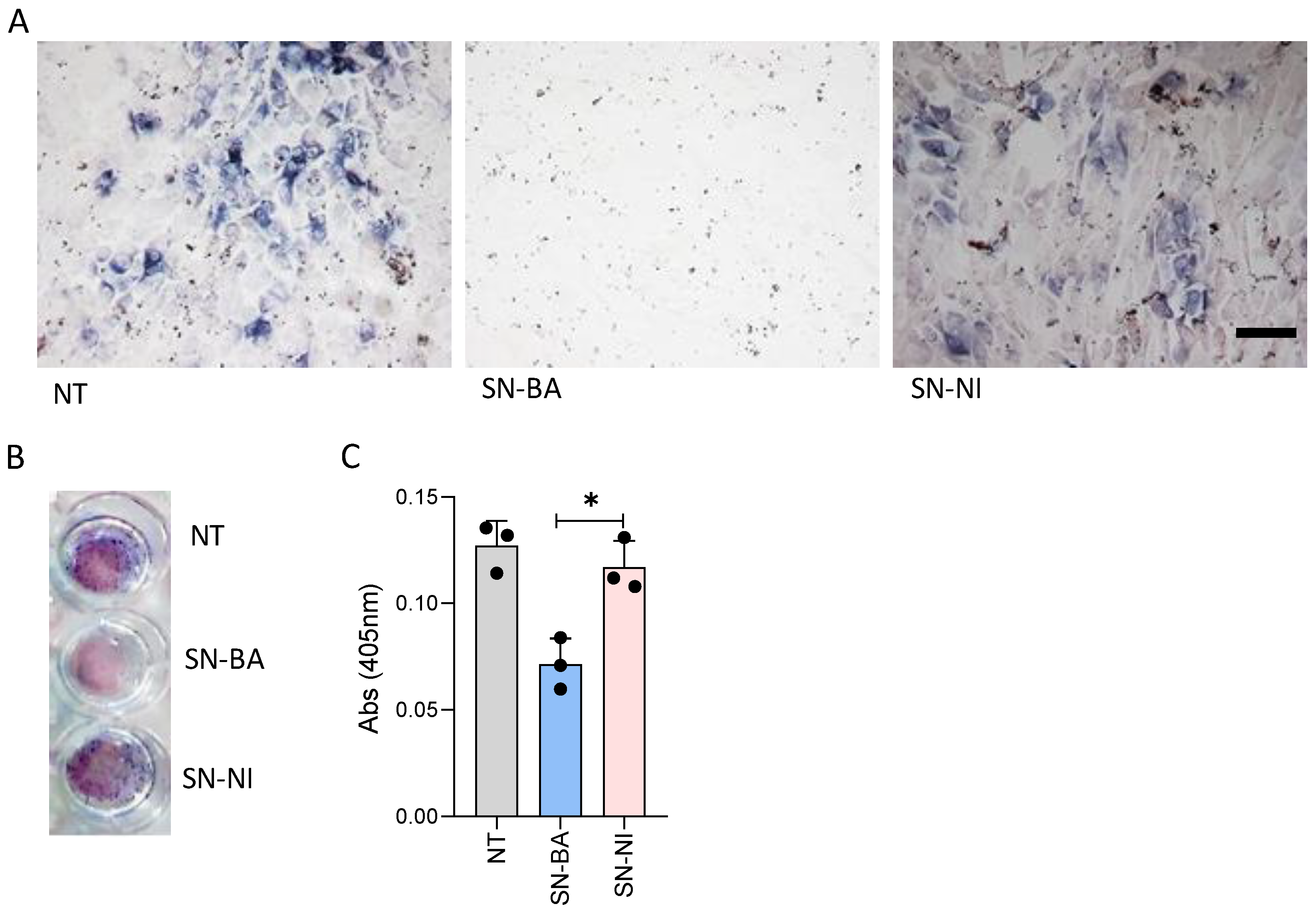
2.1. Culture Supernatants from B. abortus-Infected Adipocytes Inhibit Osteoblast Differentiation
2.2. Culture Supernatants from B. abortus-Infected Adipocytes Inhibit Osteoblast Differentiation Largely Due to IL-6
2.3. Culture Supernatants from B. abortus-Infected Adipocytes Inhibit the Osteoblast-Mediated Matrix Mineralization
2.4. Culture Supernatants from B. abortus-Infected Adipocytes Do Not Modulate Collagen Deposition by Osteoblast
2.5. Culture Supernatants from B. abortus-Infected Adipocytes Induce RANKL Expression in Osteoblasts
2.6. Supernatants from B. abortus-Infected Adipocytes Modulate Runx2 Transcription in Osteoblasts
2.7. B. abortus-Infected Osteoblasts Modulate Adipocyte Differentiation
2.8. B. abortus-Infected Osteoblasts Modulate the Transcription of Essential Pro-Adipogenic Factors
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture
4.2. Cell Culture
4.3. B. abortus Infection of Adipocytes and Osteoblasts
4.4. Stimulation with Conditioned Medium
4.5. Measurement of RANKL Expression
4.6. Measurement of IL-6 Concentration
4.7. Blocking of IL-6
4.8. Alizarin Red S Staining
4.9. Alkaline Phosphatase Staining
4.10. Assessment of Collagen Deposition by Sirius Red Staining
4.11. Assessment of Collagen Deposition by Immunofluorescence
4.12. Assessment of Adipocyte Differentiation Measuring Lipid Droplet Accumulation
4.13. mRNA Extraction and Quantitative Real-Time PCR
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pappas, G.; Akritidis, N.; Bosilkovski, M.; Tsianos, E. Brucellosis. N. Engl. J. Med. 2005, 352, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.; Sharif, H.; Abed, M.; Al-Fayez, M. Osteoarticular brucellosis: Results of bone scintigraphy in 140 patients. Am. J. Roentgenol. 1988, 150, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, M.I.; Araj, G.F.; A Majeed, S.; Lulu, A.R. Brucella arthritis: A study of 96 cases in Kuwait. Ann. Rheum. Dis. 1990, 49, 994–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giambartolomei, G.H.; Benitez, P.C.A.; Delpino, M.V. Brucella and Osteoarticular Cell Activation: Partners in Crime. Front. Microbiol. 2017, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Dudley, H.R.; Spiro, D. The Fine Structure of Bone Cells. J. Cell Biol. 1961, 11, 627–649. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 2015, 159, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004, 18, 980–982. [Google Scholar] [CrossRef]
- Meunier, P.; Aaron, J.; Edouard, C.; VlGNON, G. Osteoporosis and the Replacement of Cell Populations of the Marrow by Adipose Tissue. A quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 1971, 80, 147–154. [Google Scholar] [CrossRef]
- Verma, S.; Rajaratnam, J.H.; Denton, J.; A Hoyland, J.; Byers, R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef]
- Yeung, D.K.; Griffith, J.; Antonio, G.E.; Lee, F.K.; Woo, J.; Leung, P.C. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. J. Magn. Reson. Imaging 2005, 22, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Zvonic, S.; Floyd, E.; Kassem, M.; Nuttall, M.E. Playing with bone and fat. J. Cell. Biochem. 2006, 98, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-Y.; Pratap, J.; Javed, A.; Zaidi, S.K.; Xing, L.; Balint, E.; Dalamangas, S.; Boyce, B.; van Wijnen, A.J.; Lian, J.B.; et al. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 8650–8655. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.J.; Kim, J.A.; Kwon, S.H.; Kim, S.W.; Park, K.S.; Park, S.-W.; Kim, S.Y.; Shin, C.S. Activation of Peroxisome Proliferator-activated Receptor-γ Inhibits the Runx2-mediated Transcription of Osteocalcin in Osteoblasts. J. Biol. Chem. 2003, 278, 23270–23277. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.; Joyner, C.; Triffitt, J.; Owen, M. Adipocytic cells cultured from marrow have osteogenic potential. J. Cell Sci. 1991, 99, 131–139. [Google Scholar] [CrossRef]
- Beresford, J.; Bennett, J.; Devlin, C.; Leboy, P.; Owen, M. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 1992, 102, 341–351. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef]
- Muruganandan, S.; Ionescu, A.M.; Sinal, C.J. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. Int. J. Mol. Sci. 2020, 21, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, E.J. An Overview of Human Brucellosis. Clin. Infect. Dis. 1995, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Chihara, T.; Mizoguchi, T.; Hori, A.; Udagawa, N.; Nakamura, H.; Hasegawa, H.; Taguchi, A.; Shinohara, A.; et al. Comparing immunocompetent and immunodeficient mice as animal models for bone tissue engineering. Oral Dis. 2015, 21, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chihara, T.; Zhang, Y.; Li, X.; Shinohara, A.; Kagami, H. Effect of short-term betamethasone administration on the regeneration process of tissue-engineered bone. Histol. Histopathol. 2020, 35, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Viglietti, A.I.P.; Giambartolomei, G.H.; Quarleri, J.; Delpino, M.V. Brucella abortus Infection Modulates 3T3-L1 Adipocyte Inflammatory Response and Inhibits Adipogenesis. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef]
- Scian, R.; Barrionuevo, P.; Fossati, C.A.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus Invasion of Osteoblasts Inhibits Bone Formation. Infect. Immun. 2012, 80, 2333–2345. [Google Scholar] [CrossRef] [Green Version]
- Scian, R.; Barrionuevo, P.; Giambartolomei, G.H.; Fossati, C.A.; Baldi, P.C.; Delpino, M.V. Granulocyte-Macrophage Colony-Stimulating Factor- and Tumor Necrosis Factor Alpha-Mediated Matrix Metalloproteinase Production by Human Osteoblasts and Monocytes after Infection with Brucella abortus. Infect. Immun. 2011, 79, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Bellows, C.; Aubin, J.E.; Heersche, J. Initiation and progression of mineralization of bone nodules formed in vitro: The role of alkaline phosphatase and organic phosphate. Bone Miner. 1991, 14, 27–40. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kang, B.-S.; Lee, T.-K.; Park, W.-H.; Kim, J.-K.; Park, Y.-G.; Kim, H.-M.; Lee, Y.-C. Il-1β regulates cellular proliferation, prostaglandin e2synthesis, plasminogen activator activity, osteocalcin production, and bone resorptive activity of the mouse calvarial bone cells. Immunopharmacol. Immunotoxicol. 2002, 24, 395–407. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef] [Green Version]
- Buckwalter, J.A.; Cooper, R.R. Bone structure and function. Instr. Course Lect. 1987, 36, 27–48. [Google Scholar] [PubMed]
- Sasano, Y.; Li, H.-C.; Zhu, J.-X.; Imanaka-Yoshida, K.; Mizoguchi, I.; Kagayama, M. Immunohistochemical localization of type I collagen, fibronectin and tenascin C during embryonic osteogenesis in the dentary of mandibles and tibias in rats. Histochem. J. 2000, 32, 591–598. [Google Scholar] [CrossRef]
- Sroga, G.E.; Vashishth, D. Effects of Bone Matrix Proteins on Fracture and Fragility in Osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, H. The Unexpected Link Between Osteoclasts and the Immune System. Adv. Exp. Med. Biol. 2010, 658, 61–68. [Google Scholar] [CrossRef]
- Terrier, C.S.-P.; Gasque, P. Bone responses in health and infectious diseases: A focus on osteoblasts. J. Infect. 2017, 75, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-H.; Kwon, T.-G.; Park, H.-S.; Wozney, J.M.; Ryoo, H.-M. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem. Biophys. Res. Commun. 2003, 309, 689–694. [Google Scholar] [CrossRef]
- Carlinfante, G.; Vassiliou, D.; Svensson, O.; Wendel, M.; Heinegård, D.; Andersson, G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin. Exp. Metastasis 2003, 20, 437–444. [Google Scholar] [CrossRef]
- Kido, J.-I.; Nakamura, T.; Asahara, Y.; Sawa, T.; Kohri, K.; Nagata, T. Osteopontin in gingival crevicular fluid. J. Periodontal Res. 2001, 36, 328–333. [Google Scholar] [CrossRef]
- Ohshima, S.; Yamaguchi, N.; Nishioka, K.; Mima, T.; Ishii, T.; Umeshita-Sasai, M.; Kobayashi, H.; Shimizu, M.; Katada, Y.; Wakitani, S.; et al. Enhanced local production of osteopontin in rheumatoid joints. J. Rheumatol. 2002, 29. [Google Scholar]
- Viglietti, A.I.P.; Gentilini, M.V.; Benitez, P.C.A.; Giambartolomei, G.H.; Delpino, M.V. B. Abortus Modulates Osteoblast Function Through the Induction of Autophagy. Front. Cell. Infect. Microbiol. 2018, 8, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The Role of C/EBP Genes in Adipocyte Differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeilnejad-Ganji, S.M.; Esmaeilnejad-Ganji, S.M.R. Osteoarticular manifestations of human brucellosis: A review. World J. Orthop. 2019, 10, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Park, C.; Lee, J.; Park, D.; Jeong, S.; Yun, C.-H.; Park, O.-J.; Han, S. Regulation of Bone Cell Differentiation and Activation by Microbe-Associated Molecular Patterns. Int. J. Mol. Sci. 2021, 22, 5805. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Huang, Q.; Lin, Y.-S.; Wei, B.-Y.; Guo, Y.-S.; Sun, Z.; Wang, L.; Fan, J.; Zhang, H.-Y.; Han, Y.-H.; et al. Dose-Dependent Effect of Estrogen Suppresses the Osteo-Adipogenic Transdifferentiation of Osteoblasts via Canonical Wnt Signaling Pathway. PLoS ONE 2014, 9, e99137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimble, J.M. The function of adipocytes in the bone marrow stroma. New Biol. 1990, 2, 304–312. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Hardouin, P.; Pansini, V.; Cortet, B. Bone marrow fat. Jt. Bone Spine 2014, 81, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.A. Bone remodelling. Br. J. Orthod. 1998, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Karava, V.; Christoforidis, A.; Kondou, A.; Dotis, J.; Printza, N. Update on the Crosstalk Between Adipose Tissue and Mineral Balance in General Population and Chronic Kidney Disease. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Possetti, V.; Parente, R.; Bottazzi, B.; Inforzato, A.; Sobacchi, C. The osteoblast secretome in Staphylococcus aureus osteomyelitis. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Ma, W.; Jin, W.; He, X.; Sun, Y.; Yin, H.; Wang, Z.; Shi, S. Mycobacterium tuberculosis Induced Osteoblast Dysregulation Involved in Bone Destruction in Spinal Tuberculosis. Front. Cell. Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Cotter, E.J.; Chew, N.; Powderly, W.G.; Doran, P.P. HIV Type 1 Alters Mesenchymal Stem Cell Differentiation Potential and Cell Phenotype Ex Vivo. AIDS Res. Hum. Retrovir. 2011, 27, 187–199. [Google Scholar] [CrossRef]
- Roy, E.; Shi, W.; Duan, B.; Reid, S.P. Chikungunya Virus Infection Impairs the Function of Osteogenic Cells. Msphere 2020, 5. [Google Scholar] [CrossRef]
- Siegert, P.; Schmidt, G.; Papatheodorou, P.; Wieland, T.; Aktories, K.; Orth, J.H.C. Pasteurella multocida Toxin Prevents Osteoblast Differentiation by Transactivation of the MAP-Kinase Cascade via the Gαq/11 - p63RhoGEF - RhoA Axis. PLOS Pathog. 2013, 9, e1003385. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, X.; Zuo, B.; Zhang, L. The Role of Bone Marrow Microenvironment in Governing the Balance between Osteoblastogenesis and Adipogenesis. Aging Dis. 2016, 7, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Ayyappan, J.P.; Ganapathi, U.; Lizardo, K.; Vinnard, C.; Subbian, S.; Perlin, D.S.; Nagajyothi, J.F. Adipose Tissue Regulates Pulmonary Pathology during TB Infection. Mbio 2019, 10, e02771-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, F.B.; Villar, S.R.; Toneatto, J.; Pacini, M.F.; Márquez, J.; D’Attilio, L.; Bottasso, O.A.; Piwien-Pilipuk, G.; Pérez, A.R. Immune response triggered by Trypanosoma cruzi infection strikes adipose tissue homeostasis altering lipid storage, enzyme profile and adipokine expression. Med. Microbiol. Immunol. 2019, 208, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Scian, R.; Barrionuevo, P.; Rodriguez, A.M.; Benitez, P.C.A.; Samartino, C.G.; Fossati, C.A.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus Invasion of Synoviocytes Inhibits Apoptosis and Induces Bone Resorption through RANKL Expression. Infect. Immun. 2013, 81, 1940–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freiberger, R.N.; López, C.A.M.; Sviercz, F.A.; Cevallos, C.; Guano, A.D.; Jarmoluk, P.; Quarleri, J.; Delpino, M.V. B. abortus Infection Promotes an Imbalance in the Adipocyte–Osteoblast Crosstalk Favoring Bone Resorption. Int. J. Mol. Sci. 2023, 24, 5617. https://doi.org/10.3390/ijms24065617
Freiberger RN, López CAM, Sviercz FA, Cevallos C, Guano AD, Jarmoluk P, Quarleri J, Delpino MV. B. abortus Infection Promotes an Imbalance in the Adipocyte–Osteoblast Crosstalk Favoring Bone Resorption. International Journal of Molecular Sciences. 2023; 24(6):5617. https://doi.org/10.3390/ijms24065617
Chicago/Turabian StyleFreiberger, Rosa Nicole, Cinthya Alicia Marcela López, Franco Agustín Sviercz, Cintia Cevallos, Alex David Guano, Patricio Jarmoluk, Jorge Quarleri, and María Victoria Delpino. 2023. "B. abortus Infection Promotes an Imbalance in the Adipocyte–Osteoblast Crosstalk Favoring Bone Resorption" International Journal of Molecular Sciences 24, no. 6: 5617. https://doi.org/10.3390/ijms24065617





