Urinary Biomarkers in a Living Donor Kidney Transplantation Cohort—Predictive Value on Graft Function
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
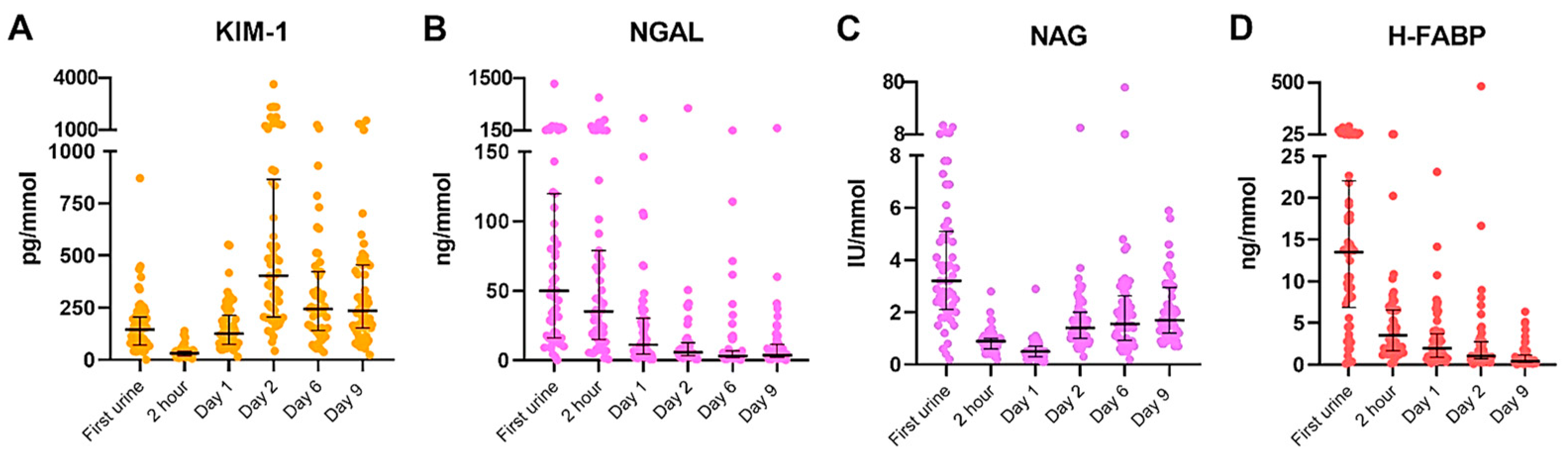
2.2. Dynamics of Urinary Biomarkers in Recipients after Transplantation
2.3. The Variability between Subjects of Urinary Biomarkers after Transplantation
2.4. The Additional Value of Urinary Biomarkers to Prediction Models for Renal Outcome
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Outcome Measures
4.3. Timepoints
4.4. Measurement of Urinary Biomarkers
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponticelli, C. Ischaemia-reperfusion injury: A major protagonist in kidney transplantation. Nephrol. Dial. Transplant. 2014, 29, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E.; Wiseman, A.C. Acute kidney injury in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2013, 22, 698–703. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World. J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N., Jr.; Poggio, E.D.; Parikh, C.R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2009, 24, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.Y.; Jeong, J.C.; Lee, Y.; Ko, K.P.; Lee, K.B.; Lee, S.; Park, S.J.; Park, J.B.; Han, M.; Lim, H.J.; et al. Pre-transplant Evaluation of Donor Urinary Biomarkers can Predict Reduced Graft Function After Deceased Donor Kidney Transplantation. Medicine 2016, 95, e3076. [Google Scholar] [CrossRef]
- Malyszko, J.; Lukaszyk, E.; Glowinska, I.; Durlik, M. Biomarkers of delayed graft function as a form of acute kidney injury in kidney transplantation. Sci. Rep. 2015, 5, 11684. [Google Scholar] [CrossRef] [PubMed]
- Halawa, A. The early diagnosis of acute renal graft dysfunction: A challenge we face. The role of novel biomarkers. Ann. Transplant. 2011, 16, 90–98. [Google Scholar]
- Kers, J.; Peters-Sengers, H.; Heemskerk, M.B.A.; Berger, S.P.; Betjes, M.G.H.; van Zuilen, A.D.; Hilbrands, L.B.; de Fijter, J.W.; Nurmohamed, A.S.; Christiaans, M.H.; et al. Prediction models for delayed graft function: External validation on The Dutch Prospective Renal Transplantation Registry. Nephrol. Dial. Transplant. 2020, 35, 1277. [Google Scholar] [CrossRef]
- Vanmassenhove, J.; Vanholder, R.; Nagler, E.; Van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol. Dial. Transplant. 2013, 28, 254–273. [Google Scholar] [CrossRef]
- Beker, B.M.; Corleto, M.G.; Fieiras, C.; Musso, C.G. Novel acute kidney injury biomarkers: Their characteristics, utility and concerns. Int. Urol. Nephrol. 2018, 50, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Obermuller, N.; Geiger, H.; Weipert, C.; Urbschat, A. Current developments in early diagnosis of acute kidney injury. Int. Urol. Nephrol. 2014, 46, 1–7. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, Y.; Lv, J.; Zhang, H.; Tang, J.; Gunaratnam, L.; Li, X.; Yang, L. Kidney injury molecule-1 expression in IgA nephropathy and its correlation with hypoxia and tubulointerstitial inflammation. Am. J. Physiol. Renal. Physiol. 2014, 306, F885–F895. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Domanski, L.; Bober, J.; Kloda, K.; Safranow, K.; Szymanska-Pasternak, J.; Romanowski, M.; Sulecka, A.; Pawlik, A.; Ciechanowski, K. N-acetyl-beta-glucosaminidase urine activity as a marker of early proximal tubule damage and a predictor of the long-term function of the transplanted kidneys. Acta Biochim. Pol. 2014, 61, 275–280. [Google Scholar] [CrossRef]
- Kotanko, P.; Margreiter, R.; Pfaller, W. Reduced renal allograft survival is related to low urinary N-acetyl-beta-D-glucosaminidase excretion during the first posttransplant month. Transplantation 1996, 61, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Cappuccilli, M.; Capelli, I.; Comai, G.; Cianciolo, G.; La Manna, G. Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Allograft Function After Renal Transplantation: Evaluation of the Current Status and Future Insights. Artif. Organs 2018, 42, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Lerut, E.; van Pelt, J.; Monbaliu, D.; Pirenne, J. Circulating AST, H-FABP, and NGAL are early and accurate biomarkers of graft injury and dysfunction in a preclinical model of kidney transplantation. Ann. Surg. 2011, 254, 784–791. [Google Scholar] [CrossRef]
- Bank, J.R.; van der Pol, P.; Vreeken, D.; Monge-Chaubo, C.; Bajema, I.M.; Schlagwein, N.; van Gijlswijk, D.J.; van der Kooij, S.W.; Reinders, M.E.J.; de Fijter, J.W.; et al. Kidney injury molecule-1 staining in renal allograft biopsies 10 days after transplantation is inversely correlated with functioning proximal tubular epithelial cells. Nephrol. Dial. Transplant. 2017, 32, 2132–2141. [Google Scholar] [CrossRef]
- Schroppel, B.; Kruger, B.; Walsh, L.; Yeung, M.; Harris, S.; Garrison, K.; Himmelfarb, J.; Lerner, S.M.; Bromberg, J.S.; Zhang, P.L.; et al. Tubular expression of KIM-1 does not predict delayed function after transplantation. J. Am. Soc. Nephrol. 2010, 21, 536–542. [Google Scholar] [CrossRef]
- Van Timmeren, M.M.; Vaidya, V.S.; van Ree, R.M.; Oterdoom, L.H.; de Vries, A.P.; Gans, R.O.; van Goor, H.; Stegeman, C.A.; Bonventre, J.V.; Bakker, S.J. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 2007, 84, 1625–1630. [Google Scholar] [CrossRef]
- Zhang, P.L.; Rothblum, L.I.; Han, W.K.; Blasick, T.M.; Potdar, S.; Bonventre, J.V. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 2008, 73, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shao, X.; Xie, Y.; Wang, Q.; Che, X.; Zhang, M.; Xu, W.; Xu, Y.; Mou, S.; Ni, Z. Kidney Injury Molecule-1 is Elevated in Nephropathy and Mediates Macrophage Activation via the Mapk Signalling Pathway. Cell Physiol. Biochem. 2017, 41, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Xu, F.; Sabbisetti, V.; Grgic, I.; Movahedi Naini, S.; Wang, N.; Chen, G.; Xiao, S.; Patel, D.; Henderson, J.M.; et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Investig. 2013, 123, 4023–4035. [Google Scholar] [CrossRef]
- Gandhi, R.; Yi, J.; Ha, J.; Shi, H.; Ismail, O.; Nathoo, S.; Bonventre, J.V.; Zhang, X.; Gunaratnam, L. Accelerated receptor shedding inhibits kidney injury molecule-1 (KIM-1)-mediated efferocytosis. Am. J. Physiol. Renal. Physiol. 2014, 307, F205–F221. [Google Scholar] [CrossRef]
- Brooks, C.R.; Bonventre, J.V. KIM-1/TIM-1 in proximal tubular cell immune response. Oncotarget 2015, 6, 44059–44060. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.R.; Yeung, M.Y.; Brooks, Y.S.; Chen, H.; Ichimura, T.; Henderson, J.M.; Bonventre, J.V. KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015, 34, 2441–2464. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Kidney injury molecule-1. Curr. Opin. Crit. Care 2010, 16, 556–561. [Google Scholar] [CrossRef]
- Yang, L.; Brooks, C.R.; Xiao, S.; Sabbisetti, V.; Yeung, M.Y.; Hsiao, L.L.; Ichimura, T.; Kuchroo, V.; Bonventre, J.V. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J. Clin. Investig. 2015, 125, 1620–1636. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ismail, O.Z.; Zhang, X.; Haig, A.; Lian, D.; Gunaratnam, L. Donor kidney injury molecule-1 promotes graft recovery by regulating systemic necroinflammation. Am. J. Transplant. 2018, 18, 2021–2028. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, C.X. Kidney injury molecule-1 (KIM-1) mediates renal epithelial cell repair via ERK MAPK signaling pathway. Mol. Cell Biochem. 2016, 416, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kuzniar, J.; Marchewka, Z.; Krasnowski, R.; Boratynska, M.; Dlugosz, A.; Klinger, M. Enzymuria and low molecular weight protein excretion as the differentiating marker of complications in the early post kidney transplantation period. Int. Urol. Nephrol. 2006, 38, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Kind, P.R. N-Acetyl-beta-D-glucosaminidase in urine of patients with renal disease, and after renal transplants and surgery. Clin. Chim. Acta 1982, 119, 89–97. [Google Scholar] [CrossRef]
- De Muro, P.; Faedda, R.; Masala, A.; Lepedda, A.J.; Zinellu, E.; Ciccarese, M.; Cossu, M.; Pala, P.G.; Satta, R.P.; Formato, M. Kidney post-transplant monitoring of urinary glycosaminoglycans/proteoglycans and monokine induced by IFN-gamma (MIG). Clin. Exp. Med. 2013, 13, 59–65. [Google Scholar] [CrossRef]
- Nauta, F.L.; Bakker, S.J.; van Oeveren, W.; Navis, G.; van der Heide, J.J.; van Goor, H.; de Jong, P.E.; Gansevoort, R.T. Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am. J. Kidney Dis. 2011, 57, 733–743. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand. J. Clin. Lab. Investig. 2008, 68 (Suppl. S241), 89–94. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, S.; Ghamdi, G.; Jaradat, M.; Tamim, H.; Aljumah, A.; Tamimi, W.; Al Dawood, A.; Binsalih, S.; Al Sayyari, A. Urinary neutrophil gelatinase-associated lipocalin and the occurrence of delayed graft function after kidney transplant. Exp. Clin. Transplant. 2014, 12, 396–400. [Google Scholar] [PubMed]
- Parikh, C.R.; Jani, A.; Mishra, J.; Ma, Q.; Kelly, C.; Barasch, J.; Edelstein, C.L.; Devarajan, P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am. J. Transplant. 2006, 6, 1639–1645. [Google Scholar] [CrossRef]
- Ramirez-Sandoval, J.C.; Herrington, W.; Morales-Buenrostro, L.E. Neutrophil gelatinase-associated lipocalin in kidney transplantation: A review. Transplant. Rev. 2015, 29, 139–144. [Google Scholar] [CrossRef]
- Buemi, A.; Musuamba, F.; Frederic, S.; Douhet, A.; De Meyer, M.; De Pauw, L.; Darius, T.; Kanaan, N.; Wallemacq, P.; Mourad, M. Is plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) determination in donors and recipients predictive of renal function after kidney transplantation? Clin. Biochem. 2014, 47, 68–72. [Google Scholar] [CrossRef]
- Schaub, J.A.; Garg, A.X.; Coca, S.G.; Testani, J.M.; Shlipak, M.G.; Eikelboom, J.; Kavsak, P.; McArthur, E.; Shortt, C.; Whitlock, R.; et al. Perioperative heart-type fatty acid binding protein is associated with acute kidney injury after cardiac surgery. Kidney Int. 2015, 88, 576–583. [Google Scholar] [CrossRef]
- Coffman, D.J.; Jay, C.L.; Sharda, B.; Garner, M.; Farney, A.C.; Orlando, G.; Reeves-Daniel, A.; Mena-Gutierrez, A.; Sakhovskaya, N.; Stratta, R., Jr.; et al. Influence of donor and recipient sex on outcomes following simultaneous pancreas-kidney transplantation in the new millennium: Single-center experience and review of the literature. Clin. Transplant. 2023, 37, e14864. [Google Scholar] [CrossRef] [PubMed]
- Lepeytre, F.; Dahhou, M.; Zhang, X.; Boucquemont, J.; Sapir-Pichhadze, R.; Cardinal, H.; Foster, B.J. Association of Sex with Risk of Kidney Graft Failure Differs by Age. J. Am. Soc. Nephrol. 2017, 28, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Nieuwenhuijs, V.B.; Seelen, M.A.J.; Berger, S.P.; van den Heuvel, M.C.; Burgerhof, J.G.M.; Ottens, P.J.; Ploeg, R.J.; Leuvenink, H.G.D.; Struys, M. Propofol-based anaesthesia versus sevoflurane-based anaesthesia for living donor kidney transplantation: Results of the VAPOR-1 randomized controlled trial. Br. J. Anaesth. 2017, 118, 720–732. [Google Scholar] [CrossRef] [PubMed]

| Donor | n = 57 |
| Age [y] | 52.3 (±11.0) |
| Male [n(%)] | 26 (45.6%) |
| BMI [kg/m2] | 27 (±3.2) |
| Active smokers [n(%)] | 16 (28.1%) |
| Cardiovascular comorbidity [n(%)] | 17 (29.8%) |
| Medication use [n(%)] | |
| Antihypertensive therapy | 15 (26.3%) |
| Statins | 7 (12.3%) |
| PPI’s | 9 (15.8%) |
| Pre-donation mGFR [mL/min] | 116 (97–134) |
| Recipient | n = 57 |
| Age [y] | 51.2 (45.0–58.5) |
| Male [n(%)] | 27 (47.4%) |
| BMI [kg/m2] | 25.4 (22.5–28.3) |
| Cardiovascular comorbidity [n(%)] | 39(68.4%) |
| Medication use [n(%)] | |
| Antihypertensive therapy | 52 (91.2%) |
| Phosphate binders | 32 (56.1%) |
| Statins | 28 (49.1%) |
| Unrelated donor [n(%)] | 29 (50.9%) |
| Pre-emptive transplantation [n(%)] | 28 (49.1%) |
| Re-transplantation [n(%)] | 7 (12.3%) |
| ≥3 HLA mismatches [n(%)] | 35 (61.4%) |
| Positive PRA [n(%)] | 7 (12.3%) |
| Ischemia times [min] | |
| WIT 1 | 4 (3–4) |
| CIT | 175.5 (156.0–187.0) |
| WIT 2 | 43.0 (± 7.3) |
| Kidney and Patient Outcomes | n = 57 |
| DGF [n(%)] | 3 (5.4%) |
| eGFR 1 month post transplantation [mL/min/1.73 m2] | 50.8 (±14.9) |
| eGFR 3 months post transplantation [mL/min/1.73 m2] | 49.6 (38.8–58.2) |
| eGFR 6 months post transplantation [mL/min/1.73 m2] | 50.4 (38.8–61.1) |
| eGFR 12 months post transplantation [mL/min/1.73 m2] | 50.2 (±14.2) |
| eGFR 24 months post transplantation [mL/min/1.73 m2] | 51.4 (±17.6) |
| Acute rejection 2 years [n(%)] | 9 (16.1%) |
| Graft loss [n(%)] | 2 (3.5%) |
| Mortality [n(%)] | 1 (1.8%) |
| Urinary Biomarker | Variable (Effect) | B (Unit) | SE of b | p-Value |
|---|---|---|---|---|
| KIM-1 | Sex mismatch (intercept) | 0.39 (male/female) | 0.14 | 0.0065 |
| WIT2 (intercept) | 0.030 (min) | 0.009 | 0.0023 | |
| NAG | Sex mismatch (intercept) | 0.28 (yes/no) | 0.08 | 0.0008 |
| Unrelated donor (intercept) | 0.19 (no/yes) | 0.08 | 0.0228 | |
| Recipient age (intercept) | 0.0079 (years) | 0.003 | 0.0213 | |
| H-FABP | CIT (intercept) | 0.0065 (min) | 0.003 | 0.0189 |
| 1-Month eGFR | ||
| Variable | p-Value | Estimate (95% CI) |
| KIM-1 day 1 | 0.010 | 7.71 (1.96–13.46) |
| NAG day 1 | 0.017 | −8.26 (−14.99–−1.53) |
| NGAL day 1 | 0.004 | −4.55 (−7.58–−1.51) |
| 3-Month eGFR | ||
| Variable | p-Value | Estimate (95% CI) |
| KIM-1 day 1 | 0.034 | 6.80 (0.54–13.06) |
| 6-Month eGFR | ||
| Variable | p-Value | Estimate (95% CI) |
| KIM-1 day 1 | 0.025 | 6.57 (0.86–12.27) |
| NAG day 1 | 0.006 | −9.65 (−16.37–−2.94) |
| NAG day 2 | 0.020 | 8.89 (1.47–16.31) |
| 12-Month eGFR | ||
| Variable | p-Value | Estimate (95% CI) |
| KIM-1 day 1 | 0.008 | 7.33 (1.99–12.66) |
| NAG day 2 | 0.022 | 8.42 (1.24–15.60) |
| 24-Month eGFR | ||
| Variable | p-Value | Estimate (95% CI) |
| KIM-1 day 1 | 0.025 | 7.82 (1.00–14.63) |
| NAG day 1 | 0.037 | 8.87 (−17.20–−0.55) |
| 1-Month eGFR | ||||
| Variable | Adjusted R2 | p-Value | Estimate (95% CI) | |
| Crude model | Donor sex | 0.255 | 0.009 | −9.79 (−16.98–2.60) |
| Donor age | 0.007 | −0.46 (−0.80–−0.13) | ||
| Addition of biomarker | KIM day 1 | 0.323 | 0.018 | 6.39 (1.16–11.62) |
| NAG day 1 | 0.259 | 0.089 | −5.83 (−12.56–0.93) | |
| NGAL day 1 | 0.259 | 0.003 | −4.39 (−7.16–1.62) | |
| Addition of===>1 biomarker | KIM day 1 | 0.396 | 0.001 | 8.70 (3.63–13.77) |
| NAG day 1 | 0.117 | −4.64 (−10.50–1.12) | ||
| NGAL day 1 | 0.125 | −2.20 (−5.03–0.63) | ||
| 3-Month eGFR | ||||
| Variable | Adjusted R2 | p-Value | Estimate (95% CI) | |
| Crude model | Donor age | 0.203 | <0.001 | −0.68 (−1.04–−0.33) |
| Addition of biomarker | KIM-1 day 1 | 0.272 | 0.014 | 7.06 (1.52–12.60) |
| 6-Month eGFR | ||||
| Variable | Adjusted R2 | p-Value | Estimate (95% CI) | |
| Crude model | Donor age | 0.246 | <0.001 | −0.70 (−1.02–−0.38) |
| Addition of biomarker | KIM-1 day 1 | 0.317 | 0.007 | 6.82 (1.91–11.73) |
| NAG day 1 | 0.276 | 0.050 | −6.42 (−12.85–0.01) | |
| NAG day 2 | 0.319 | 0.006 | 9.18 (2.79–15.57) | |
| Addition of >1 biomarker | KIM-1 day 1 | 0.373 | 0.090 | 4.59 (−0.74–9.91) |
| NAG day 1 | 0.056 | −5.83 (−11.80–0.15) | ||
| NAG day 2 | 0.037 | 7.44 (0.48–14.40) | ||
| 12-Month eGFR | ||||
| Variable | Adjusted R2 | p-Value | Estimate (95% CI) | |
| Crude model | Donor age | 0.120 | 0.005 | −0.48 (−0.82–−0.15) |
| Addition of biomarker | KIM-1 day 1 | 0.240 | 0.004 | 7.51 (2.56–12.45) |
| NAG day 2 | 0.189 | 0.013 | 8.62 (1.89–15.36) | |
| Addition of >1 biomarker | KIM-1 day 1 | 0.264 | 0.035 | 5.64 (0.40–10.88) |
| NAG day 2 | 0.045 | 7.31 (0.17–14.45) | ||
| 24-Month eGFR | ||||
| Variable | Adjusted R2 | p-Value | Estimate (95% CI) | |
| Crude model | Donor age | 0.106 | 0.009 | −0.57 (−0.98–−0.15) |
| Addition of biomarker | KIM-1 day 1 | 0.182 | 0.016 | 8.00 (1.57–14.43) |
| NAG day 1 | 0.118 | 0.137 | −6.40 (−14.91–2.10) | |
| Addition of >1 biomarker | KIM-1 day 1 | 0.200 | 0.019 | 8.12 (1.37–14.86) |
| NAG day 1 | 0.182 | −5.48 (−13.62–2.66) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huisman, G.J.J.; Spraakman, N.A.; Koomen, J.V.; Talsma, A.M.; Pol, R.A.; Berger, S.P.; Leuvenink, H.G.D.; Struys, M.M.R.F.; Nieuwenhuijs-Moeke, G.J. Urinary Biomarkers in a Living Donor Kidney Transplantation Cohort—Predictive Value on Graft Function. Int. J. Mol. Sci. 2023, 24, 5649. https://doi.org/10.3390/ijms24065649
Huisman GJJ, Spraakman NA, Koomen JV, Talsma AM, Pol RA, Berger SP, Leuvenink HGD, Struys MMRF, Nieuwenhuijs-Moeke GJ. Urinary Biomarkers in a Living Donor Kidney Transplantation Cohort—Predictive Value on Graft Function. International Journal of Molecular Sciences. 2023; 24(6):5649. https://doi.org/10.3390/ijms24065649
Chicago/Turabian StyleHuisman, G. J. Julia, Nora A. Spraakman, Jeroen V. Koomen, A. Marrit Talsma, Robert A. Pol, Stefan P. Berger, Henri G. D. Leuvenink, Michel M. R. F. Struys, and Gertrude J. Nieuwenhuijs-Moeke. 2023. "Urinary Biomarkers in a Living Donor Kidney Transplantation Cohort—Predictive Value on Graft Function" International Journal of Molecular Sciences 24, no. 6: 5649. https://doi.org/10.3390/ijms24065649
APA StyleHuisman, G. J. J., Spraakman, N. A., Koomen, J. V., Talsma, A. M., Pol, R. A., Berger, S. P., Leuvenink, H. G. D., Struys, M. M. R. F., & Nieuwenhuijs-Moeke, G. J. (2023). Urinary Biomarkers in a Living Donor Kidney Transplantation Cohort—Predictive Value on Graft Function. International Journal of Molecular Sciences, 24(6), 5649. https://doi.org/10.3390/ijms24065649







