The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models
Abstract
:1. Introduction
2. Results
2.1. Initial Characterization and Seeding Density Optimization of the Four Mucosa Cell Lines
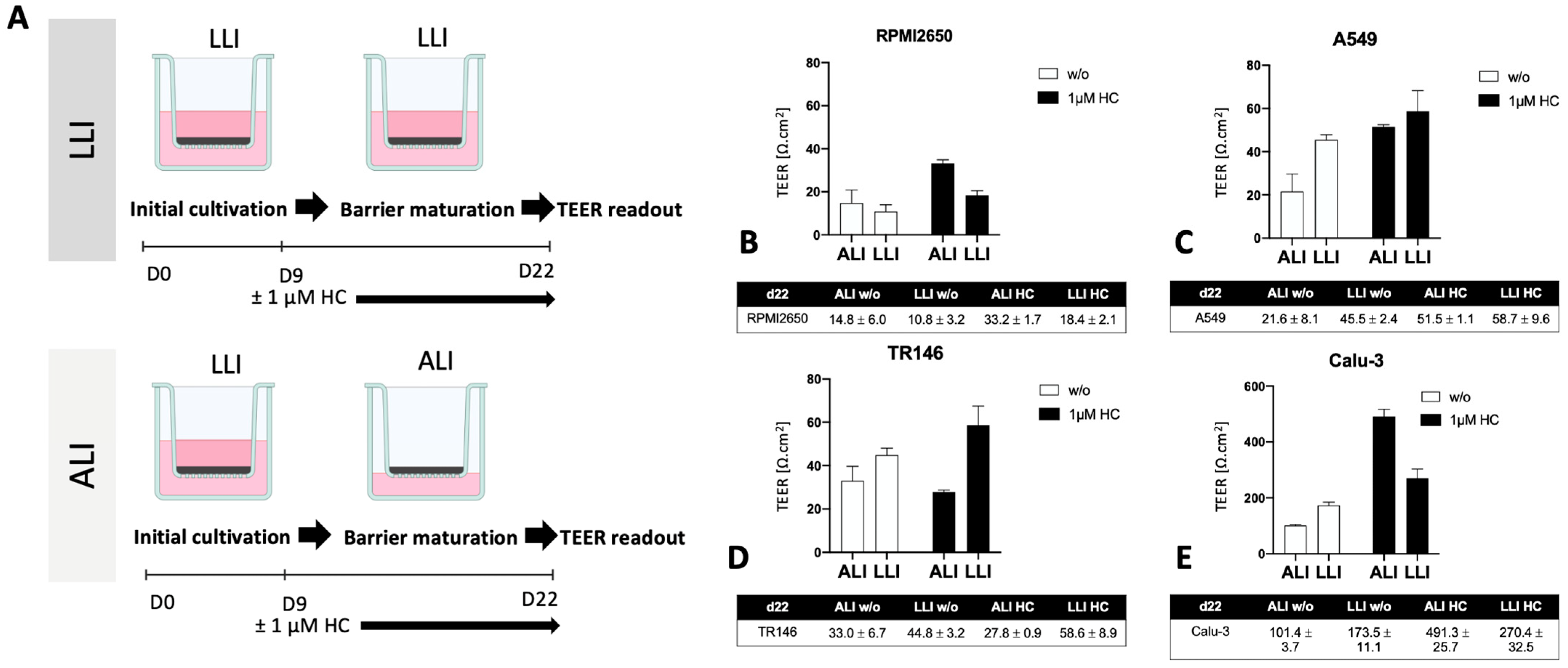
2.2. Optimization of the Liquid-Liquid Interface (LLI)-Based Cultivation Approach and Transfer to Air-Liquid Interface (ALI) Cultivation of Human Mucosa Barrier Models
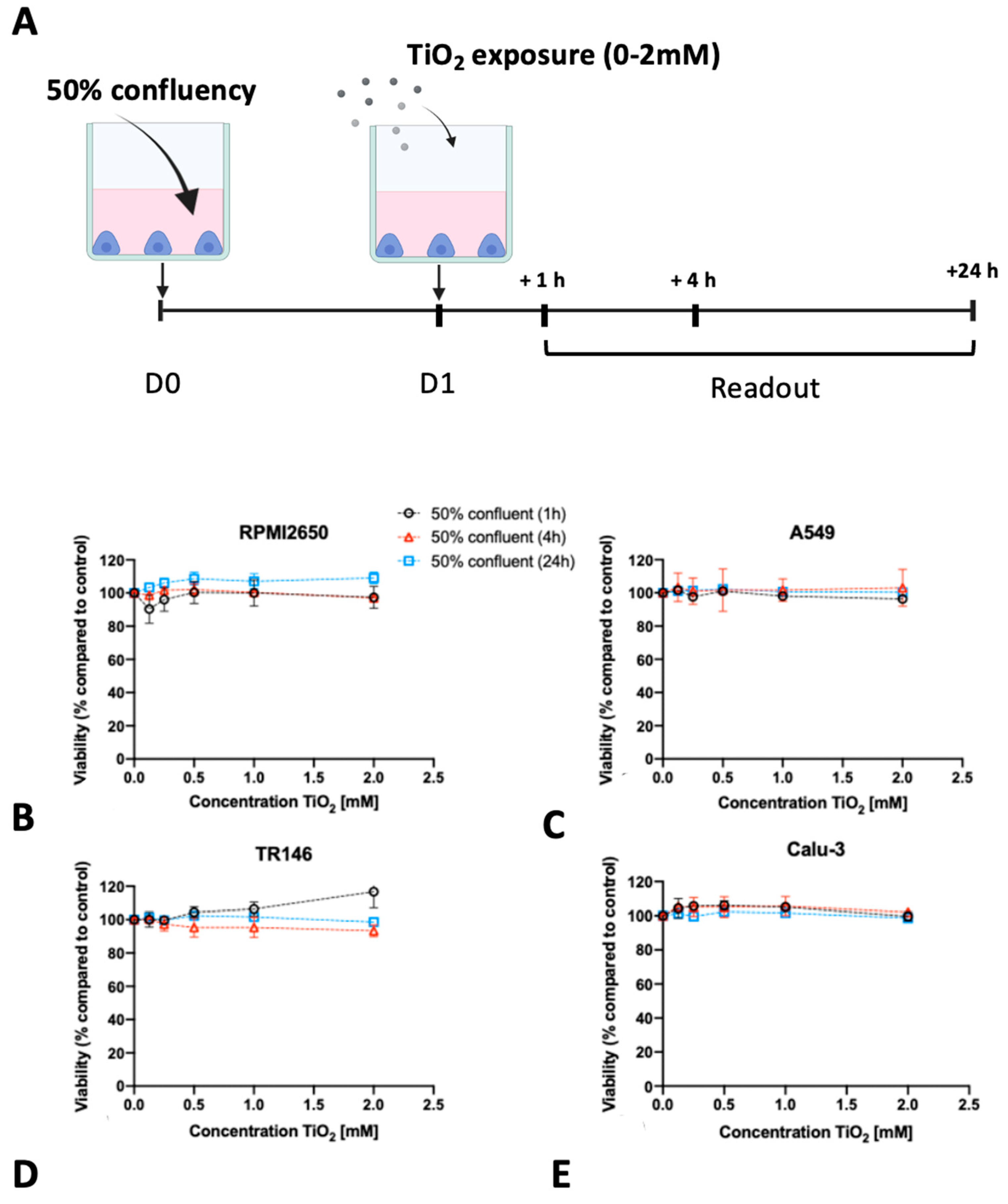
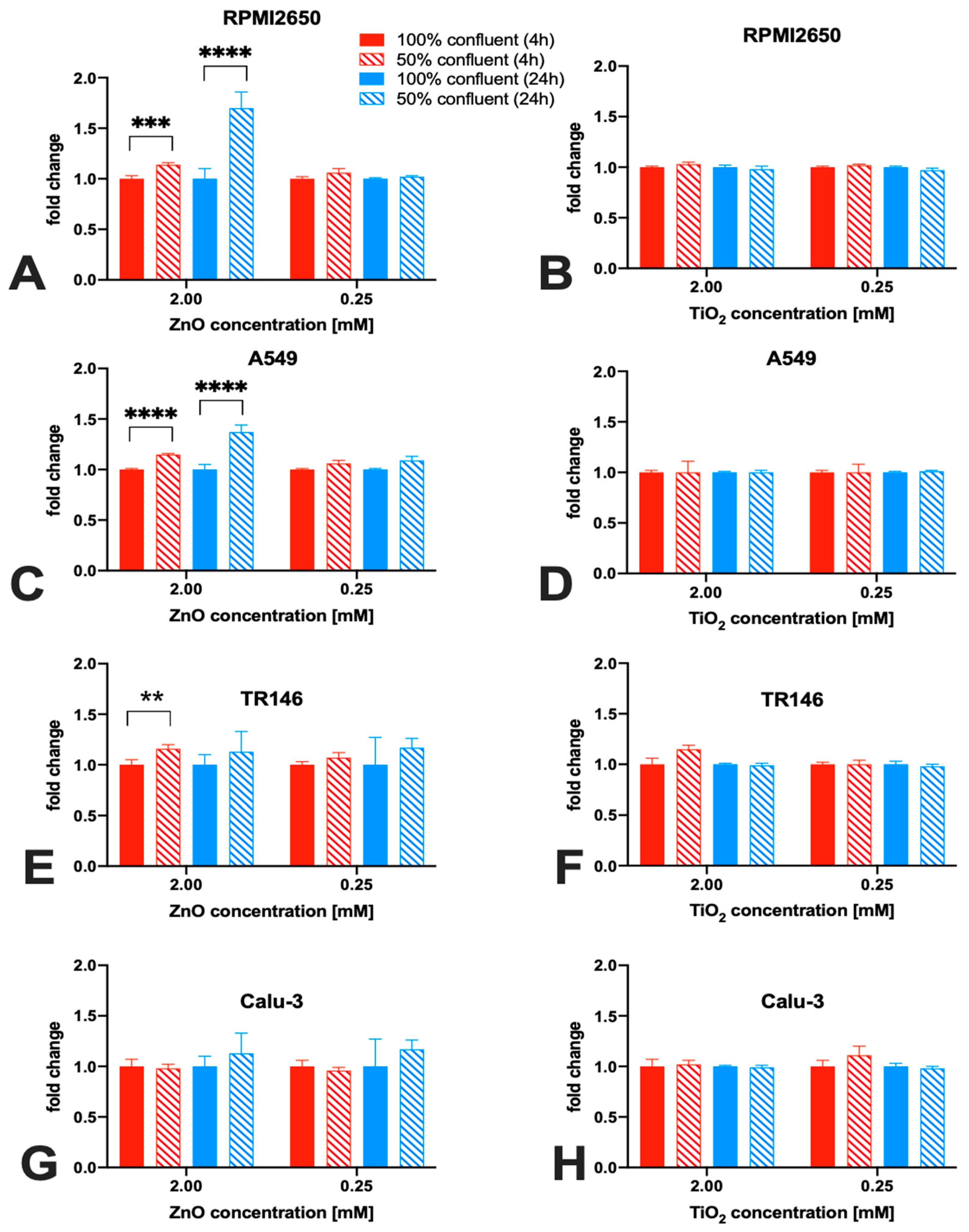
2.3. Impact of Barrier Cell Confluency on the Dose-Response Readout of Human Barrier Models Exposed to TiO2 and ZnO Nanoparticles
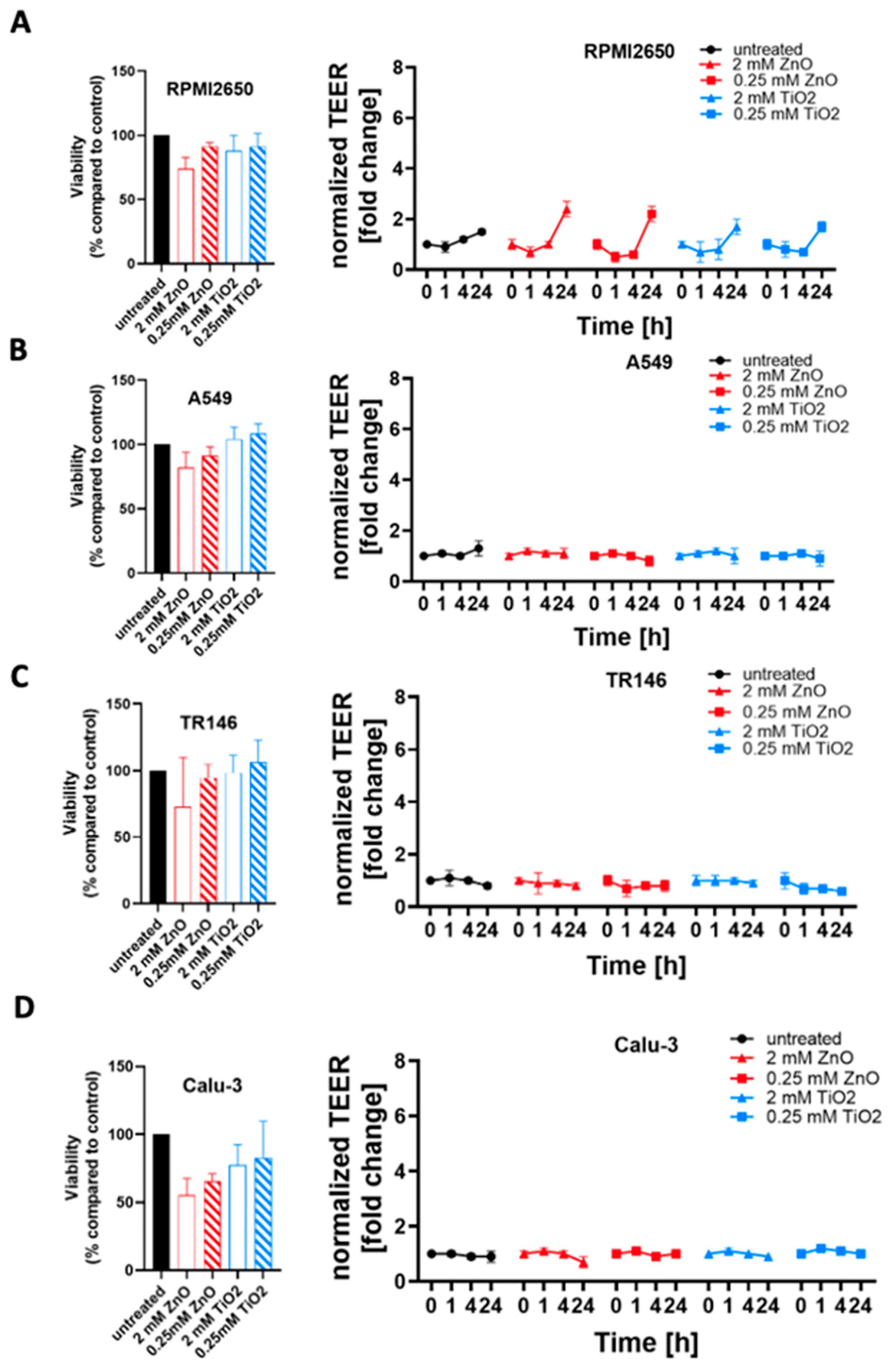
2.4. Toxicological Evaluation of Mature Mucosa Barrier Models Challenged with Subtoxic and Toxic ZnO and TiO2 Nanoparticle Concentrations
3. Discussion and Prospects
4. Materials and Methods
4.1. Cell lines and Culture Conditions
- RPMI2650, (Sigma-Aldrich, Vienna Austria, Cat. Nr. 88031602) originating from squamous cell carcinoma of nasal epithelium, was cultured in EMEM (Sigma-Aldrich, Vienna, Austria, Cat. Nr. M0325);
- A549 (ATCC, Manassas, Virginia, USA, Cat. Nr. CCL-185) originating from adenocarcinoma from alveolar lung epithelium, were cultured in RPMI1640 (Sigma-Aldrich, Vienna, Austria, Cat. Nr.R8758);
- TR146, (Sigma-Aldrich, Vienna, Austria, Cat. Nr. 10032305) originating from squamous cell carcinoma of buccal (oral) epithelium, were cultured in Ham’s F12 (Sigma-Aldrich, Vienna, Austria, Cat. Nr. 51651C);
- Calu-3, (Sigma-Aldrich, Vienna, Austria, Cat. Nr. HTB-55, kindly provided by IMC FH Krems) originating from adenocarcinoma from bronchial lung epithelium, were cultured in EMEM (Sigma-Aldrich, Vienna, Austria, Cat. Nr. M0325).
4.2. Barrier Formation Studies
4.3. Evaluation of Tight Junction Formation via Immunocytochemistry
4.4. Toxicological Evaluation of Zinc Oxide and Titanium Dioxide Nanoparticles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef] [Green Version]
- Xiang, M.; Aguerre-Chariol, O.; Morgeneyer, M.; Philippe, F.; Liu, Y.; Bressot, C. Uncertainty assessment for the airborne nanoparticle collection efficiency of a TEM grid-equipped sampling system by Monte-Carlo calculation. Adv. Powder Technol. 2021, 32, 1793–1801. [Google Scholar] [CrossRef]
- Xiang, M.; Morgeneyer, M.; Aguerre-Chariol, O.; Philippe, F.; Bressot, C. Airborne nanoparticle collection efficiency of a TEM grid-equipped sampling system. Aerosol Sci. Technol. 2021, 55, 526–538. [Google Scholar] [CrossRef]
- Shandilya, N.; Le Bihan, O.; Bressot, C.; Morgeneyer, M. Evaluation of the Particle Aerosolization from n-TiO2Photocatalytic Nanocoatings under Abrasion. J. Nanomater. 2014, 2014, 185080. [Google Scholar] [CrossRef] [Green Version]
- Bressot, C.; Shandilya, N.; Nogueira, E.S.D.C.; Cavaco-Paulo, A.; Morgeneyer, M.; Le Bihan, O.; Aguerre-Chariol, O. Exposure Assessment Based Recommendations to Improve Nanosafety at Nanoliposome Production Sites. J. Nanomater. 2015, 2015, 931405. [Google Scholar] [CrossRef] [Green Version]
- Philippe, F.; Morgeneyer, M.; Xiang, M.; Manokaran, M.; Berthelot, B.; Chen, Y.M.; Charles, P.; Guingand, F.; Bressot, C. Representativeness of airborne brake wear emission for the automotive industry: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2021, 235, 2651–2666. [Google Scholar] [CrossRef]
- Philippe, F.; Xiang, M.; Morgeneyer, M.; Chen, Y.-M.; Charles, P.; Guingand, F.; Bressot, C. Emission rate assessment of airborne brake particles by characterization of the pad and disc surfaces from a pin-on-disc tribometer. Toxicol. Res. Appl. 2020, 4, 239784732097778. [Google Scholar] [CrossRef]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Lippmann, M.; Yeates, D.B.; Albert, R.E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef] [Green Version]
- Morozesk, M.; Souza, I.D.C.; Fernandes, M.N.; Soares, D.C.F. Airborne particulate matter in an iron mining city: Characterization, cell uptake and cytotoxicity effects of nanoparticles from PM2.5, PM10 and PM20 on human lung cells. Environ. Adv. 2021, 6, 100125. [Google Scholar] [CrossRef]
- Wang, S.; Alenius, H.; El-Nezami, H.; Karisola, P. A New Look at the Effects of Engineered ZnO and TiO2 Nanoparticles: Evidence from Transcriptomics Studies. Nanomaterials 2022, 12, 1247. [Google Scholar] [CrossRef]
- Majumder, N.; Goldsmith, W.T.; Kodali, V.K.; Velayutham, M.; Friend, S.A.; Khramtsov, V.V.; Nurkiewicz, T.R.; Erdely, A.; Zeidler-Erdely, P.C.; Castranova, V.; et al. Oxidant-induced epithelial alarmin pathway mediates lung inflammation and functional decline following ultrafine carbon and ozone inhalation co-exposure. Redox Biol. 2021, 46, 102092. [Google Scholar] [CrossRef] [PubMed]
- García-Salvador, A.; Katsumiti, A.; Rojas, E.; Aristimuño, C.; Betanzos, M.; Martínez-Moro, M.; Moya, S.E.; Goñi-De-cerio, F. A complete in vitro toxicological assessment of the biological effects of cerium oxide nanoparticles: From acute toxicity to multi-dose subchronic cytotoxicity study. Nanomaterials 2021, 11, 1577. [Google Scholar] [CrossRef]
- Di Giampaolo, L.; Zaccariello, G.; Benedetti, A.; Vecchiotti, G.; Caposano, F.; Sabbioni, E.; Groppi, F.; Manenti, S.; Niu, Q.; Maria, A.; et al. Genotoxicity and Immunotoxicity of Titanium Dioxide-Embedded Mesoporous Silica Nanoparticles (TiO2@MSN) in Primary Peripheral Human Blood Mononuclear Cells (PBMC). Nanomaterials 2021, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Howe, J.L.C.; Yu, Z.; Leong, D.T.; Chu, J.J.H.; Loo, J.S.C.; Ng, K.W. Exposure to Titanium Dioxide Nanoparticles Induces Autophagy in Primary Human Keratinocytes. Small 2013, 9, 387–392. [Google Scholar] [CrossRef]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef]
- Tucci, P.; Porta, G.; Agostini, M.; Dinsdale, D.; Iavicoli, I.; Cain, K.; Finazzi-Agró, A.; Melino, G.; Willis, A. Metabolic effects of TiO2 nanoparticles, a common component of sunscreens and cosmetics, on human keratinocytes. Cell Death Dis. 2013, 4, e549. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice. Nanoscale Res. Lett. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2015, 22, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Nazari, H.; Heirani-Tabasi, A.; Ghorbani, S.; Eyni, H.; Razavi Bazaz, S.; Khayati, M.; Gheidari, F.; Moradpour, K.; Kehtari, M.; Ahmadi Tafti, S.M.; et al. Microfluidic-Based Droplets for Advanced Regenerative Medicine: Current Challenges and Future Trends. Biosensors 2022, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, Y.; Yin, S.; Song, B.; Wei, L.; Chen, L.; Shao, L. Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner. Int. J. Nanomed. 2017, 12, 8085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 2699–2711. [Google Scholar] [CrossRef]
- Wu, W.; Samet, J.M.; Peden, D.B.; Bromberg, P.A. Phosphorylation of p65 is required for zinc oxide nanoparticle-induced interleukin 8 expression in human bronchial epithelial cells. Environ. Health Perspect. 2010, 118, 982–987. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, I.L.; Huang, Y.J. Titanium oxide shell coatings decrease the cytotoxicity of ZnO nanoparticles. Chem. Res. Toxicol. 2011, 24, 303–313. [Google Scholar] [CrossRef]
- Yuan, J.H.; Chen, Y.; Zha, H.X.; Song, L.J.; Li, C.Y.; Li, J.Q.; Xia, X.H. Determination, characterization and cytotoxicity on HELF cells of ZnO nanoparticles. Colloids Surf. B. Biointerfaces 2010, 76, 145–150. [Google Scholar] [CrossRef]
- Berntsen, P.; Park, C.Y.; Rothen-Rutishauser, B.; Tsuda, A.; Sager, T.M.; Molina, R.M.; Donaghey, T.C.; Alencar, A.M.; Kasahara, D.I.; Ericsson, T.; et al. Biomechanical effects of environmental and engineered particles on human airway smooth muscle cells. J. R. Soc. Interface 2010, 7, S331–S340. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Li, F.; Tang, Y.; Yang, S.D.; Li, J.Z.; Yuan, Z.Q.; Liu, Y.; Zhou, X.F.; Liu, C.; Zhang, X.N. Stepwise pH-responsive nanoparticles for enhanced cellular uptake and on-demand intracellular release of doxorubicin. Int. J. Nanomed. 2017, 12, 4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.P.; Lai, C.S.; Hung, C.J.; Dhaiveegan, P.; Tsai, M.L.; Chiu, C.L.; Fang, J.M. Subchronic oral toxicity evaluation of gold nanoparticles in male and female mice. Heliyon 2021, 7, e06577. [Google Scholar] [CrossRef]
- Li, H.; Huang, T.; Wang, Y.; Pan, B.; Zhang, L.; Zhang, Q.; Niu, Q. Toxicity of alumina nanoparticles in the immune system of mice. Nanomedicine 2020, 15, 927–946. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, Y.; He, K.; Li, H.; Gao, F.; Moehling, T.J.; Wu, X.; Duncan, J.; Niu, Q. Exposure to alumina nanoparticles in female mice during pregnancy induces neurodevelopmental toxicity in the offspring. Front. Pharmacol. 2018, 9, 253. [Google Scholar] [CrossRef]
- Elkhadrawy, B.; Abou-Zeid, S.; El-Borai, N.; El-Sabbagh, H.; El-Bialy, B.E.-S. Potential Toxic Effects of Aluminum Nanoparticles: An overview. J. Curr. Vet. Res. 2021, 3, 94–106. [Google Scholar] [CrossRef]
- Alghriany, A.A.I.; Omar, H.E.D.M.; Mahmoud, A.M.; Atia, M.M. Assessment of the Toxicity of Aluminum Oxide and Its Nanoparticles in the Bone Marrow and Liver of Male Mice: Ameliorative Efficacy of Curcumin Nanoparticles. ACS Omega 2022, 7, 13841–13852. [Google Scholar] [CrossRef]
- Castell, J.V.; Donato, M.T.; Gómez-Lechón, M.J. Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp. Toxicol. Pathol. 2005, 57, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.M.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. Vitr. 2018, 47, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Seagrave, J.C.; McDonald, J.D.; Mauderly, J.L. In vitro versus in vivo exposure to combustion emissions. Exp. Toxicol. Pathol. 2005, 57, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.G.; Karg, E.; Lentner, B.; Dittrich, V.; Brandenberger, C.; Rothen-Rutishauser, B.; Schulz, H.; Ferron, G.A.; Schmid, O. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Part. Fibre Toxicol. 2009, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.E.; Biola-Clier, M.; Ambrose, S.; et al. Air-Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomaterials 2020, 11, 65. [Google Scholar] [CrossRef]
- Leibrock, L.; Wagener, S.; Singh, A.V.; Laux, P.; Luch, A. Nanoparticle induced barrier function assessment at liquid–liquid and air–liquid interface in novel human lung epithelia cell lines. Toxicol. Res. 2019, 8, 1016–1027. [Google Scholar] [CrossRef] [Green Version]
- Ritter, D.; Knebel, J.; Niehof, M.; Loinaz, I.; Marradi, M.; Gracia, R.; te Welscher, Y.; van Nostrum, C.F.; Falciani, C.; Pini, A.; et al. In vitro inhalation cytotoxicity testing of therapeutic nanosystems for pulmonary infection. Toxicol. Vitr. 2020, 63, 104714. [Google Scholar] [CrossRef]
- Ritter, D.; Knebel, J.W.; Aufderheide, M. In vitro exposure of isolated cells to native gaseous compounds—Development and validation of an optimized system for human lung cells. Exp. Toxicol. Pathol. 2001, 53, 373–386. [Google Scholar] [CrossRef]
- Doryab, A.; Taskin, M.B.; Stahlhut, P.; Schröppel, A.; Orak, S.; Voss, C.; Ahluwalia, A.; Rehberg, M.; Hilgendorff, A.; Stöger, T.; et al. A Bioinspired in vitro Lung Model to Study Particokinetics of Nano-/Microparticles Under Cyclic Stretch and Air-Liquid Interface Conditions. Front. Bioeng. Biotechnol. 2021, 9, 42. [Google Scholar] [CrossRef]
- Leibrock, L.B.; Jungnickel, H.; Tentschert, J.; Katz, A.; Toman, B.; Petersen, E.J.; Bierkandt, F.S.; Singh, A.V.; Laux, P.; Luch, A. Parametric optimization of an air-liquid interface system for flow through inhalation exposure to nanoparticles: Assessing dosimetry and intracellular uptake of CeO2 nanoparticles. Nanomaterials 2020, 10, 2369. [Google Scholar] [CrossRef]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G. The Nose Revisited: A Brief Review of the Comparative Structure, Function, and Toxicologic Pathology of the Nasal Epithelium. Toxicol. Pathol. 2006, 34, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 2001, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, N.; Kubo, H.; Suzuki, T.; Ota, C.; Hegab, A.E.; He, M.; Suzuki, S.; Suzuki, T.; Yamada, M.; Kondo, T.; et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab. Investig. 2011, 91, 363–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, B.Q.; Finkbeiner, W.E.; Wine, J.J.; Mrsny, R.J.; Widdicombe, J.H. Calu-3: A human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am. J. Physiol. Cell. Mol. Physiol. 1994, 266, L493–L501. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Wolpert, E.B.; DeMaio, L.; Harhaj, N.S.; Scaduto, R.C. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J. Neurochem. 2002, 80, 667–677. [Google Scholar] [CrossRef]
- Pozzoli, M.; Ong, H.X.; Morgan, L.; Sukkar, M.; Traini, D.; Young, P.M.; Sonvico, F. Application of RPMI 2650 nasal cell model to a 3D printed apparatus for the testing of drug deposition and permeation of nasal products. Eur. J. Pharm. Biopharm. 2016, 107, 223–233. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Birch, N.P.; Suresh, V. An optimised human cell culture model for alveolar epithelial transport. PLoS ONE 2016, 11, e0165225. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.K.; Rahman, Q.; Kashyap, M.P.; Singh, A.K.; Jain, G.; Jahan, S.; Lohani, M.; Lantow, M.; Pant, A.B. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum. Exp. Toxicol. 2013, 32, 153–166. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Blank, F.; Rothen-Rutishauser, B.M.; Schurch, S.; Gehr, P. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J. Aerosol Med. Depos. Clear. Eff. Lung 2006, 19, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Munis, A.M.; Hyde, S.C.; Gill, D.R. A human surfactant B deficiency air-liquid interface cell culture model suitable for gene therapy applications. Mol. Ther.—Methods Clin. Dev. 2021, 20, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Mathias, N.R.; Timoszyk, J.; Stetsko, P.I.; Megill, J.R.; Smith, R.L.; Wall, D.A. Permeability characteristics of Calu-3 human bronchial epithelial cells: In vitro-in vitro correlation to predict lung absorption in rats. J. Drug Target. 2002, 10, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Avery, M.L.; Yazdanian, M.; Audus, K.L. Characterization of the Calu-3 cell line as a tool to screen pulmonary drug delivery. Int. J. Pharm. 2000, 208, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bol, L.; Galas, J.C.; Hillaireau, H.; Le Potier, I.; Nicolas, V.; Haghiri-Gosnet, A.M.; Fattal, E.; Taverna, M. A microdevice for parallelized pulmonary permeability studies. Biomed. Microdevices 2014, 16, 277–285. [Google Scholar] [CrossRef]
- Joy, A.P.; Cowley, E.A. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am. J. Respir. Cell Mol. Biol. 2008, 38, 143–152. [Google Scholar] [CrossRef]
- Min, K.A.; Rosania, G.R.; Kim, C.K.; Shin, M.C. Functional and cytometric examination of different human lung epithelial cell types as drug transport barriers. Arch. Pharm. Res. 2016, 39, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Reichl, S.; Becker, K. Cultivation of RPMI 2650 cells as an in-vitro model for human transmucosal nasal drug absorption studies: Optimization of selected culture conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Rižner, T.L.; Hevir-Kene, N.; Peternel, L.; Kristan, K. The characterization of the human nasal epithelial cell line RPMI 2650 under different culture conditions and their optimization for an appropriate in vitro nasal model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef]
- Lin, G.C.; Leitgeb, T.; Vladetic, A.; Friedl, H.P.; Rhodes, N.; Rossi, A.; Roblegg, E.; Neuhaus, W. Optimization of an oral mucosa in vitro model based on cell line TR146. Tissue Barriers 2020, 8, 1748459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, J.; Nielsen, E.B.; Brøndum-Nielsen, K.; Christensen, M.E.; Olin, H.D.; Tommerup, N.; Rassing, M.R. Filter-grown TR146 cells as an in vitro model of human buccal epithelial permeability. Eur. J. Oral Sci. 1999, 107, 138–146. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; He, R.; Vandebriel, R.J.; Gremmer, E.R.; Zwart, E.; Vermeulen, J.P.; Fokkens, P.; Boere, J.; Gosens, I.; Cassee, F.R. An air-liquid interface bronchial epithelial model for realistic, repeated inhalation exposure to airborne particles for toxicity testing. J. Vis. Exp. 2020, 2020, e61210. [Google Scholar] [CrossRef]
- Barosova, H.; Meldrum, K.; Karakocak, B.B.; Balog, S.; Doak, S.H.; Petri-Fink, A.; Clift, M.J.D.; Rothen-Rutishauser, B. Inter-laboratory variability of A549 epithelial cells grown under submerged and air-liquid interface conditions. Toxicol. Vitr. 2021, 75, 105178. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhao, X.; Xiong, S.; Ng, K.W.; Boey, F.Y.C.; Loo, J.S.C. Cytotoxicity of zinc oxide (ZnO) nanoparticles is influenced by cell density and culture format. Arch. Toxicol. 2011, 85, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Leroux, M.M.; Doumandji, Z.; Chézeau, L.; Gaté, L.; Nahle, S.; Hocquel, R.; Zhernovkov, V.; Migot, S.; Ghanbaja, J.; Bonnet, C.; et al. Toxicity of TiO2 nanoparticles: Validation of alternative models. Int. J. Mol. Sci. 2020, 21, 4855. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Long, J.; Ding, Y.; Zou, X.; Liao, G.; Cao, Y. A comparative study of toxicity of TiO2, ZnO, and Ag nanoparticles to human aortic smooth-muscle cells. Int. J. Nanomed. 2018, 13, 8037–8049. [Google Scholar] [CrossRef] [Green Version]
- Čepin, M.; Hribar, G.; Caserman, S.; Orel, Z.C. Morphological impact of zinc oxide particles on the antibacterial activity and human epithelia toxicity. Mater. Sci. Eng. C 2015, 52, 204–211. [Google Scholar] [CrossRef]
- Sticker, D.; Rothbauer, M.; Lechner, S.; Hehenberger, M.T.; Ertl, P. Multi-layered, membrane-integrated microfluidics based on replica molding of a thiol-ene epoxy thermoset for organ-on-a-chip applications. Lab Chip 2015, 15, 4542–4554. [Google Scholar] [CrossRef]
- Schuller, P.; Rothbauer, M.; Kratz, S.R.A.; Höll, G.; Taus, P.; Schinnerl, M.; Genser, J.; Bastus, N.; Moriones, O.H.; Puntes, V.; et al. A lab-on-a-chip system with an embedded porous membrane-based impedance biosensor array for nanoparticle risk assessment on placental Bewo trophoblast cells. Sens. Actuators B Chem. 2020, 312, 127946. [Google Scholar] [CrossRef]
- Charwat, V.; Olmos Calvo, I.; Rothbauer, M.; Kratz, S.R.A.; Jungreuthmayer, C.; Zanghellini, J.; Grillari, J.; Ertl, P. Combinatorial in Vitro and in Silico Approach to Describe Shear-Force Dependent Uptake of Nanoparticles in Microfluidic Vascular Models. Anal. Chem. 2018, 90, 3651–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothbauer, M.; Praisler, I.; Docter, D.; Stauber, R.H.; Ertl, P. Microfluidic Impedimetric Cell Regeneration Assay to Monitor the Enhanced Cytotoxic Effect of Nanomaterial Perfusion. Biosensors 2015, 5, 736–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Cell Line | Cell Length [µm, mean ± sdev] | Confluency [cells/cm2] | Doubling Time [h] |
|---|---|---|---|
| RPMI2650 | 19.3 ± 2.3 | 6 × 105 | 41.2 ± 9.3 |
| A549 | 47.4 ± 5.2 | 4 × 105 | 34.6 ± 9.0 |
| TR146 | 222.3 ± 25.9 | 1 × 105 | 63.8 ± 17.7 |
| Calu-3 | 79.1 ± 16.6 | 1.8 × 105 | 137.3 ± 20.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuetz, H.; Reihs, E.I.; Neuhaus, W.; Pflüger, M.; Hundsberger, H.; Ertl, P.; Resch, C.; Bauer, G.; Povoden, G.; Rothbauer, M. The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models. Int. J. Mol. Sci. 2023, 24, 5634. https://doi.org/10.3390/ijms24065634
Stuetz H, Reihs EI, Neuhaus W, Pflüger M, Hundsberger H, Ertl P, Resch C, Bauer G, Povoden G, Rothbauer M. The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models. International Journal of Molecular Sciences. 2023; 24(6):5634. https://doi.org/10.3390/ijms24065634
Chicago/Turabian StyleStuetz, Helene, Eva I. Reihs, Winfried Neuhaus, Maren Pflüger, Harald Hundsberger, Peter Ertl, Christian Resch, Gerald Bauer, Günter Povoden, and Mario Rothbauer. 2023. "The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models" International Journal of Molecular Sciences 24, no. 6: 5634. https://doi.org/10.3390/ijms24065634
APA StyleStuetz, H., Reihs, E. I., Neuhaus, W., Pflüger, M., Hundsberger, H., Ertl, P., Resch, C., Bauer, G., Povoden, G., & Rothbauer, M. (2023). The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models. International Journal of Molecular Sciences, 24(6), 5634. https://doi.org/10.3390/ijms24065634







