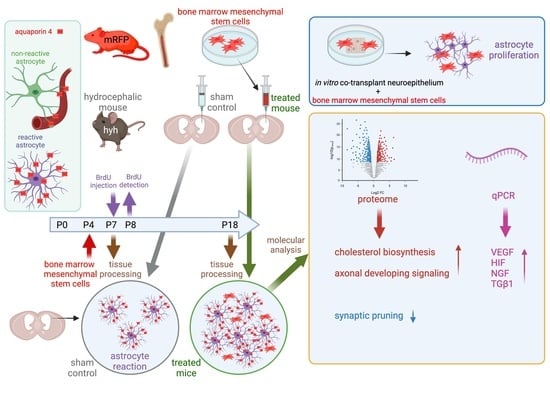
Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy
Abstract
:1. Introduction
2. Results
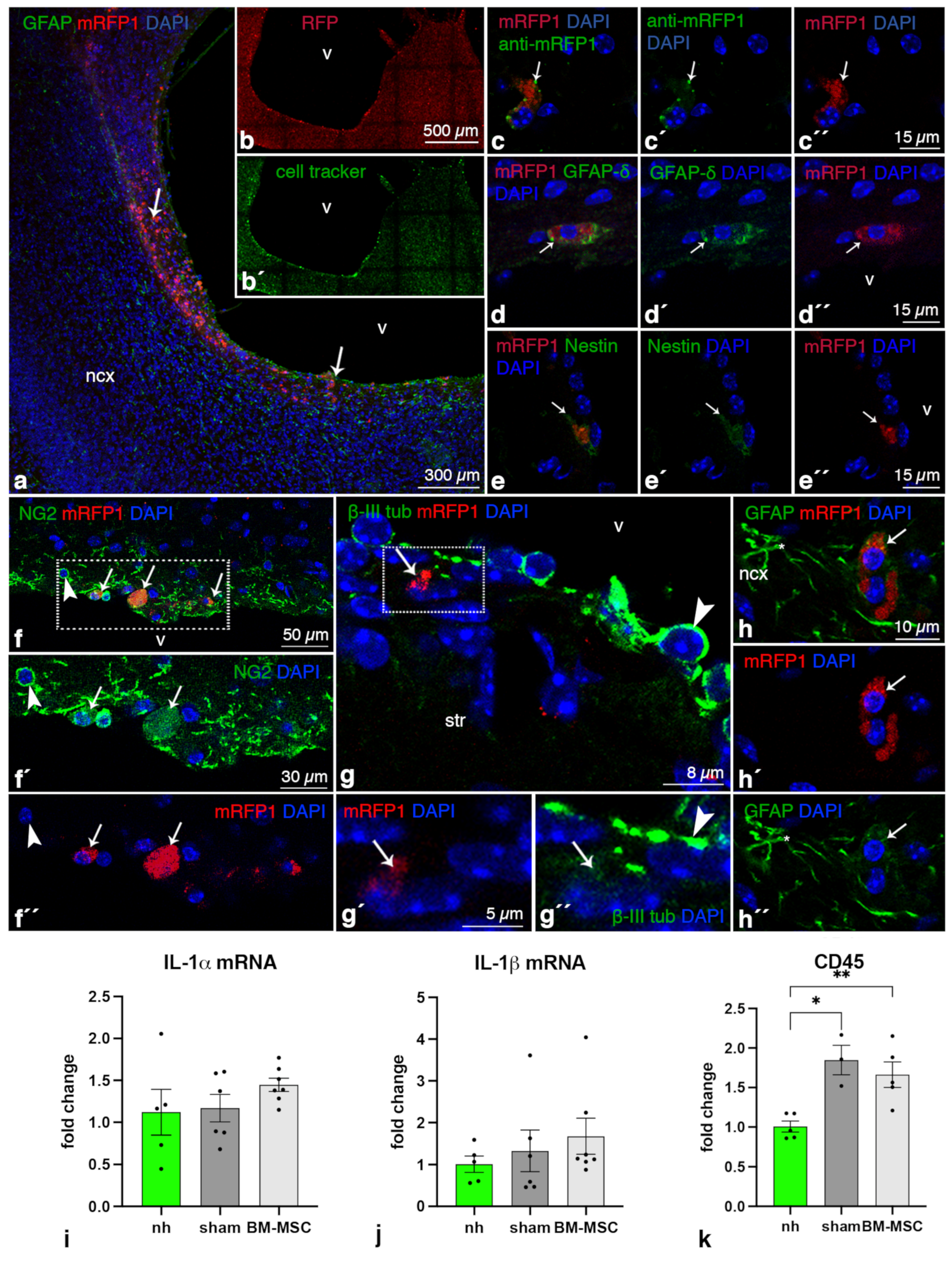
2.1. BM-MSCs Injected into the Ventricles Integrate and Survive Fourteen Days in the Damaged Periventricular Walls
2.2. BM-MSCs Do Not Transdifferentiate into Neural Cells after Transplantation
2.3. BM-MSCs Do Not Induce an Inflammatory Reaction after Transplantation
2.4. Higher Levels of NGF, VEGF, HIF1α and TGFβ1 Are Present in the Cerebral Tissue of Hydrocephalic Transplanted Mice
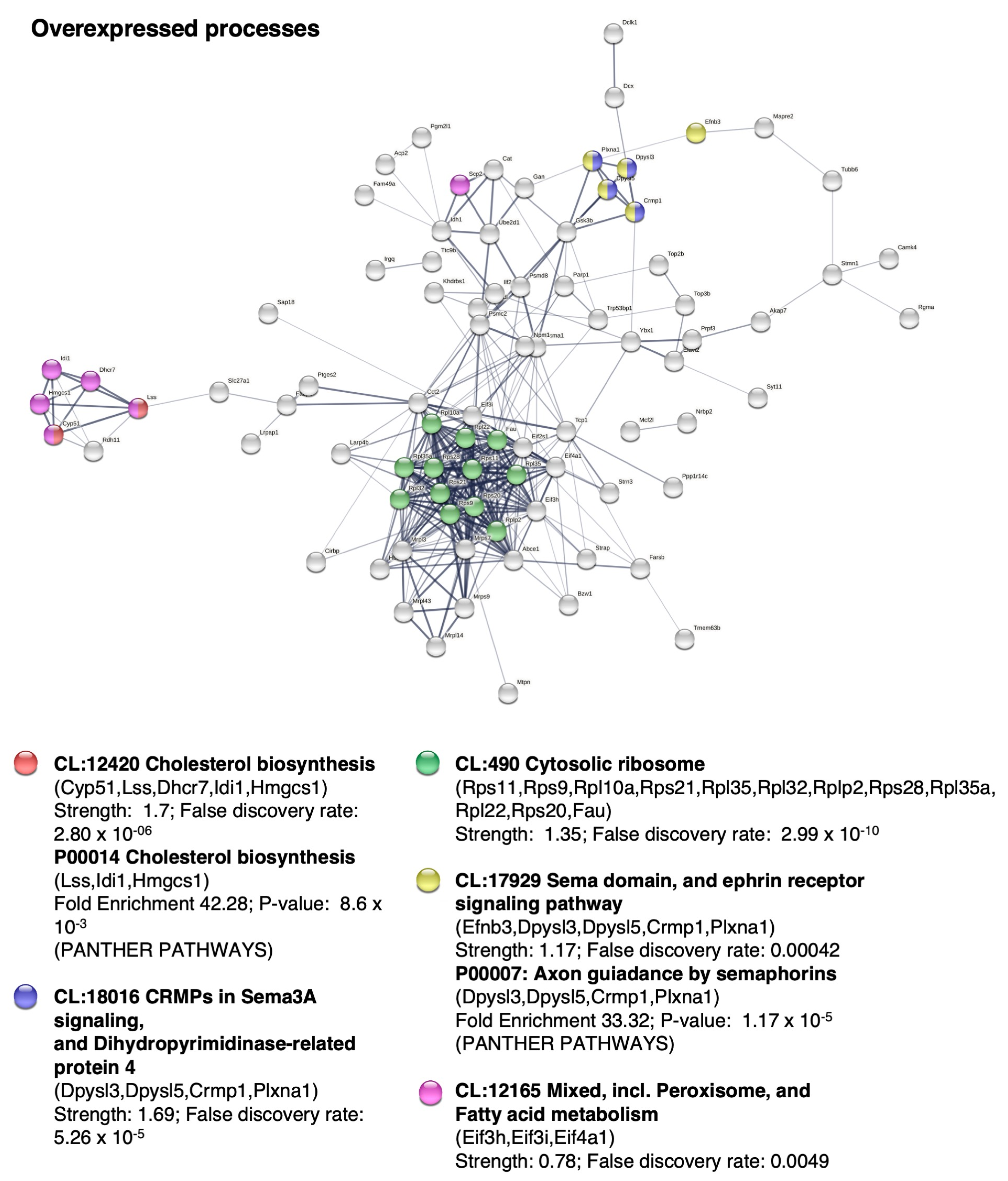
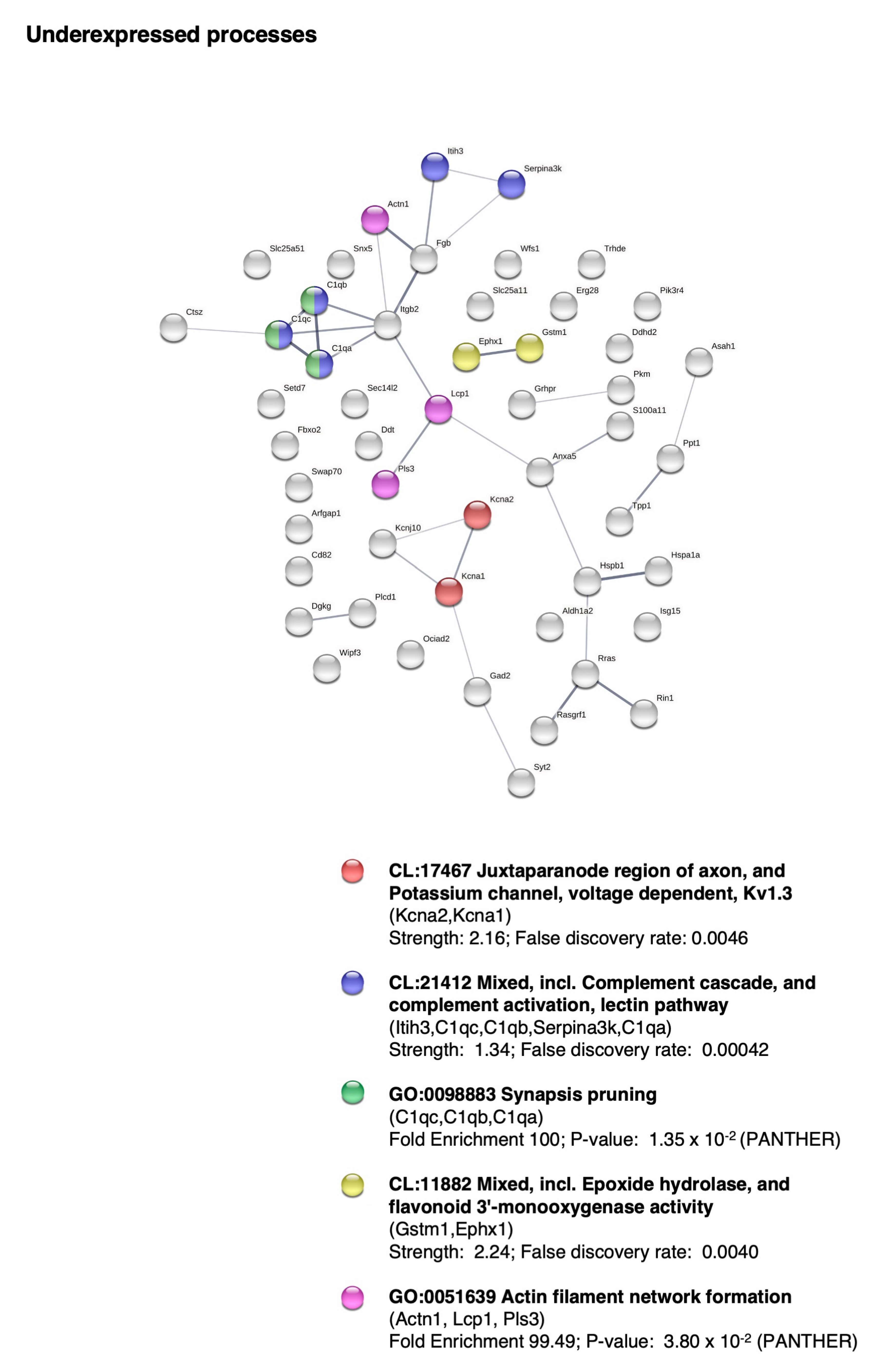
2.5. BM-MSC Treatment Induces Changes in the Proteome Related to Neural Development
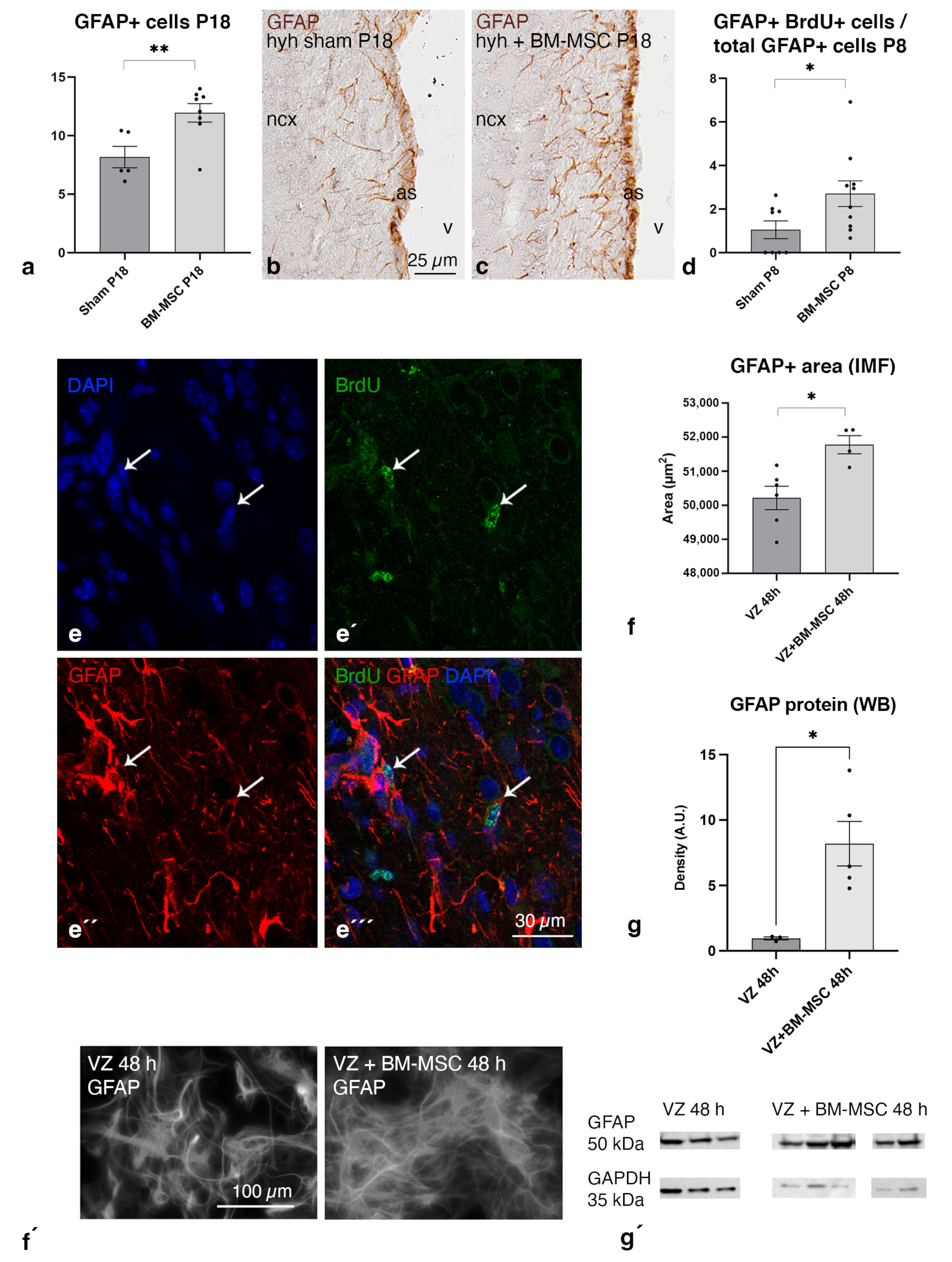
2.6. BM-MSC Treatment Induces the Proliferation of Astrocytes Present in the Periventricular White Matter
2.7. BM-MSCs Stimulate the Astrocyte Proliferation in the Ventricular Zone In Vitro
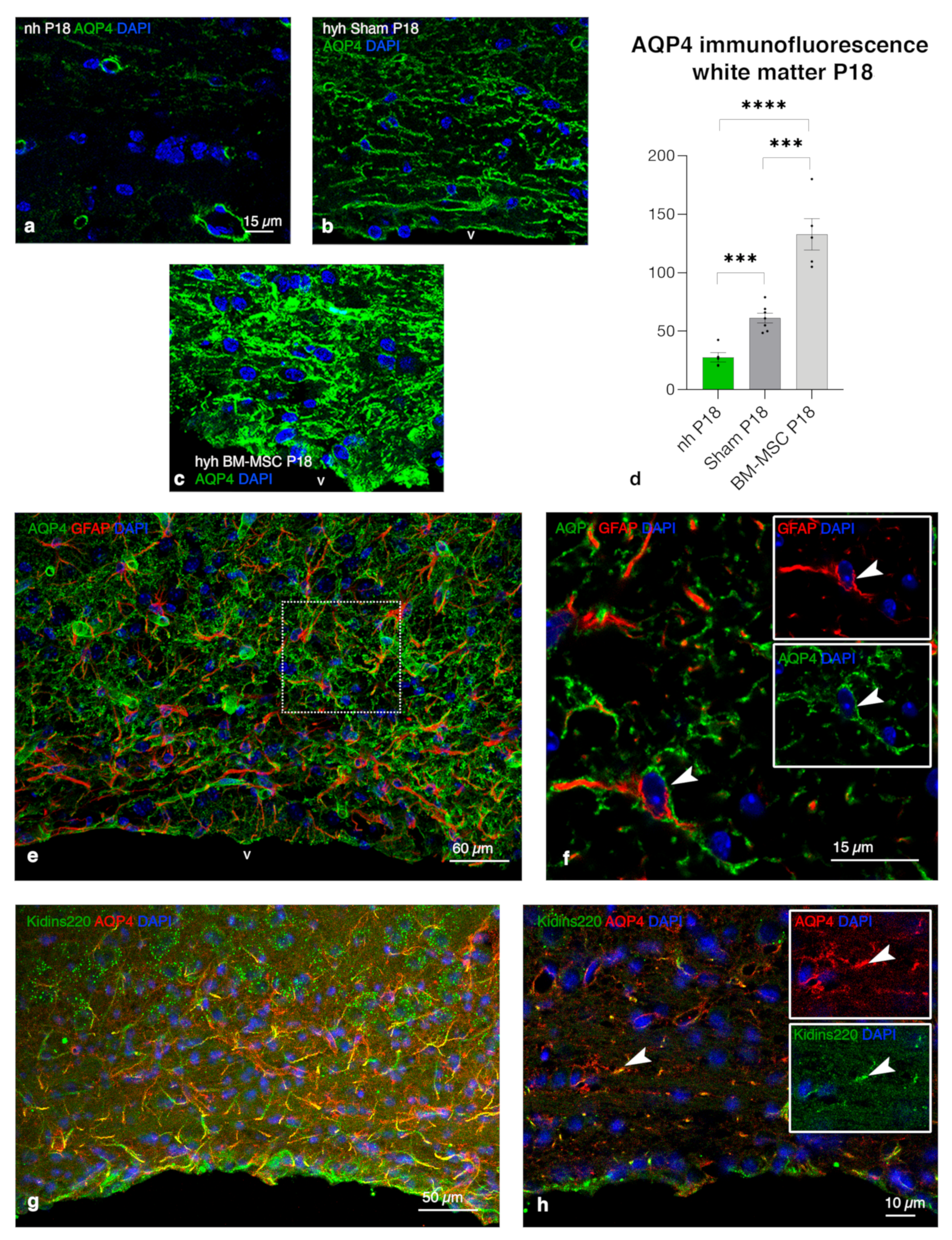
2.8. Upregulation of AQP4 Expression in the Periventricular White Matter of Hydrocephalic Treated Hyh Mice
3. Discussion
3.1. Transplanted BM-MSC Integrated without Rejection into the Periventricular Astrocyte Reaction
3.2. BM-MSCs Stimulate Proliferation of Astrocytes Overexpressing AQP4 in the Periventricular Edema
3.3. An In Vitro Model to Study the Stimulation of Astrocytes by BM-MSCs
3.4. Overexpression of AQP4 as a Potential Neuroprotective Effect on the Periventricular Edema
3.5. BM-MSC Environment Can Play a Neuroprotector Role and Be Implied in AQP4 Upregulation
3.6. Other Neuroprotective Effects of BM-MSCs beyond AQP4 Upregulation
3.7. Limitations of the Study
4. Materials and Methods
4.1. Experimental Animals
4.2. Bone Marrow-Derived Mesenchymal Stem Cells Isolation, Culture, and Characterization
4.3. Bone Marrow-Derived Mesenchymal Stem Cell Transplantation
4.4. Immunofluorescence and Immunocytochemistry
4.5. Bromodeoxyuridine Labeling
4.6. In Vitro Assay
4.7. Real-Time PCR
4.8. Mass Spectrometry Analysis of Proteins
4.9. Image Analysis and Quantification
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahle, K.T.; Kulkarni, A.V.; Limbrick, D.D.; Warf, B.C. Hydrocephalus in Children. Lancet 2016, 387, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Furey, C.G.; Antwi, P.; Kahle, K.T. Congenital Hydrocephalus. In Cerebrospinal Fluid Disorders; Limbrick, D.D., Leonard, J.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 87–113. ISBN 978-3-319-97927-4. [Google Scholar]
- Garcia-Bonilla, M.; McAllister, J.; Limbrick, D. Genetics and Molecular Pathogenesis of Human Hydrocephalus. Neurol. India 2021, 69, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Shirane, R.; Sato, S.; Sato, K.; Kameyama, M.; Ogawa, A.; Yoshimoto, T.; Hatazawa, J.; Ito, M. Cerebral Blood Flow and Oxygen Metabolism in Infants with Hydrocephalus. Childs Nerv. Syst. 1992, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.C. Pathophysiology of Hydrocephalus. In Pediatric Hydrocephalus; Cinalli, G., Sainte-Rose, C., Maixner, W.J., Eds.; Springer Milan: Milano, Italy, 2005; pp. 65–77. ISBN 978-88-470-2173-0. [Google Scholar]
- McAllister, J.P., 2nd. Pathophysiology of Congenital and Neonatal Hydrocephalus. Semin. Fetal Neonatal Med. 2012, 17, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sansone, J.M.; Iskandar, B.J. Endoscopic Cerebral Aqueductoplasty: A Trans-Fourth Ventricle Approach. J. Neurosurg. 2005, 103, 388–392. [Google Scholar] [CrossRef]
- McAllister, J.P.; Williams, M.A.; Walker, M.L.; Kestle, J.R.W.; Relkin, N.R.; Anderson, A.M.; Gross, P.H.; Browd, S.R. Hydrocephalus Symposium Expert Panel An Update on Research Priorities in Hydrocephalus: Overview of the Third National Institutes of Health-Sponsored Symposium “Opportunities for Hydrocephalus Research: Pathways to Better Outcomes”. J. Neurosurg. 2015, 123, 1427–1438. [Google Scholar] [CrossRef] [Green Version]
- Tourdias, T.; Dragonu, I.; Fushimi, Y.; Deloire, M.S.A.; Boiziau, C.; Brochet, B.; Moonen, C.; Petry, K.G.; Dousset, V. Aquaporin 4 Correlates with Apparent Diffusion Coefficient and Hydrocephalus Severity in the Rat Brain: A Combined MRI-Histological Study. Neuroimage 2009, 47, 659–666. [Google Scholar] [CrossRef]
- Skjolding, A.D.; Holst, A.V.; Broholm, H.; Laursen, H.; Juhler, M. Differences in Distribution and Regulation of Astrocytic Aquaporin-4 in Human and Rat Hydrocephalic Brain. Neuropathol. Appl. Neurobiol. 2013, 39, 179–191. [Google Scholar] [CrossRef]
- Skjolding, A.D.; Rowland, I.J.; Søgaard, L.V.; Praetorius, J.; Penkowa, M.; Juhler, M. Hydrocephalus Induces Dynamic Spatiotemporal Regulation of Aquaporin-4 Expression in the Rat Brain. Cereb. Fluid Res. 2010, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Owler, B.K.; Pitham, T.; Wang, D. Aquaporins: Relevance to Cerebrospinal Fluid Physiology and Therapeutic Potential in Hydrocephalus. Cereb. Fluid Res. 2010, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Verkman, A.S.; Tradtrantip, L.; Smith, A.J.; Yao, X. Aquaporin Water Channels and Hydrocephalus. Pediatr. Neurosurg. 2017, 52, 409–416. [Google Scholar] [CrossRef]
- Desai, B.; Hsu, Y.; Schneller, B.; Hobbs, J.G.; Mehta, A.I.; Linninger, A. Hydrocephalus: The Role of Cerebral Aquaporin-4 Channels and Computational Modeling Considerations of Cerebrospinal Fluid. Focus 2016, 41, E8. [Google Scholar] [CrossRef] [Green Version]
- Henzi, R.; Vío, K.; Jara, C.; Johanson, C.E.; McAllister, J.P.; Rodríguez, E.M.; Guerra, M. Neural Stem Cell Therapy of Foetal Onset Hydrocephalus Using the HTx Rat as Experimental Model. Cell Tissue Res. 2020, 381, 141–161. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal Stem Cells Prevent Hydrocephalus after Severe Intraventricular Hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef] [Green Version]
- García-Bonilla, M.; Ojeda-Pérez, B.; García-Martín, M.L.; Muñoz-Hernández, M.C.; Vitorica, J.; Jiménez, S.; Cifuentes, M.; Santos-Ruíz, L.; Shumilov, K.; Claros, S.; et al. Neocortical Tissue Recovery in Severe Congenital Obstructive Hydrocephalus after Intraventricular Administration of Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 121. [Google Scholar] [CrossRef]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal Stem Cell-Derived Factors: Immuno-Modulatory Effects and Therapeutic Potential. Biofactors 2017, 43, 633–644. [Google Scholar] [CrossRef]
- Sordi, V.; Malosio, M.L.; Marchesi, F.; Mercalli, A.; Melzi, R.; Giordano, T.; Belmonte, N.; Ferrari, G.; Leone, B.E.; Bertuzzi, F.; et al. Bone Marrow Mesenchymal Stem Cells Express a Restricted Set of Functionally Active Chemokine Receptors Capable of Promoting Migration to Pancreatic Islets. Blood 2005, 106, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Volkman, R.; Offen, D. Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 2017, 35, 1867–1880. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by Mesenchymal Stem Cells: Mechanisms and Clinical Applications. Stem Cell Res. Ther. 2010, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Bronson, R.T.; Lane, P.W. Hydrocephalus with Hop Gait (Hyh): A New Mutation on Chromosome 7 in the Mouse. Brain Res. Dev. Brain Res. 1990, 54, 131–136. [Google Scholar] [CrossRef]
- Jiménez, A.J.; Tomé, M.; Páez, P.; Wagner, C.; Rodríguez, S.; Fernández-Llebrez, P.; Rodríguez, E.M.; Pérez-Fígares, J.M. A Programmed Ependymal Denudation Precedes Congenital Hydrocephalus in the Hyh Mutant Mouse. J. Neuropathol. Exp. Neurol. 2001, 60, 1105–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C.; Bátiz, L.F.; Rodríguez, S.; Jiménez, A.J.; Páez, P.; Tomé, M.; Pérez-Fígares, J.M.; Rodríguez, E.M. Cellular Mechanisms Involved in the Stenosis and Obliteration of the Cerebral Aqueduct of Hyh Mutant Mice Developing Congenital Hydrocephalus. J. Neuropathol. Exp. Neurol. 2003, 62, 1019–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Páez, P.; Bátiz, L.F.; Roales-Buján, R.; Rodríguez-Pérez, L.M.; Rodríguez, S.; Jiménez, A.J.; Rodríguez, E.M.; Pérez-Fígares, J.M. Patterned Neuropathologic Events Occurring in Hyh Congenital Hydrocephalic Mutant Mice. J. Neuropathol. Exp. Neurol. 2007, 66, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- García-Bonilla, M.; García-Martín, M.L.; Muñoz-Hernández, M.C.; Domínguez-Pinos, D.; Martínez-León, M.I.; Peñalver, A.; Castilla, L.; Alonso, F.J.; Márquez, J.; Shumilov, K.; et al. A Distinct Metabolite Profile Correlates with Neurodegenerative Conditions and the Severity of Congenital Hydrocephalus. J. Neuropathol. Exp. Neurol. 2018, 77, 1122–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roales-Bujan, R.; Páez, P.; Guerra, M.; Rodríguez, S.; Vío, K.; Ho-Plagaro, A.; García-Bonilla, M.; Rodríguez-Pérez, L.M.; Domínguez-Pinos, M.D.; Rodríguez, E.M.; et al. Astrocytes Acquire Morphological and Functional Characteristics of Ependymal Cells Following Disruption of Ependyma in Hydrocephalus. Acta Neuropathol. 2012, 124, 531–546. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Pinos, M.D.; Páez, P.; Jiménez, A.J.; Weil, B.; Arráez, M.A.; Pérez-Fígares, J.M.; Rodríguez, E.M. Ependymal Denudation and Alterations of the Subventricular Zone Occur in Human Fetuses with a Moderate Communicating Hydrocephalus. J. Neuropathol. Exp. Neurol. 2005, 64, 595–604. [Google Scholar] [CrossRef] [Green Version]
- McAllister, J.P.; Guerra, M.M.; Ruiz, L.C.; Jimenez, A.J.; Dominguez-Pinos, D.; Sival, D.; den Dunnen, W.; Morales, D.M.; Schmidt, R.E.; Rodriguez, E.M.; et al. Ventricular Zone Disruption in Human Neonates With Intraventricular Hemorrhage. J. Neuropathol. Exp. Neurol. 2017, 76, 358–375. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.J.; Domínguez-Pinos, M.D.; Guerra, M.M.; Fernández-Llebrez, P.; Pérez-Fígares, J.M. Structure and Function of the Ependymal Barrier and Diseases Associated with Ependyma Disruption. Tissue Barriers 2014, 2, e28426. [Google Scholar] [CrossRef]
- Bátiz, L.F.; Páez, P.; Jiménez, A.J.; Rodríguez, S.; Wagner, C.; Pérez-Fígares, J.M.; Rodríguez, E.M. Heterogeneous Expression of Hydrocephalic Phenotype in the Hyh Mice Carrying a Point Mutation in Alpha-SNAP. Neurobiol. Dis. 2006, 23, 152–168. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Zacharek, A.; Chen, J.; Li, A.; Cui, X.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/TIE2 and VEGF/FLK1 Induced by MSC Treatment Amplifies Angiogenesis and Vascular Stabilization after Stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.P.; Matias, I.; Siqueira, M.; Stipursky, J.; Gomes, F.C.A. Astrocytes and the TGF-Β1 Pathway in the Healthy and Diseased Brain: A Double-Edged Sword. Mol. Neurobiol. 2019, 56, 4653–4679. [Google Scholar] [CrossRef]
- Castaneyra-Ruiz, L.; McAllister, J.P.; Morales, D.M.; Brody, S.L.; Isaacs, A.M.; Limbrick, D.D. Preterm Intraventricular Hemorrhage in Vitro: Modeling the Cytopathology of the Ventricular Zone. Fluids Barriers CNS 2020, 17, 46. [Google Scholar] [CrossRef]
- Del Puerto, A.; Pose-Utrilla, J.; Simón-García, A.; López-Menéndez, C.; Jiménez, A.J.; Porlan, E.; Pajuelo, L.S.M.; Cano-García, G.; Martí-Prado, B.; Sebastián-Serrano, Á.; et al. Kidins220 Deficiency Causes Ventriculomegaly via SNX27-Retromer-Dependent AQP4 Degradation. Mol. Psychiatry 2021, 26, 6411–6426. [Google Scholar] [CrossRef]
- Del Bigio, M.R. Calcium-Mediated Proteolytic Damage in White Matter of Hydrocephalic Rats? J. Neuropathol. Exp. Neurol. 2000, 59, 946–954. [Google Scholar] [CrossRef] [Green Version]
- Braun, K.P.; Dijkhuizen, R.M.; de Graaf, R.A.; Nicolay, K.; Vandertop, W.P.; Gooskens, R.H.; Tulleken, K.A. Cerebral Ischemia and White Matter Edema in Experimental Hydrocephalus: A Combined in Vivo MRI and MRS Study. Brain Res. 1997, 757, 295–298. [Google Scholar] [CrossRef]
- Castejón, O.J. Submicroscopic Pathology of Human and Experimental Hydrocephalic Cerebral Cortex. Folia Neuropathol. 2010, 48, 159–174. [Google Scholar]
- Jiménez, A.J.; Rodríguez-Pérez, L.M.; Domínguez-Pinos, M.D.; Gómez-Roldán, M.C.; García-Bonilla, M.; Ho-Plagaro, A.; Roales-Buján, R.; Jiménez, S.; Roquero-Mañueco, M.C.; Martínez-León, M.I.; et al. Increased Levels of Tumour Necrosis Factor Alpha (TNFα) but Not Transforming Growth Factor-Beta 1 (TGFβ1) Are Associated with the Severity of Congenital Hydrocephalus in the Hyh Mouse. Neuropathol. Appl. Neurobiol. 2014, 40, 911–932. [Google Scholar] [CrossRef]
- Jiménez, A.J.; García-Verdugo, J.M.; González, C.A.; Bátiz, L.F.; Rodríguez-Pérez, L.M.; Páez, P.; Soriano-Navarro, M.; Roales-Buján, R.; Rivera, P.; Rodríguez, S.; et al. Disruption of the Neurogenic Niche in the Subventricular Zone of Postnatal Hydrocephalic Hyh Mice. J. Neuropathol. Exp. Neurol. 2009, 68, 1006–1020. [Google Scholar] [CrossRef] [Green Version]
- Pirzad Jahromi, G.; Shabanzadeh Pirsaraei, A.; Sadr, S.S.; Kaka, G.; Jafari, M.; Seidi, S.; Charish, J. Multipotent Bone Marrow Stromal Cell Therapy Promotes Endogenous Cell Proliferation Following Ischemic Stroke. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1158–1167. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.-X.; Liang, H.; Xi, Z.; Pan, J.; Yang, Y.; Sun, Q.; Yang, G.; Sun, Y.; Bian, L. Bone Marrow Mesenchymal Stem Cells Transplantation Alleviates Brain Injury after Intracerebral Hemorrhage in Mice through the Hippo Signaling Pathway. Aging 2020, 12, 6306–6323. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Li, Y.; Chopp, M. Astrocytic Endogenous Glial Cell Derived Neurotrophic Factor Production Is Enhanced by Bone Marrow Stromal Cell Transplantation in the Ischemic Boundary Zone after Stroke in Adult Rats. Glia 2010, 58, 1074–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippidis, A.S.; Kalani, M.Y.S.; Rekate, H.L. Hydrocephalus and Aquaporins: Lessons Learned from the Bench. Child’s Nerv. Syst. 2011, 27, 27–33. [Google Scholar] [CrossRef]
- Guo, J.; Mi, X.; Zhan, R.; Li, M.; Wei, L.; Sun, J. Aquaporin 4 Silencing Aggravates Hydrocephalus Induced by Injection of Autologous Blood in Rats. Med. Sci. Monit. 2018, 24, 4204–4212. [Google Scholar] [CrossRef]
- Castaneyra-Ruiz, L.; Morales, D.M.; McAllister, J.P.; Brody, S.L.; Isaacs, A.M.; Strahle, J.M.; Dahiya, S.M.; Limbrick, D.D. Blood Exposure Causes Ventricular Zone Disruption and Glial Activation In Vitro. J. Neuropathol. Exp. Neurol. 2018, 77, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Yang, G.-Y. Aquaporin-4: A Potential Therapeutic Target for Cerebral Edema. Int. J. Mol. Sci. 2016, 17, 1413. [Google Scholar] [CrossRef] [Green Version]
- Milhorat, T.H. Classification of the Cerebral Edemas with Reference to Hydrocephalus and Pseudotumor Cerebri. Child’s Nerv. Syst. 1992, 8, 301–306. [Google Scholar] [CrossRef]
- Iencean, S.M. Brain Edema—A New Classification. Med. Hypotheses 2003, 61, 106–109. [Google Scholar] [CrossRef]
- Das, M.; Mayilsamy, K.; Mohapatra, S.S.; Mohapatra, S. Mesenchymal Stem Cell Therapy for the Treatment of Traumatic Brain Injury: Progress and Prospects. Rev. Neurosci. 2019, 30, 839–855. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Ma, X.-L.; Wang, J.; Zhao, L.-J.; Du, L.; Wang, L.-Y.; Wang, X.-R.; Liu, K.-D. Effect of Bone Marrow Stromal Cell Transplantation on Neurologic Function and Expression of VEGF in Rats with Focal Cerebral Ischemia. Mol. Med. Rep. 2014, 10, 2299–2305. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.S.; Ahn, S.Y.; Jeon, H.B.; Sung, D.K.; Kim, E.S.; Sung, S.I.; Yoo, H.S.; Choi, S.J.; Oh, W.I.; Park, W.S. Critical Role of Vascular Endothelial Growth Factor Secreted by Mesenchymal Stem Cells in Hyperoxic Lung Injury. Am. J. Respir. Cell Mol. Biol. 2014, 51, 391–399. [Google Scholar] [CrossRef]
- Chuang, T.J.; Lin, K.C.; Chio, C.C.; Wang, C.C.; Chang, C.P.; Kuo, J.R. Effects of Secretome Obtained from Normoxia-Preconditioned Human Mesenchymal Stem Cells in Traumatic Brain Injury Rats. J. Trauma Acute Care Surg. 2012, 73, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.R.; Suh, H.; Yu, J.H.; Kim, H.H.; Seo, J.H.; Seo, C.H. Astroglial Activation by an Enriched Environment after Transplantation of Mesenchymal Stem Cells Enhances Angiogenesis after Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2016, 17, 1550. [Google Scholar] [CrossRef] [Green Version]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A Review of Therapeutic Effects of Mesenchymal Stem Cell Secretions and Induction of Secretory Modification by Different Culture Methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Pisani, F.; Cammalleri, M.; Dal Monte, M.; Locri, F.; Mola, M.G.; Nicchia, G.P.; Frigeri, A.; Bagnoli, P.; Svelto, M. Potential Role of the Methylation of VEGF Gene Promoter in Response to Hypoxia in Oxygen-Induced Retinopathy: Beneficial Effect of the Absence of AQP4. J. Cell. Mol. Med. 2018, 22, 613–627. [Google Scholar] [CrossRef]
- Kaur, C.; Sivakumar, V.; Yong, Z.; Lu, J.; Foulds, W.S.; Ling, E.A. Blood-Retinal Barrier Disruption and Ultrastructural Changes in the Hypoxic Retina in Adult Rats: The Beneficial Effect of Melatonin Administration. J. Pathol. 2007, 212, 429–439. [Google Scholar] [CrossRef]
- Kaur, C.; Sivakumar, V.; Zhang, Y.; Ling, E.A. Hypoxia-Induced Astrocytic Reaction and Increased Vascular Permeability in the Rat Cerebellum. Glia 2006, 54, 826–839. [Google Scholar] [CrossRef]
- Zou, Y.Y.; Lu, J.; Poon, D.J.F.; Kaur, C.; Cao, Q.; Teo, A.L.; Ling, E.A. Combustion Smoke Exposure Induces Up-Regulated Expression of Vascular Endothelial Growth Factor, Aquaporin 4, Nitric Oxide Synthases and Vascular Permeability in the Retina of Adult Rats. Neuroscience 2009, 160, 698–709. [Google Scholar] [CrossRef]
- Do, P.T.; Wu, C.-C.; Chiang, Y.-H.; Hu, C.-J.; Chen, K.-Y. Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects. Int. J. Mol. Sci. 2021, 22, 10045. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chen, Y.; Li, L.; Jiang, J.; Wu, G.; Zuo, Y.; Zhang, J.H.; Feng, H.; Yan, X.; Liu, F. Decorin Alleviated Chronic Hydrocephalus via Inhibiting TGF-Β1/Smad/CTGF Pathway after Subarachnoid Hemorrhage in Rats. Brain Res. 2016, 1630, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Botfield, H.; Gonzalez, A.M.; Abdullah, O.; Skjolding, A.D.; Berry, M.; McAllister, J.P.; Logan, A. Decorin Prevents the Development of Juvenile Communicating Hydrocephalus. Brain 2013, 136, 2842–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazawa, K.; Tada, T. Elevation of Transforming Growth Factor-Beta 1 Level in Cerebrospinal Fluid of Patients with Communicating Hydrocephalus after Subarachnoid Hemorrhage. Stroke 1994, 25, 1400–1404. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Pattisapu, J.; Tarnuzzer, R.; Fernandez-Valle, C.; Gibson, J. TGF-Β1 Expression Is Reduced in Hydrocephalic H-Tx Rat Brain. Eur. J. Pediatr. Surg. 1999, 9, 35–38. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Fathali, N.; Doyle, K.P.; Williams, A.M.; Han, J.; Buckwalter, M.S. Astrocytic Transforming Growth Factor-Beta Signaling Reduces Subacute Neuroinflammation after Stroke in Mice: Astrocytic TGFβ Reduces Neuroinflammation. Glia 2014, 62, 1227–1240. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yang, G.-Y.; Ahlemeyer, B.; Pang, L.; Che, X.-M.; Culmsee, C.; Klumpp, S.; Krieglstein, J. Transforming Growth Factor-Β1 Increases Bad Phosphorylation and Protects Neurons Against Damage. J. Neurosci. 2002, 22, 3898–3909. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Fahnestock, M. Differential Expression of Nerve Growth Factor Transcripts in Glia and Neurons and Their Regulation by Transforming Growth Factor-Β1. Mol. Brain Res. 2002, 105, 115–125. [Google Scholar] [CrossRef]
- Semkova, I.; Krieglstein, J. Neuroprotection Mediated via Neurotrophic Factors and Induction of Neurotrophic Factors. Brain Res. Rev. 1999, 30, 176–188. [Google Scholar] [CrossRef]
- Saher, G.; Stumpf, S.K. Cholesterol in Myelin Biogenesis and Hypomyelinating Disorders. Biochim. Biophys. Acta 2015, 1851, 1083–1094. [Google Scholar] [CrossRef]
- Bashaw, G.J.; Klein, R. Signaling from Axon Guidance Receptors. Cold Spring Harb. Perspect. Biol. 2010, 2, a001941. [Google Scholar] [CrossRef] [Green Version]
- Huber, A.B.; Kolodkin, A.L.; Ginty, D.D.; Cloutier, J.F. Signaling at the Growth Cone: Ligand-Receptor Complexes and the Control of Axon Growth and Guidance. Annu. Rev. Neurosci. 2003, 26, 509–563. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Liao, T.; Liu, J.; Fan, Z.; Zeng, Q.; Zhou, J.; Pei, H.; Xi, J.; He, L.; Chen, L.; et al. Clinical-Grade Human Umbilical Cord-Derived Mesenchymal Stem Cells Reverse Cognitive Aging via Improving Synaptic Plasticity and Endogenous Neurogenesis. Cell Death Dis. 2017, 8, e2996. [Google Scholar] [CrossRef] [Green Version]
- Batiz, L.F.; Roales-Buján, R.; Rodríguez-Pérez, L.M.; Matas, I.M.; Páez, P.; Roque, M.; Jiménez, A.J.; Ramos, C.; Pérez-Fígares, J.M. A Simple PCR-Based Genotyping Method for M105I Mutation of Alpha-SNAP Enhances the Study of Early Pathological Changes in Hyh Phenotype. Mol. Cell. Probes 2009, 23, 281–290. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]


| Protein Name | Sum PEP Score | AR | AR p Value |
|---|---|---|---|
| 28S ribosomal protein S7. mitochondrial | 19.568 | 1.666 | 0.008 |
| 39S ribosomal protein L3. mitochondrial | 9.29 | 1.598 | 2.701 × 10−5 |
| Amyloid-beta A4 precursor protein-binding family A member 1 | 59.91 | 1.691 | 0.0002 |
| Bisphosphoglycerate mutase | 14.636 | 1.905 | 0.004 |
| Claudin domain-containing protein 1 | 14.01 | 1.907 | 0.019 |
| Cold-inducible RNA-binding protein | 36.025 | 2.136 | 0.011 |
| Constitutive coactivator of peroxisome proliferator-activated receptor gamma | 8.253 | 1.749 | 0.019 |
| Coxsackievirus and adenovirus receptor homolog | 110.023 | 1.743 | 0.003 |
| DNA topoisomerase 2-beta | 98.163 | 1.557 | 0.007 |
| DNA topoisomerase 3-beta-1 | 5.518 | 1.95 | 0.012 |
| Dynein light chain Tctex-type 1 | 17.169 | 1.734 | 0.013 |
| Electroneutral sodium bicarbonate exchanger 1 | 90.13 | 1.566 | 0.023 |
| Ephrin-B3 | 12.103 | 2.089 | 0.0002 |
| Fatty acyl-CoA reductase 1 | 10.939 | 2.961 | 0.002 |
| Gigaxonin (OS = Mus musculus) | 13.586 | 1.506 | 0.012 |
| GPI inositol-deacylase | 68.486 | 1.318 | 0.008 |
| Guanine nucleotide exchange factor DBS | 6.267 | 2.914 | 0.028 |
| Interferon-inducible double-stranded RNA-dependent protein kinase activator A | 44.187 | 1.548 | 0.003 |
| Isoform 2 of peroxisomal acyl-coenzyme A oxidase 1 | 149.85 | 1.749 | 0.001 |
| KIF1-binding protein | 88.274 | 1.865 | 0.002 |
| Kinesin-like protein KIF21B | 169.817 | 2.724 | 0.0002 |
| La-related protein 4B | 9.525 | 1.557 | 0.019 |
| MARCKS-related protein | 58.881 | 1.753 | 0.001 |
| Mitofusin-1 | 6.777 | 1.538 | 0.034 |
| Neuron navigator 1 | 245.817 | 1.6 | 0.003 |
| Neuronal migration protein doublecortin | 63.588 | 2.29 | 0.0005 |
| Nuclear autoantigenic sperm protein | 20.927 | 1.73 | 0.016 |
| Nuclease-sensitive element-binding protein 1 | 137.43 | 1.675 | 0.037 |
| Poly [ADP-ribose] polymerase 1 | 24.147 | 2.214 | 0.003 |
| Protein phosphatase 1 regulatory subunit 14C | 12.2 | 1.893 | 0.012 |
| Shootin-1 | 39.062 | 1.601 | 0.005 |
| Synaptotagmin-11 | 15.866 | 1.588 | 0.016 |
| Tetratricopeptide repeat protein 9B | 29.372 | 1.846 | 0.035 |
| Transcription factor BTF3 | 15.06 | 1.679 | 0.012 |
| Translation initiation factor eIF-2B subunit delta | 27.65 | 1.557 | 0.023 |
| Tubulin beta-6 chain | 296.149 | 2.272 | 0.001 |
| U4/U6 small nuclear ribonucleoprotein Prp3 | 11.174 | 2.032 | 0.003 |
| Protein | Sum PEP Score | AR | AR p Value |
|---|---|---|---|
| ATP-sensitive inward rectifier potassium channel 10 | 19.789 | 0.563 | 0.0008 |
| cAMP-specific 3′.5′-cyclic phosphodiesterase 4A | 41.45 | 0.576 | 0.014 |
| Cathepsin Z | 13.292 | 0.382 | 0.031 |
| Complement C1q subcomponent subunit A | 22.698 | 0.466 | 0.002 |
| Complement C1q subcomponent subunit B | 30.001 | 0.701 | 0.014 |
| Complement C1q subcomponent subunit C | 30.796 | 0.419 | 0.002 |
| Fibrinogen beta chain | 190.931 | 0.498 | 0.02 |
| Fibrinogen gamma chain | 108.708 | 0.442 | 0.013 |
| Heat shock protein beta-1 | 31.513 | 0.383 | 0.01 |
| Inter-alpha-trypsin inhibitor heavy chain H3 | 17.338 | 0.444 | 0.008 |
| Probable ergosterol biosynthetic protein 28 | 4.042 | 0.205 | 0.024 |
| Protein S100-A11 | 8.482 | 0.507 | 0.049 |
| Serine protease inhibitor A3K | 117.573 | 0.332 | 0.004 |
| Switch-associated protein 70 | 7.563 | 0.464 | 0.009 |
| Antibody | Source, Reference | Type | Dilution, Use |
|---|---|---|---|
| AQP4 | Sigma-Aldrich, A5971 | Rabbit polyclonal | 1:200, I, WB |
| AQP4 | Sigma-Aldrich AMAB90537 | Mouse monoclonal | 1:100, I |
| BDNF | Abcam, ab108319 | Rabbit monoclonal | 1:250, I |
| BrdU | DHSB, G3G4 | Mouse monoclonal | 1:1000, I |
| GAPDH | Abcam, ab9485 | Rabbit polyclonal | 1:1000, WB |
| GAPDH | Cell Signaling, 5174S | Rabbit monoclonal | 1:500, WB |
| GDNF | Santa Cruz, sc-328 | Rabbit polyclonal | 1:100, I |
| GFAP | Sigma-Aldrich, G-A-5 | Mouse monoclonal | 1:1000, I |
| GFAP | Sigma-Aldrich, C9205 | Mouse monoclonal (Cy3 conjugate) | 1:1000, WB |
| GFAP | Abcam, ab7260 | Rabbit polyclonal | 1:400, I in vitro |
| δ-GFAP | Merck Millipore, AB9598 | Rabbit polyclonal | 1:500, I |
| Kidins220 | Teresa Iglesias [36] | Rabbit polyclonal (TIV24 batch) | 1:500, I |
| NG2 | Abcam, ab5320 | Rabbit polyclonal | 1:200, I |
| NGF | Abcam, ab6199 | Rabbit polyclonal | 1:500, I |
| Rat-401 (nestin) | DHSB, AB 2235915 | Mouse monoclonal | 1:100, I |
| mRFP1 | Chromotek, 5F8 | Rat monoclonal | 1:500, I |
| β–III tubulin | Promega, A6712 | Mouse monoclonal | 1:5000, I |
| VEGF | Abcam, ab46154 | Rabbit polyclonal | 1:1000, I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Bonilla, M.; Ojeda-Pérez, B.; Shumilov, K.; Rodríguez-Pérez, L.-M.; Domínguez-Pinos, D.; Vitorica, J.; Jiménez, S.; Ramírez-Lorca, R.; Echevarría, M.; Cárdenas-García, C.; et al. Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy. Int. J. Mol. Sci. 2023, 24, 5640. https://doi.org/10.3390/ijms24065640
García-Bonilla M, Ojeda-Pérez B, Shumilov K, Rodríguez-Pérez L-M, Domínguez-Pinos D, Vitorica J, Jiménez S, Ramírez-Lorca R, Echevarría M, Cárdenas-García C, et al. Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy. International Journal of Molecular Sciences. 2023; 24(6):5640. https://doi.org/10.3390/ijms24065640
Chicago/Turabian StyleGarcía-Bonilla, María, Betsaida Ojeda-Pérez, Kirill Shumilov, Luis-Manuel Rodríguez-Pérez, Dolores Domínguez-Pinos, Javier Vitorica, Sebastián Jiménez, Reposo Ramírez-Lorca, Miriam Echevarría, Casimiro Cárdenas-García, and et al. 2023. "Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy" International Journal of Molecular Sciences 24, no. 6: 5640. https://doi.org/10.3390/ijms24065640
APA StyleGarcía-Bonilla, M., Ojeda-Pérez, B., Shumilov, K., Rodríguez-Pérez, L.-M., Domínguez-Pinos, D., Vitorica, J., Jiménez, S., Ramírez-Lorca, R., Echevarría, M., Cárdenas-García, C., Iglesias, T., Gutiérrez, A., McAllister, J. P., II, Limbrick, D. D., Jr., Páez-González, P., & Jiménez, A. J. (2023). Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy. International Journal of Molecular Sciences, 24(6), 5640. https://doi.org/10.3390/ijms24065640