Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence
Abstract
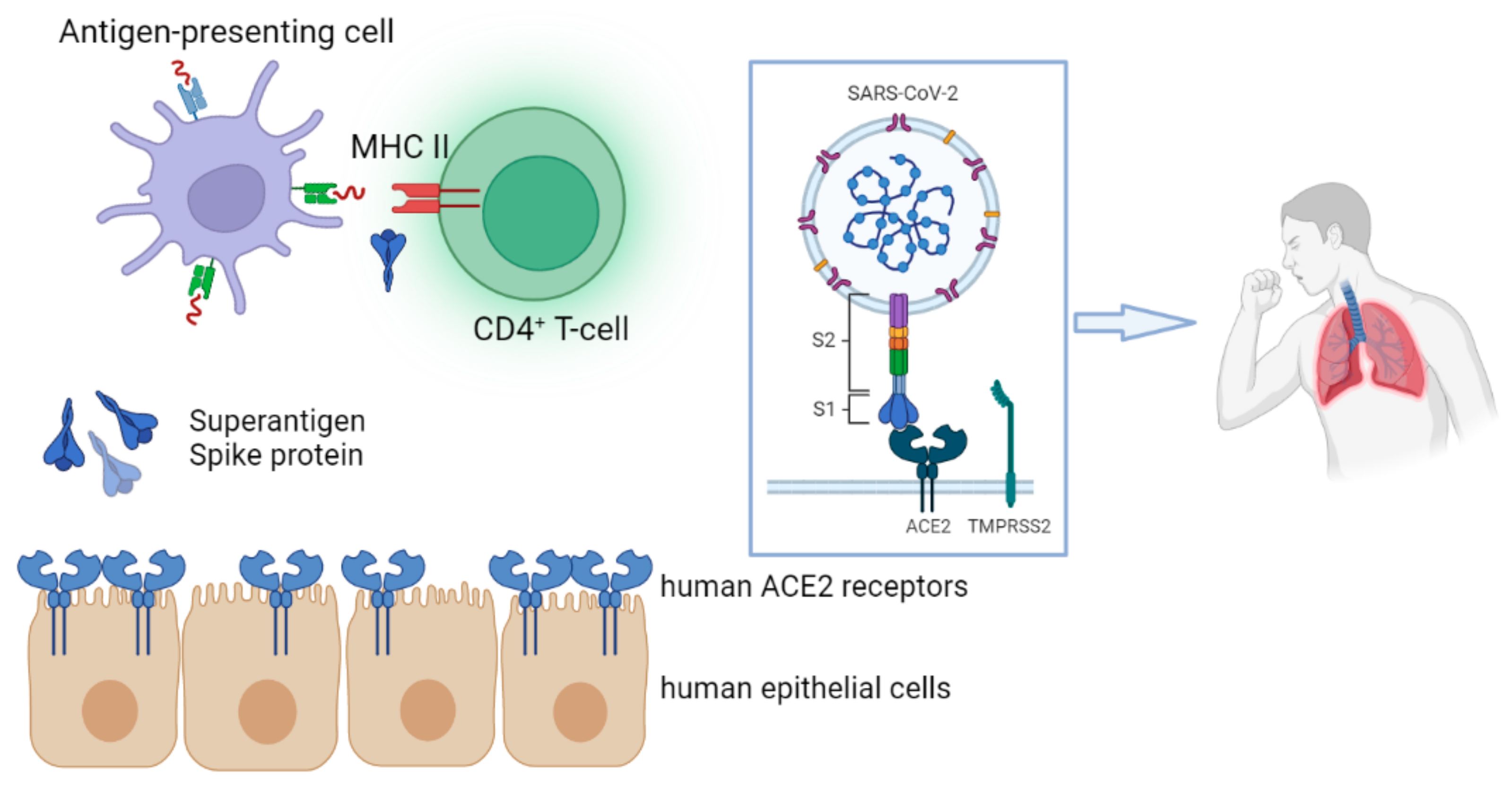
:1. Introduction
2. Incidence of MIS-C
3. Innate Immune Responses in MIS-C
4. Humoral Immune Responses in MIS-C
5. Cellular Immune Response in MIS-C
6. SARS-CoV-2 Spike Protein as a Superantigen in MIS-C
7. MIS-C and COVID-19 Vaccination
8. Genetic Factors Associated with MIS-C
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Systems Science and Engineering. COVID-19 Map—Johns Hopkins Coronavirus Resource Center; Johns Hopkins Coronavirus Resource Center: Baltimore, MD, USA, 2021. [Google Scholar]
- CDC. CDC COVID Data Tracker: Total Cases and Deaths by Race/Ethnicity, Age, and Sex. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#demographics (accessed on 16 September 2022).
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, Y.; Shi, Y.; Chen, Y.; Liu, E. Why multisystem inflammatory syndrome in children has been less commonly described in Asia? Transl. Pediatr. 2020, 9, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Abraham, D.R.; Faleye, A.; McCulloch, M.; Rabie, H.; Scott, C. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc. Health 2020, 4, e38. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.W.; Zachariah, P.; Gorelik, M.; Boneparth, A.; Kernie, S.G.; Orange, J.S.; Milner, J.D. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 2020, 324, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- CDC COVID Data Tracker: Multisystem Inflammatory Syndrome in Children (MIS-C). Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 12 June 2022).
- Cloete, J.; Kruger, A.; Masha, M.; du Plessis, N.M.; Mawela, D.; Tshukudu, M.; Manyane, T.; Komane, L.; Venter, M.; Jassat, W.; et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: A multicentre observational study. Lancet Child Adolesc. Health 2022, 6, 294–302. [Google Scholar] [CrossRef]
- CDC. HAN Archive-00442|Health Alert Network (HAN). 2021. Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 14 September 2022).
- BC Centre for Disease Control. Multi-System Inflammatory Syndrome in Children and Adolescents (MIS-C); BC Centre for Disease Control: Vancouver, BC, Canada, 2020. [Google Scholar]
- BCirks, B.T.; Rowe, S.J.; Jiang, S.Y.; Brooks, R.M.; Mulreany, M.P.; Hoffner, W.; Jones, O.Y.; Hickey, P.W. Sixteen Weeks Later: Expanding the Risk Period for Multisystem Inflammatory Syndrome in Children. J. Pediatr. Infect. Dis. Soc. 2021, 10, 686–690. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Cases of inflammatory syndrome in children surge after urgent alert. BMJ 2020, 369, m1990. [Google Scholar] [CrossRef]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.M.; Hayes, D.A.; Singh-Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem inflammatory syndrome in children and Kawasaki disease: A critical comparison. Nat. Rev. Rheumatol. 2021, 17, 731–748. [Google Scholar] [CrossRef]
- Carter, M.J.; Fish, M.; Jennings, A.; Doores, K.J.; Wellman, P.; Seow, J.; Acors, S.; Graham, C.; Timms, E.; Kenny, J.; et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020, 26, 1701–1707. [Google Scholar] [CrossRef]
- Lee, P.Y.; Day-Lewis, M.; Henderson, L.A.; Friedman, K.G.; Lo, J.; Roberts, J.E.; Lo, M.S.; Platt, C.D.; Chou, J.; Hoyt, K.J.; et al. Distinct clinical and immunological features of SARS-CoV-2–induced multisystem inflammatory syndrome in children. J. Clin. Investig. 2020, 130, 5942–5950. [Google Scholar] [CrossRef]
- Miller, A.D.; Zambrano, L.D.; Yousaf, A.R.; Abrams, J.Y.; Meng, L.; Wu, M.J.; Melgar, M.; E Oster, M.; Cato, S.E.G.; Belay, E.D.; et al. Multisystem Inflammatory Syndrome in Children—United States, February 2020–July 2021. Clin. Infect. Dis. 2021, 75, e1165–e1175. [Google Scholar] [CrossRef]
- Eleftheriou, I.; Maritsi, D.; Lampidi, S.; Charisi, K.; Vantsi, P.; Skourti, K.; Filippatos, F.; Amplianitis, I.; Dimou, D.; Papadopoulou-Legbelou, K.; et al. Decreasing Incidence of the Multisystem Inflammatory Syndrome in Children Over 3 Pandemic Waves. Pediatr. Infect. Dis. J. 2023, 42, 122–124. [Google Scholar] [CrossRef]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA 2022, 327, 2452–2454. [Google Scholar] [CrossRef]
- Filippatos, F.; Tatsi, E.-B.; Dellis, C.; Koukou, D.-M.; Papagiannopoulos, C.; Margeli, A.; Siahanidou, T.; Kanaka-Gantenbein, C.; Syriopoulou, V.; Michos, A. SARS-CoV-2 seroepidemiology in paediatric population during Delta and Omicron predominance. Epidemiology Infect. 2022, 150, e177. [Google Scholar] [CrossRef]
- Holm, M.; Espenhain, L.; Glenthøj, J.; Schmidt, L.S.; Nordly, S.B.; Hartling, U.B.; Nygaard, U. Risk and Phenotype of Multisystem Inflammatory Syndrome in Vaccinated and Unvaccinated Danish Children Before and During the Omicron Wave. JAMA Pediatr. 2022, 176, 821. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef]
- Abrams, J.Y.; E Oster, M.; E Godfred-Cato, S.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; A Tsang, C.; Pierce, T.J.; Kennedy, J.L.; et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child Adolesc. Health 2021, 5, 323–331. [Google Scholar] [CrossRef]
- Sahoo, D.; Katkar, G.D.; Shimizu, C.; Kim, J.; Khandelwal, S.; Tremoulet, A.H.; Kanegaye, J.; Pediatric Emergency Medicine Kawasaki Disease Research Group; Bocchini, J.; Das, S.; et al. An AI-guided invariant signature places MIS-C with Kawasaki disease in a continuum of host immune responses. bioRxiv, 2021. [Google Scholar] [CrossRef]
- Seery, V.; Raiden, S.C.; Algieri, S.C.; Grisolía, N.A.; Filippo, D.; De Carli, N.; Di Lalla, S.; Cairoli, H.; Chiolo, M.J.; Meregalli, C.N.; et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. Ebiomedicine 2021, 67, 103357. [Google Scholar] [CrossRef]
- Boribong, B.P.; LaSalle, T.J.; Bartsch, Y.C.; Ellett, F.; Loiselle, M.E.; Davis, J.P.; Gonye, A.L.; Sykes, D.B.; Hajizadeh, S.; Kreuzer, J.; et al. Neutrophil Profiles of Pediatric COVID-19 and Multisystem Inflammatory Syndrome in Children. bioRxiv, 2021. [Google Scholar] [CrossRef]
- Ramaswamy, A.; Brodsky, N.N.; Sumida, T.S.; Comi, M.; Asashima, H.; Hoehn, K.B.; Li, N.; Liu, Y.; Shah, A.; Ravindra, N.G.; et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity 2021, 54, 1083–1095.e7. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Stürzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. Ebiomedicine 2020, 58, 102925. [Google Scholar] [CrossRef] [PubMed]
- de Cevins, C.; Luka, M.; Smith, N.; Meynier, S.; Magérus, A.; Carbone, F.; García-Paredes, V.; Barnabei, L.; Batignes, M.; Boullé, A.; et al. A monocyte/dendritic cell molecular signature of SARS-CoV-2-related multisystem inflammatory syndrome in children with severe myocarditis. Med 2021, 2, 1072–1092.e7. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Shraim, R.; Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Canna, S.W.; Henrickson, S.E.; McNerney, K.O.; Balamuth, F.; et al. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat. Commun. 2021, 12, 7222. [Google Scholar] [CrossRef]
- Diorio, C.; Henrickson, S.E.; Vella, L.A.; McNerney, K.O.; Chase, J.M.; Burudpakdee, C.; Lee, J.H.; Jasen, C.; Balamuth, F.; Barrett, D.M.; et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J. Clin. Investig. 2020, 130, 5967–5975. [Google Scholar] [CrossRef]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Caldarale, F.; Giacomelli, M.; Garrafa, E.; Tamassia, N.; Morreale, A.; Poli, P.; Timpano, S.; Baresi, G.; Zunica, F.; Cattalini, M.; et al. Plasmacytoid Dendritic Cells Depletion and Elevation of IFN-γ Dependent Chemokines CXCL9 and CXCL10 in Children With Multisystem Inflammatory Syndrome. Front. Immunol. 2021, 12, 654587. [Google Scholar] [CrossRef]
- Akindele, N.P.; Kouo, T.; Karaba, A.H.; Gordon, O.; Fenstermacher, K.Z.J.; Beaudry, J.; Rubens, J.H.; Atik, C.C.; Zhou, W.; Ji, H.; et al. Distinct Cytokine and Chemokine Dysregulation in Hospitalized Children With Acute Coronavirus Disease 2019 and Multisystem Inflammatory Syndrome With Similar Levels of Nasopharyngeal Severe Acute Respiratory Syndrome Coronavirus 2 Shedding. J. Infect. Dis. 2021, 224, 606–615. [Google Scholar] [CrossRef]
- Diorio, C.; McNerney, K.O.; Lambert, M.; Paessler, M.; Anderson, E.M.; Henrickson, S.E.; Chase, J.; Liebling, E.J.; Burudpakdee, C.; Lee, J.H.; et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020, 4, 6051–6063. [Google Scholar] [CrossRef]
- Choi, N.H.; Fremed, M.; Starc, T.; Weller, R.; Cheung, E.; Ferris, A.; Silver, E.S.; Liberman, L. MIS-C and Cardiac Conduction Abnormalities. Pediatrics 2020, 146, e2020009738. [Google Scholar] [CrossRef]
- Perez-Toledo, M.; Faustini, S.E.; Jossi, S.E.; Shields, A.M.; Marcial-Juarez, E.; Kanthimathinathan, H.K.; Allen, J.D.; Watanabe, Y.; Goodall, M.; Willcox, B.E.; et al. SARS-CoV-2-specific IgG1/IgG3 but not IgM in children with Pediatric Inflammatory Multi-System Syndrome. Pediatr. Allergy Immunol. 2021, 32, 1125–1129. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2020, 22, 25–31. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 27, 454–462. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, e20202486. [Google Scholar] [CrossRef]
- Pfeifer, J.; Thurner, B.; Kessel, C.; Fadle, N.; Kheiroddin, P.; Regitz, E.; Hoffmann, M.-C.; Kos, I.A.; Preuss, K.-D.; Fischer, Y.; et al. Autoantibodies against interleukin-1 receptor antagonist in multisystem inflammatory syndrome in children: A multicentre, retrospective, cohort study. Lancet Rheumatol. 2022, 4, e329–e337. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Castagnoli, R.; Shimizu, C.; Delmonte, O.M.; Dobbs, K.; Discepolo, V.; Vecchio, A.L.; Guarino, A.; Licciardi, F.; Ramenghi, U.; et al. Autoantibodies Against Proteins Previously Associated With Autoimmunity in Adult and Pediatric Patients With COVID-19 and Children With MIS-C. Front. Immunol. 2022, 13, 841126. [Google Scholar] [CrossRef]
- Wang, X.; Gui, J. Cell-mediated immunity to SARS-CoV-2. Pediatr. Investig. 2020, 4, 281–291. [Google Scholar] [CrossRef]
- Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Diorio, C.; Kuri-Cervantes, L.; Alanio, C.; Pampena, M.B.; Wu, J.E.; Chen, Z.; et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared with adult and pediatric COVID-19. Sci. Immunol. 2021, 6, 7570. [Google Scholar] [CrossRef]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune Responses to SARS-CoV-2 Infection in Hospitalized Pediatric and Adult Patients. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Porritt, R.A.; Paschold, L.; Rivas, M.N.; Cheng, M.H.; Yonker, L.M.; Chandnani, H.; Lopez, M.; Simnica, D.; Schultheiß, C.; Santiskulvong, C.; et al. HLA class I-associated expansion of TRBV11-2 T cells in Multisystem Inflammatory Syndrome in Children. J. Clin. Investig. 2021, 131, e146614. [Google Scholar] [CrossRef]
- Filippatos, F.; Tzanoudaki, M.; Tatsi, E.-B.; Efthymiou, V.; Liatsis, M.; Syriopoulou, V.; Michos, A. Multiparametric Investigation Of Spike-Protein Specific T-cell Cytokine Expression Profile In Children With Symptomatic COVID-19 Or Multisystem Inflammatory Syndrome. Open Forum Infect. Dis. 2022, 9, 1093. [Google Scholar] [CrossRef]
- Cheng, M.H.; Zhang, S.; Porritt, R.A.; Noval Rivas, M.; Paschold, L.; Willscher, E.; Binder, M.; Arditi, M.; Bahar, I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 25254–25262. [Google Scholar] [CrossRef] [PubMed]
- Moreews, M.; Le Gouge, K.; Khaldi-Plassart, S.; Pescarmona, R.; Mathieu, A.-L.; Malcus, C.; Djebali, S.; Bellomo, A.; Dauwalder, O.; Perret, M.; et al. Polyclonal expansion of TCR Vb 21.3 + CD4 + and CD8 + T cells is a hallmark of multisystem inflammatory syndrome in children. Sci. Immunol. 2021, 6, eabh1516. [Google Scholar] [CrossRef] [PubMed]
- Hoste, L.; Roels, L.; Naesens, L.; Bosteels, V.; Vanhee, S.; Dupont, S.; Bosteels, C.; Browaeys, R.; Vandamme, N.; Verstaen, K.; et al. TIM3+TRBV11-2 T cells and IFNγ signature in patrolling monocytes and CD16+ NK cells delineate MIS-C. J. Exp. Med. 2021, 219, e20211381. [Google Scholar] [CrossRef]
- Rivas, M.N.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 2022, 13, 3480. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef]
- Esteve-Sole, A.; Anton, J.; Pino-Ramirez, R.M.; Sanchez-Manubens, J.; Fumadó, V.; Fortuny, C.; Rios-Barnes, M.; Sanchez-De-Toledo, J.; Girona-Alarcón, M.; Mosquera, J.M.; et al. Similarities and differences between the immunopathogenesis of COVID-19–related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021, 131, e144554. [Google Scholar] [CrossRef]
- Rehaume, L.M.; Hancock, R.E.W. Neutrophil-derived defensins as modulators of innate immune function. Crit. Rev. Immunol. 2008, 28, 185–200. [Google Scholar] [CrossRef]
- Diaz, F.; Bustos B, R.; Yagnam, F.; Karsies, T.J.; Vásquez-Hoyos, P.; Jaramillo-Bustamante, J.-C.; Gonzalez-Dambrauskas, S.; Drago, M.; Cruces, P. Comparison of Interleukin-6 Plasma Concentration in Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Pediatric Sepsis. Front. Pediatr. 2021, 9, 756083. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Whitworth, H.B.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef]
- Polycarpou, A.; Grigoriadou, S.; Klavinskis, L.; Sacks, S. Does the Lectin Complement Pathway Link Kawasaki Disease and SARS-CoV-2? Front. Immunol. 2021, 11, 604512. [Google Scholar] [CrossRef]
- Rodriguez-Smith, J.J.; Verweyen, E.L.; Clay, G.M.; Esteban, Y.M.; de Loizaga, S.R.; Baker, E.J.; Do, T.; Dhakal, S.; Lang, S.M.; A Grom, A.; et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: A cohort study. Lancet Rheumatol. 2021, 3, e574–e584. [Google Scholar] [CrossRef]
- Filippatos, F.; Tatsi, E.; Michos, A. Immune response to SARS-CoV-2 in children: A review of the current knowledge. Pediatr. Investig. 2021, 5, 217–228. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020, 72, 1791–1805. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef]
- Duarte-Neto, A.N.; Caldini, E.G.; Gomes-Gouvêa, M.S.; Kanamura, C.T.; Monteiro, R.A.D.A.; Ferranti, J.F.; Ventura, A.M.C.; Regalio, F.A.; Fiorenzano, D.M.; Gibelli, M.A.B.C.; et al. An autopsy study of the spectrum of severe COVID-19 in children: From SARS to different phenotypes of MIS-C. Eclinicalmedicine 2021, 35, 100850. [Google Scholar] [CrossRef]
- Sananez, I.; Raiden, S.C.; Algieri, S.C.; Uranga, M.; Grisolía, N.A.; Filippo, D.; De Carli, N.; Di Lalla, S.; Cairoli, H.; Chiolo, M.J.; et al. A poor and delayed anti-SARS-CoV2 IgG response is associated to severe COVID-19 in children. Ebiomedicine 2021, 72, 103615. [Google Scholar] [CrossRef]
- Tang, J.; Ravichandran, S.; Lee, Y.; Grubbs, G.; Coyle, E.M.; Klenow, L.; Genser, H.; Golding, H.; Khurana, S. Antibody affinity maturation and plasma IgA associate with clinical outcome in hospitalized COVID-19 patients. Nat. Commun. 2021, 12, 1221. [Google Scholar] [CrossRef]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021, 147, 545–557.e9. [Google Scholar] [CrossRef]
- Dogan, M.; Kozhaya, L.; Placek, L.; Gunter, C.; Yigit, M.; Hardy, R.; Plassmeyer, M.; Coatney, P.; Lillard, K.; Bukhari, Z.; et al. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun. Biol. 2021, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.A.; Subramony, A.; Sweberg, T.; Schneider, J.; Shah, S.; Rubin, L.; Schleien, C.; Epstein, S.; Johnson, J.C.; Kessel, A.; et al. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J. Pediatr. 2020, 224, 141–145. [Google Scholar] [CrossRef] [PubMed]
- De Rose, D.U.; Pugnaloni, F.; Calì, M.; Ronci, S.; Caoci, S.; Maddaloni, C.; Martini, L.; Santisi, A.; Dotta, A.; Auriti, C. Multisystem Inflammatory Syndrome in Neonates Born to Mothers with SARS-CoV-2 Infection (MIS-N) and in Neonates and Infants Younger Than 6 Months with Acquired COVID-19 (MIS-C): A Systematic Review. Viruses 2022, 14, 750. [Google Scholar] [CrossRef]
- Pawar, R.; Gavade, V.; Patil, N.; Mali, V.; Girwalkar, A.; Tarkasband, V.; Loya, S.; Chavan, A.; Nanivadekar, N.; Shinde, R.; et al. Neonatal Multisystem Inflammatory Syndrome (MIS-N) Associated with Prenatal Maternal SARS-CoV-2: A Case Series. Children 2021, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, J.M.; Murphy, E.A.; Yee, J.; Cagino, K.A.; Friedlander, R.L.; Glynn, S.M.; Matthews, K.C.; Jurkiewicz, M.; Sukhu, A.C.; Zhao, Z.; et al. Severe acute respiratory syndrome coronavirus 2 serology levels in pregnant women and their neonates. Am. J. Obstet. Gynecol. 2021, 225, 73.e1–73.e7. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Jafarzadeh, S.; Nozari, P.; Mokhtari, P.; Nemati, M. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. Scand. J. Immunol. 2020, 93, e12967. [Google Scholar] [CrossRef]
- Sánchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sánchez-Alonso, S.; Sánchez-Azofra, A.; Marcos-Jiménez, A.; Ávalos, E.; Alcaraz-Serna, A.; Santos, I.D.L.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020, 130, 6290–6300. [Google Scholar] [CrossRef]
- Kvedaraite, E.; Hertwig, L.; Sinha, I.; Ponzetta, A.; Myrberg, I.H.; Lourda, M.; Dzidic, M.; Akber, M.; Klingström, J.; Folkesson, E.; et al. Major alterations in the mononuclear phagocyte landscape associated with COVID-19 severity. Proc. Natl. Acad. Sci. 2021, 118, e2018587118. [Google Scholar] [CrossRef]
- Hsieh, L.; Grifoni, A.; Sidney, J.; Shimizu, C.; Shike, H.; Ramchandar, N.; Moreno, E.; Tremoulet, A.H.; Burns, J.C.; Franco, A. Characterization of SARS-CoV-2 and common cold coronavirus-specific T-cell responses in MIS-C and Kawasaki disease children. Eur. J. Immunol. 2021, 52, 123–137. [Google Scholar] [CrossRef]
- Schmitt, N.; Ueno, H. Regulation of human helper T cell subset differentiation by cytokines. Curr. Opin. Immunol. 2015, 34, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Dowling, D.J.; Levy, O. Ontogeny of early life immunity. Trends Immunol. 2014, 35, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, N.D.; Comella, P.H.; Cheng, E.; Lepow, L.; Beckmann, A.G.; Tyler, S.R.; Mouskas, K.; Simons, N.W.; Hoffman, G.E.; Francoeur, N.J.; et al. Downregulation of exhausted cytotoxic T cells in gene expression networks of multisystem inflammatory syndrome in children. Nat. Commun. 2021, 12, 4854. [Google Scholar] [CrossRef]
- Schlievert, P. Role of Superantigens in Human Disease. J. Infect. Dis. 1993, 167, 997–1002. [Google Scholar] [CrossRef]
- Kum, W.W.; Laupland, K.B.; Chow, A.W. Defining a novel domain of staphylococcal toxic shock syndrome toxin-1 critical for major histocompatibility complex class II binding, superantigenic activity, and lethality. Can. J. Microbiol. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Xia, X. Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design. Viruses 2021, 13, 109. [Google Scholar] [CrossRef]
- Rivas, M.N.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. COVID-19–associated multisystem inflammatory syndrome in children (MIS-C): A novel disease that mimics toxic shock syndrome—the superantigen hypothesis. J. Allergy Clin. Immunol. 2020, 147, 57–59. [Google Scholar] [CrossRef]
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic Shock Syndrome and Bacterial Superantigens: An Update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef]
- Salzman, M.B.; Huang, C.-W.; O’Brien, C.M.; Castillo, R.D. Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Recher, M.; Hubert, H.; Javouhey, E.; Fléchelles, O.; Leteurtre, S.; Angoulvant, F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA 2022, 327, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years — United States, July–December 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Buchhorn, R.; Meyer, C.; Schulze-Forster, K.; Junker, J.; Heidecke, H. Autoantibody Release in Children after Corona Virus mRNA Vaccination: A Risk Factor of Multisystem Inflammatory Syndrome? Vaccines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Health Service Executive. Clinical Guidance for COVID-19 Vaccination; CDC: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#covid19-vaccination-misc-misa (accessed on 13 December 2022).
- Chou, J.; Platt, C.D.; Habiballah, S.; Nguyen, A.A.; Elkins, M.; Weeks, S.; Peters, Z.; Day-Lewis, M.; Novak, T.; Armant, M.; et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J. Allergy Clin. Immunol. 2021, 148, 732–738.e1. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef]
- Holman, R.C.; Belay, E.D.; Christensen, K.Y.; Folkema, A.M.; Steiner, C.A.; Schonberger, L.B. Hospitalizations for Kawasaki Syndrome Among Children in the United States, 1997–2007. Pediatr. Infect. Dis. J. 2010, 29, 483–488. [Google Scholar] [CrossRef]
- Belay, E.D.; Abrams, J.; Oster, M.E.; Giovanni, J.; Pierce, T.; Meng, L.; Prezzato, E.; Balachandran, N.; Openshaw, J.J.; Rosen, H.E.; et al. Trends in Geographic and Temporal Distribution of US Children With Multisystem Inflammatory Syndrome During the COVID-19 Pandemic. JAMA Pediatr. 2021, 175, 837–845. [Google Scholar] [CrossRef]
- Nguyen, A.; David, J.K.; Maden, S.K.; Wood, M.A.; Weeder, B.R.; Nellore, A.; Thompson, R.F. Human Leukocyte Antigen Susceptibility Map for Severe Acute Respiratory Syndrome Coronavirus 2. J. Virol. 2020, 94, e00510-20. [Google Scholar] [CrossRef] [Green Version]
- Alexander, W.S.; Starr, R.; Fenner, J.E.; Scott, C.L.; Handman, E.; Sprigg, N.S.; Corbin, J.E.; Cornish, A.L.; Darwiche, R.; Owczarek, C.M.; et al. SOCS1 Is a Critical Inhibitor of Interferon γ Signaling and Prevents the Potentially Fatal Neonatal Actions of this Cytokine. Cell 1999, 98, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.Y.; Platt, C.D.; Weeks, S.; Grace, R.F.; Maher, G.; Gauthier, K.; Devana, S.; Vitali, S.; Randolph, A.G.; McDonald, D.R.; et al. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J. Allergy Clin. Immunol. 2020, 146, 1194–1200.e1. [Google Scholar] [CrossRef]
| Immune System Aspects | Immunological Factors in MIS-C | References |
|---|---|---|
| Innate immunity | Lymphopenia, neutrophilia, elevated inflammatory markers, thrombocytopenia and low eosinophil counts | [22,23,24] |
| Increased expression of CD11b, CD66b, LAIR-1, and PD-L1 in neutrophils | [25] | |
| CCRL2, ELMO2, GPR84, IRF7, IFIT3, and MX1 genes upregulation | [26] | |
| Reduced antigen-presenting cells | [27] | |
| Increased neutrophil extracellular traps | [28,29] | |
| Increased signaling pathways (NF-kB, VEGF, IFN, IL-1, IL-6, and IL-17) | [30] | |
| NK cells: increased expression of CCL4, NCR1 | [27,31] | |
| Evelated cytokines (TNF, TRAIL, IL-7, IL-2, IL-13, IFN-g, IFN-a2, IL-17A, Granzyme B, IL-8, IL-10) | [15,16,27,30,32,33,34,35] | |
| Elevated chemokines (CCL2, CCL3, CCL4, CXCL1, CXCL5, CXCL6, CXCL9, CXCL10, CXCL11, CX3CL1, CX10, CCL20) | [15,16,27,30,32,33,34,35] | |
| Increased expression of IFN-stimulated genes in dendritic cells and monocytes: JAK2, STAT1, STAT2, IFITM1, IFITM2, IFI35, IFIT1, IFIT3, MX1, IRF1 | [30] | |
| Increased phospholipase A2 (PLA2G2A) | [31] | |
| Increased soluble C5b-9 | [36] | |
| Humoral Immunity | Higher SARS-CoV-2 and IgA antibody levels, lower IgM compared to non-MIS-C patients | [37,38,39] |
| Higher SARS-CoV-2 anti-S IgG compared to anti-N IgG levels in MIS-C | [33,39,40] | |
| Comparable SARS-CoV-2 anti-S IgG, anti-S IgM and anti-N IgG levels to non-MIS-C patients | [39] | |
| Increased autoantibodies against Type I IFNs, IL-1Ra | [41,42,43] | |
| Cellular immunity | Increased CD4+ and CD8+ T cells | [44,45,46] |
| Increased B-cell plasmablasts | [45] | |
| Increased CD8+ T cells cytotoxicity | [47] | |
| Increased CX3CR1+CD8+ T cells | [45] | |
| Increased CD8+ T cell-mediated IFN-gamma | [48] | |
| Superantigen | High affinity of SARS-CoV-2 Spike protein to TCR | [49] |
| Εnhancement of TRBV11-2 in T cells | [47,50,51,52] |
| Immunological Factors | MIS-C | Kawasaki Disease | References |
|---|---|---|---|
| Absolute neutrophil counts | ↓ | ↑ | [24] |
| Absolute lymphocyte counts | ↓ | ↑ | [24] |
| Absolute platelet counts | ↓ | ↑ | [24] |
| Absolute eosinophil counts | ↓ | ↑ | [24] |
| IFN-gamma CXCL9 values | ↑ | ↓ | [53] |
| IL-1 and IL-8 | ↑ | ↑ | [24] |
| TNF-a, IFN-gamma and IL-10 | ↑ | ↑ | [24,54] |
| IL-17 | ↑ | ↑ | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippatos, F.; Tatsi, E.-B.; Michos, A. Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 5711. https://doi.org/10.3390/ijms24065711
Filippatos F, Tatsi E-B, Michos A. Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence. International Journal of Molecular Sciences. 2023; 24(6):5711. https://doi.org/10.3390/ijms24065711
Chicago/Turabian StyleFilippatos, Filippos, Elizabeth-Barbara Tatsi, and Athanasios Michos. 2023. "Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence" International Journal of Molecular Sciences 24, no. 6: 5711. https://doi.org/10.3390/ijms24065711
APA StyleFilippatos, F., Tatsi, E.-B., & Michos, A. (2023). Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence. International Journal of Molecular Sciences, 24(6), 5711. https://doi.org/10.3390/ijms24065711








