Subsets of Eosinophils in Asthma, a Challenge for Precise Treatment
Abstract
:1. Introduction
2. Development, Phenotypes, and Functional Characteristics of the Eosinophil Lineage: Generation of Eosinophil Endotypes
3. Trafficking of Eosinophils from the Bone Marrow to the Peripheral Tissues
4. The Conventional Concept of Eosinophils’ Role in Tissues
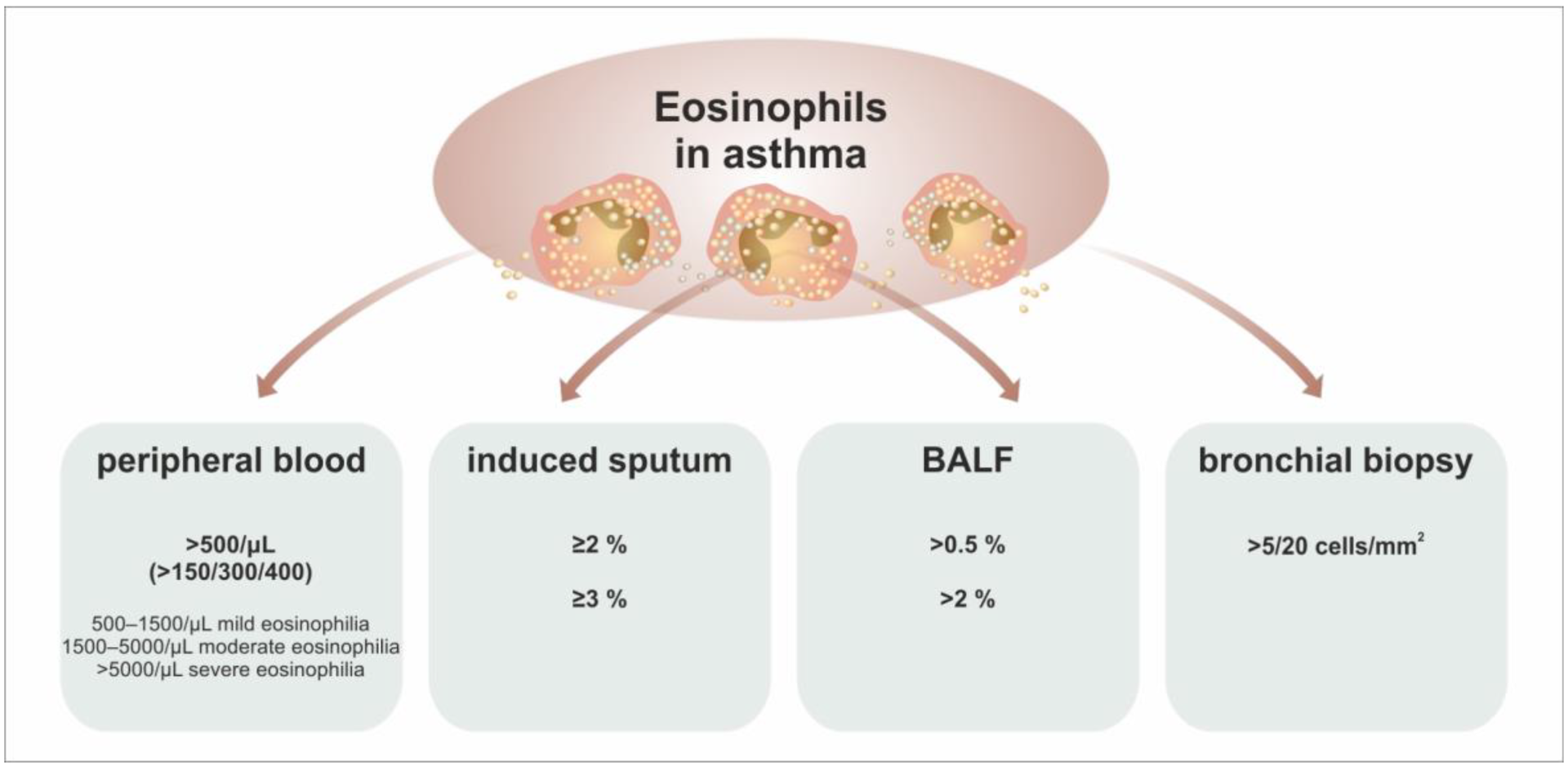
5. Eosinophils as a Biomarker in Asthma
6. Anti-Eosinophil Targeted Therapy in Asthma
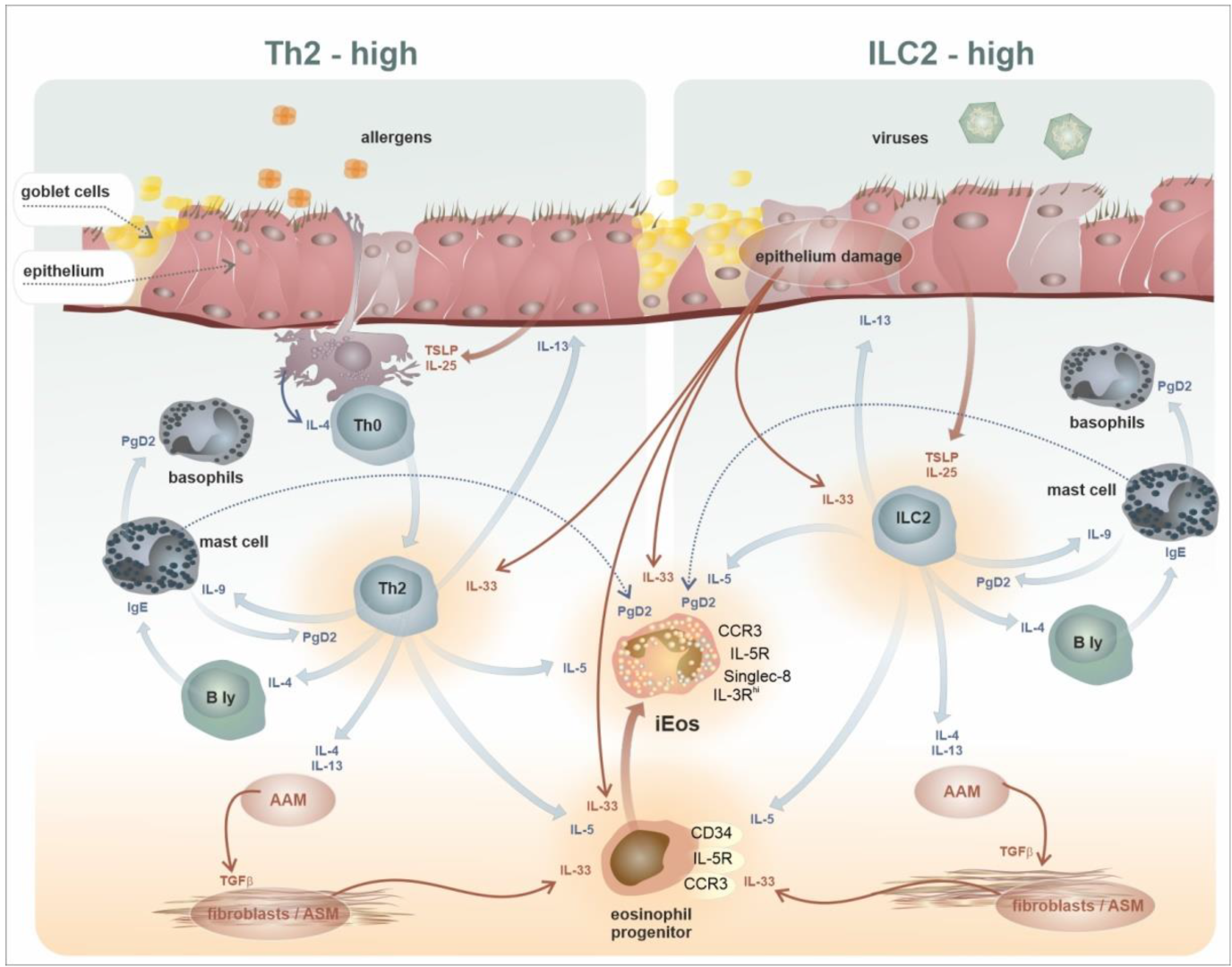
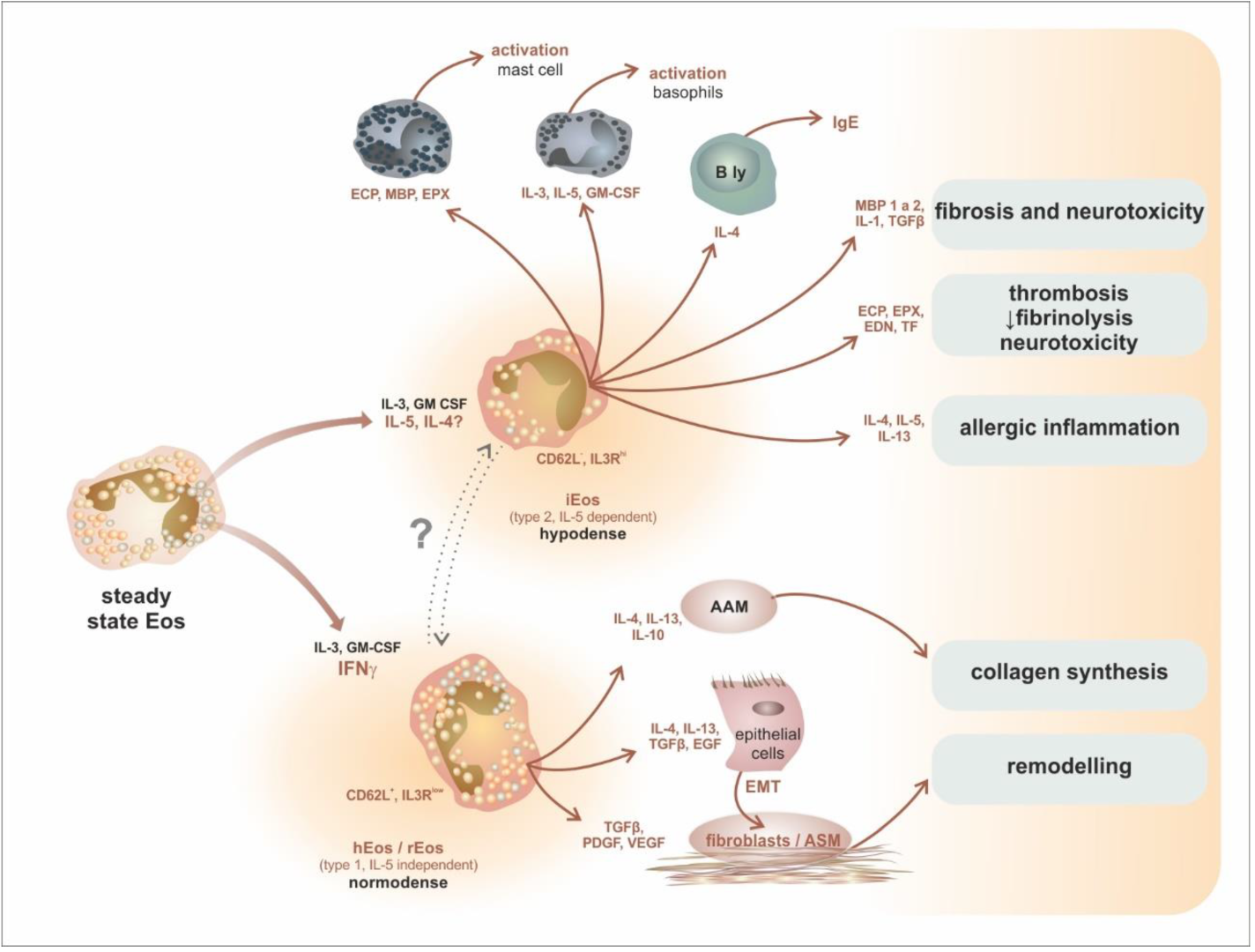
7. Intratissue Kinetics and Functional Development of Eosinophils, the Novel Concept of Eosinophil Functions and Plasticity
8. What Questions Regarding Eosinophils in Asthma Remain to Be Solved
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hulst, G.; Bureau, F.; Desmet, C.J. Eosinophils as drivers of severe eosinophilic asthma: Endotypes or plasticity? Int. J. Mol. Sci. 2021, 22, 10150. [Google Scholar] [CrossRef] [PubMed]
- Loktionov, A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J. Gastroenterol. 2019, 25, 3503–3526. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, M.E. A hidden residential cell in the lung. J. Clin. Investig. 2016, 126, 3185–3187. [Google Scholar] [CrossRef] [Green Version]
- Marichal, T.; Mesnil, C.; Bureau, F. Homeostatic Eosinophils: Characteristics and Functions. Front. Med. 2017, 4, 101. [Google Scholar] [CrossRef] [Green Version]
- Abdala-Valencia, H.; Coden, M.E.; Chiarella, S.E.; Jacobsen, E.A.; Bochner, B.S.; Lee, J.J.; Berdnikovs, S. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 2018, 104, 95–108. [Google Scholar] [CrossRef]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 179–209. [Google Scholar] [CrossRef] [Green Version]
- Rühle, P.F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Development of a modular assay for detailed immunophenotyping of peripheral human whole blood samples by multicolor flow cytometry. Int. J. Mol. Sci. 2016, 17, 1316. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, H.F.; Phipps, S.; Foster, P.S. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007, 119, 1303–1310. [Google Scholar] [CrossRef]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Roufosse, F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Hassani, M.; Koenderman, L. Immunological and hematological effects of IL-5(Rα)-targeted therapy: An overview. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 1979–1988. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Chen, Y.H.; Peterson, L.A.C. GATA family transcriptional factors: Emerging suspects in hematologic disorders. Exp. Hematol. Oncol. 2015, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bochner, B.S. The eosinophil: For better or worse, in sickness and in health. Ann. Allergy Asthma Immunol. 2018, 121, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Tavernier, J.; Van der Heyden, J.; Verhee, A.; Brusselle, G.; Van Ostade, X.; Vandekerckhove, J.; North, J.; Rankin, S.M.; Kay, A.B.; Robinson, D.S. Interleukin 5 regulates the isoform expression of its own receptor alpha-subunit. Blood 2000, 95, 1600–1607. [Google Scholar] [CrossRef]
- Sehmi, R.; Wardlaw, A.J.; Cromwell, O.; Kurihara, K.; Waltmann, P.; Kay, A.B. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood 1992, 79, 2952–2959. [Google Scholar] [CrossRef] [Green Version]
- Melo, R.C.N.; Weller, P.F. Contemporary understanding of the secretory granules in human eosinophils. J. Leukoc. Biol. 2018, 104, 85–93. [Google Scholar] [CrossRef]
- Munitz, A.; Levi-Schaffer, F. Inhibitory receptors on eosinophils: A direct hit to a possible Achilles heel? J. Allergy Clin. Immunol. 2007, 119, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Rådinger, M.; Lötvall, J. Eosinophil progenitors in allergy and asthma—Do they matter? Pharmacol. Ther. 2009, 121, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.P.; Lemiere, C.; Martin, J.; et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdnikovs, S. The twilight zone: Plasticity and mixed ontogeny of neutrophil and eosinophil granulocyte subsets. Semin. Immunopathol. 2021, 43, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Besse, J.A.; Mingler, M.K.; Fulkerson, P.C.; Rothenberg, M.E. Eosinophil adoptive transfer system to directly evaluate pulmonary eosinophil trafficking in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 6067–6072. [Google Scholar] [CrossRef] [Green Version]
- Winkel, P.; Statland, B.E.; Saunders, A.M.; Osborn, H.; Kupperman, H. Within-day physiologic variation of leukocyte types in healthy subjects as assayed by two automated leukocyte differential analyzers. Am. J. Clin. Pathol. 1981, 75, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Kanda, A.; Yun, Y.; Van Bui, D.; Nguyen, L.M.; Kobayashi, Y.; Suzuki, K.; Mitani, A.; Sawada, S.; Hamada, S.; Asako, M.; et al. The multiple functions and subpopulations of eosinophils in tissues under steady-state and pathological conditions. Allergol. Int. 2021, 70, 9–18. [Google Scholar] [CrossRef]
- Januskevicius, A.; Gosens, R.; Sakalauskas, R.; Vaitkiene, S.; Janulaityte, I.; Halayko, A.J.; Hoppenot, D.; Malakauskas, K. Suppression of eosinophil integrins prevents remodeling of airway smooth muscle in asthma. Front. Physiol. 2017, 7, 680. [Google Scholar] [CrossRef] [Green Version]
- Michail, S.; Mezoff, E.; Abernathy, F. Role of selectins in the intestinal epithelial migration of eosinophils. Pediatr. Res. 2005, 58, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, M.E. Eotaxin: An essential mediator of Eosinophil trafficking into mucosal tissues. Am. J. Respir. Cell Mol. Biol. 1999, 21, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Castan, L.; Magnan, A.; Bouchaud, G. Chemokine receptors in allergic diseases. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.M.; Bochner, B.S. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol. Res. 2010, 2, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Klion, A.D.; Horny, H.P.; Roufosse, F.; Gotlib, J.; Weller, P.F.; Hellmann, A.; Metzgeroth, G.; Leiferman, K.M.; Arock, M.; et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012, 130, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Kephart, G.M.; Morikawa, A.; Gleich, G.J. Eosinophil infiltration and degranulation in normal human tissues: Evidence for eosinophil degranulation in normal gastrointestinal tract. Int. Arch. Allergy Immunol. 2001, 125, 55–58. [Google Scholar] [CrossRef]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Huang, L.; Beiting, D.P.; Gebreselassie, N.G.; Gagliardo, L.F.; Ruyechan, M.C.; Lee, N.A.; Lee, J.J.; Appleton, J.A. Eosinophils and IL-4 Support Nematode Growth Coincident with an Innate Response to Tissue Injury. PLoS Pathog. 2015, 11, e1005347. [Google Scholar] [CrossRef]
- Stein, L.H.; Redding, K.M.; Lee, J.J.; Nolan, T.J.; Schad, G.A.; Lok, J.B.; Abraham, D. Eosinophils utilize multiple chemokine receptors for chemotaxis to the parasitic nematode strongyloides stercoralis. J. Innate Immun. 2009, 1, 618–630. [Google Scholar] [CrossRef] [Green Version]
- Fabre, V.; Beiting, D.P.; Bliss, S.K.; Gebreselassie, N.G.; Gagliardo, L.F.; Lee, N.A.; Lee, J.J.; Appleton, J.A. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 2009, 182, 1577–1583. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. Immunol. 2013, 13, 9–22. [Google Scholar] [CrossRef]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.; Uller, L. Theirs but to die and do: Primary lysis of eosinophils and free eosinophil granules in asthma. Am. J. Respir. Crit. Care Med. 2014, 189, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Bulir, D.C.; Radford, K.; Kjarsgaard, M.; Huang, C.M.; Jacobsen, E.A.; Ochkur, S.I.; Catuneanu, A.; Lamothe-Kipnes, H.; Mahony, J.; et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. 2018, 141, 1269–1279. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.; Ochkur, S.I.; Protheroe, C.; Radford, K.; Efthimiadis, A.; Lee, N.A.; Lee, J.J. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, M.; Thomas, S.R.; Radford, K.; Dvorkin-Gheva, A.; Davydchenko, S.; Kjarsgaard, M.; Svenningsen, S.; Almas, S.; Felix, L.C.; Stearns, J.; et al. Sputum Antineutrophil Cytoplasmic Antibodies in Serum Antineutrophil Cytoplasmic Antibody-Negative Eosinophilic Granulomatosis with Polyangiitis. Am. J. Respir. Crit. Care Med. 2019, 199, 158–170. [Google Scholar] [CrossRef]
- Klion, A.D.; Nutman, T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004, 113, 30–37. [Google Scholar] [CrossRef]
- Drake, M.G.; Bivins-Smith, E.R.; Proskocil, B.J.; Nie, Z.; Scott, G.D.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am. J. Respir. Cell Mol. Biol. 2016, 55, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, G.A.; Yacoub, M.R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. Biomed. Res. Int. 2018, 2018, 28. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil extracellular traps and inflammatory pathologies-untangling the web! Front. Immunol. 2018, 9, 2763. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Long, H.; Liao, W.; Wang, L.; Lu, Q. A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System. Transfus. Med. Hemotherapy 2016, 43, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Padigel, U.M.; Lee, J.J.; Nolan, T.J.; Schad, G.A.; Abraham, D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect. Immun. 2006, 74, 3232–3238. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.K.; Fichtinger, P.S.; Kelly, J.T.; Lee, W.M.; Gern, J.E.; Jarjour, N.N. Interaction between allergy and innate immunity: Model for eosinophil regulation of epithelial cell interferon expression. Ann. Allergy Asthma Immunol. 2013, 111, 25–31.e1. [Google Scholar] [CrossRef] [Green Version]
- Prehn, A.; Seger, R.A.; Faber, J.; Torresani, T.; Molinari, L.; Gerber, A.; Sennhauser, F.H. The relationship of serum-eosinophil cationic protein and eosinophil count to disease activity in children with bronchial asthma. Pediatr. Allergy Immunol. 1998, 9, 197–203. [Google Scholar] [CrossRef]
- Rodriguez, L.; Pekkarinen, P.T.; Lakshmikanth, T.; Tan, Z.; Consiglio, C.R.; Pou, C.; Chen, Y.; Mugabo, C.H.; Nguyen, N.A.; Nowlan, K.; et al. Systems-Level Immunomonitoring from Acute to Recovery Phase of Severe COVID-19. Cell Rep. Med. 2020, 1, 100078. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Mukherjee, M.; Zhao, N.; Huang, C.; Radford, K.; Ashkar, A.A.; Nair, P. Asthma exacerbations on benralizumab are largely non-eosinophilic. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 375–379. [Google Scholar] [CrossRef]
- Varricchi, G.; Bagnasco, D.; Borriello, F.; Heffler, E.; Canonica, G.W. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: Evidence and unmet needs. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 186–200. [Google Scholar] [CrossRef] [Green Version]
- Humbles, A.A.; Lloyd, C.M.; McMillan, S.J.; Friend, D.S.; Xanthou, G.; McKenna, E.E.; Ghiran, S.; Gerard, N.P.; Yu, C.; Orkin, S.H.; et al. A Critical Role for Eosinophils in Allergic Airways Remodeling. Science 2004, 305, 1776–1779. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Maes, T.; Bracke, K.R. Eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 2013, 19, 977–979. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Koppelman, G.H. Biologic Therapies for Severe Asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Terl, M.; Sedlák, V.; Cap, P.; Dvořáková, R.; Kašák, V.; Kočí, T.; Novotna, B.; Seberova, E.; Panzner, P.; Zindr, V. Asthma management: A new phenotype-based approach using presence of eosinophilia and allergy. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 1279–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barnéon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Saglani, S.; Lloyd, C.M. Novel concepts in airway inflammation and remodelling in asthma. Eur. Respir. J. 2015, 46, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, A.J.; Dunnette, S.; Gleich, G.J.; Collins, J.V.; Kay, A.B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am. Rev. Respir. Dis. 1988, 137, 62–69. [Google Scholar] [CrossRef]
- Oddera, S.; Silvestri, M.; Balbo, A.; Jovovich, B.O.; Penna, R.; Crimi, E.; Rossi, G.A. Airway eosinophilic inflammation, epithelial damage, and bronchial hyperresponsiveness in patients with mild-moderate, stable asthma. Allergy 1996, 51, 100–107. [Google Scholar]
- Jatakanon, A.; Lim, S.; Barnes, P.J. Changes in sputum eosinophils predict loss of asthma control. Am. J. Respir. Crit. Care Med. 2000, 161, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Pizzichini, M.M.M.; Pizzichini, E.; Clelland, L.; Efthimiadis, A.; Pavord, I.; Dolovich, J.; Hargreave, F.E. Prednisone-dependent asthma: Inflammatory indices in induced sputum. Eur. Respir. J. 1999, 13, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.; Morgan, A.; Shaw, D.E.; Parker, D.; Green, R.; Brightling, C.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007, 62, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, K.; Leigh, R.; Hargreave, F.E. Sputum eosinophil count to assess compliance with corticosteroid therapy in asthma. J. Allergy Clin. Immunol. 1999, 104, 502–503. [Google Scholar] [CrossRef]
- Global Strategy for Asthma Management and Prevention (2022 Update); Global Initiative for Asthma: Fontana, WI, USA, 2022; pp. 1–186. Available online: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (accessed on 7 January 2022).
- Global Initiative for Asthma (GINA). Difficult-to-Treat Severe Asthma in Adolescent and Adult Patients GINA Pocket Guide for Health Professionals Diagnosis and Management. 2019. Available online: https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf (accessed on 30 April 2019).
- Arron, J.R.; Choy, D.F.; Scheerens, H.; Matthews, J.G. Noninvasive biomarkers that predict treatment benefit from biologic therapies in asthma. Ann. Am. Thorac. Soc. 2013, 10, S206–S213. [Google Scholar] [CrossRef]
- Hastie, A.T.; Moore, W.C.; Li, H.; Rector, B.M.; Ortega, V.E.; Pascual, R.M.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J. Allergy Clin. Immunol. 2013, 132, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Leckie, M.J.; ten Brinke, A.; Khan, J.; Diamant, Z.; O’Connor, B.J.; Walls, C.M.; Mathur, A.K.; Cowley, H.C.; Chung, K.F.; Djukanovic, R.; et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000, 356, 2144–2148. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.; Pizzichini, M.M.M.; Kjarsgaard, M.; Inman, M.D.; Efthimiadis, A.; Pizzichini, E.; Hargreave, F.E.; O’Byrne, P.M. Mepolizumab for Prednisone-Dependent Asthma with Sputum Eosinophilia. N. Engl. J. Med. 2009, 360, 985–993. [Google Scholar] [CrossRef]
- Pavord, I.D.; Haldar, P.; Bradding, P.; Wardlaw, A.J. Mepolizumab in refractory eosinophilic asthma. Thorax 2010, 65, 370. [Google Scholar] [CrossRef] [Green Version]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.; O’Byrne, P.M. Measuring Eosinophils to Make Treatment Decisions in Asthma. Chest 2016, 150, 485–487. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, P.; Visca, D.; Cherubino, F.; Zampogna, E.; Lucini, E.; Saderi, L.; Sotgiu, G.; Spanevello, A. Do blood eosinophils strictly reflect airway inflammation in COPD? Comparison with asthmatic patients. Respir. Res. 2019, 20, 145. [Google Scholar] [CrossRef] [Green Version]
- Chlumský, J.; Striz, I.; Terl, M.; Vondracek, J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J. Int. Med. Res. 2006, 34, 129–139. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Montanari, G.; Patricelli, G.; Cavazza, A.; Galeone, C.; Ruggiero, P.; Bagnasco, D.; Facciolongo, N. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther. Clin. Risk Manag. 2019, 15, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, M.; Nair, P. Blood or sputum eosinophils to guide asthma therapy? Lancet Respir. Med. 2015, 3, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, S.; Takahashi, K.; Kwong, F.N.K.; Xie, J.; Hoda, U.; Sun, K.; Elyasigomari, V.; Agapow, P.; Loza, M.; Baribaud, F.; et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur. Respir. J. 2019, 53, 1800938. [Google Scholar] [CrossRef]
- Kostikas, K.; Brindicci, C.; Patalano, F. Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD. Curr. Drug Targets 2018, 19, 1882–1896. [Google Scholar] [CrossRef]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Terl, M.; Pohunek, P.; Kuhn, M.; Bystron, J. Four seasons of Czech asthma study: Asthma characteristics and management reality in the Czech Republic. J. Asthma 2020, 57, 898–910. [Google Scholar] [CrossRef]
- Hekking, P.P.W.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The prevalence of severe refractory asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef]
- FitzGerald, M.; Bateman, E.D.; Boulet, L.-P.; Cruz, A.A.; Haahtela, T.; Levy, M.L.; O’Byrne, P.; Paggiaro, P.; Pedersen, S.E.; Soto-Quiroz, M.; et al. Global Strategy for Asthma Management and Prevention (GINA 2015). 2015. Available online: https://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdf (accessed on 30 July 2015).
- Menzella, F.; Galeone, C.; Ruggiero, P.; Bagnasco, D.; Catellani, C.; Facciolongo, N. Biologics and Bronchial Thermoplasty for severe refractory asthma treatment: From eligibility criteria to real practice. A cross-sectional study. Pulm. Pharmacol. Ther. 2020, 60, 101874. [Google Scholar] [CrossRef]
- Chand, N.; Harrison, J.E.; Rooney, S.; Pillar, J.; Jakubicki, R.; Nolan, K.; Diamantis, W.; Duane Sofia, R. Anti-IL-5 monoclonal antibody inhibits allergic late phase bronchial eosinophilia in guinea pigs: A therapeutic approach. Eur. J. Pharmacol. 1992, 211, 121–123. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Chupp, G.L.; Bradford, E.S.; Albers, F.C.; Bratton, D.J.; Wang-Jairaj, J.; Nelsen, L.M.; Trevor, J.L.; Magnan, A.; ten Brinke, A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): A randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir. Med. 2017, 5, 390–400. [Google Scholar] [CrossRef]
- Khatri, S.; Moore, W.; Gibson, P.G.; Leigh, R.; Bourdin, A.; Maspero, J.; Barros, M.; Buhl, R.; Howarth, P.; Albers, F.C.; et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. 2019, 143, 1742–1751.e7. [Google Scholar] [CrossRef] [Green Version]
- Lugogo, N.; Domingo, C.; Chanez, P.; Leigh, R.; Gilson, M.J.; Price, R.G.; Yancey, S.W.; Ortega, H.G. Long-term Efficacy and Safety of Mepolizumab in Patients With Severe Eosinophilic Asthma: A Multi-center, Open-label, Phase IIIb Study. Clin. Ther. 2016, 38, 2058–2070.e1. [Google Scholar] [CrossRef] [Green Version]
- Galkin, D.; Liu, M.C.; Chipps, B.E.; Chapman, K.R.; Muñoz, X.; Angel Bergna, M.; Azmi, J.; Mouneimne, D.; Joksaite, S.; Albers, F.C. Efficacy and Safety of Mepolizumab in Uncontrolled Patients with Severe Eosinophilic Asthma Following a Switch from Omalizumab (OSMO Study): Exacerbation and Safety Outcomes. J. Allergy Clin. Immunol. 2018, 141, AB409. [Google Scholar] [CrossRef]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef]
- Moiseev, S.; Zagvozdkina, E.; Kazarina, V.; Bulanov, N.; Novikov, P. Mepolizumab in patients with eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 2019, 144, 621. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [Green Version]
- Roufosse, F.; Kahn, J.-E.; Rothenberg, M.E.; Wardlaw, A.J.; Klion, A.D.; Kirby, S.Y.; Gilson, M.J.; Bentley, J.H.; Bradford, E.S.; Yancey, S.W.; et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 146, 1397–1405. [Google Scholar] [CrossRef]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase 3 Study. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corren, J.; Weinstein, S.; Janka, L.; Zangrilli, J.; Garin, M. Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. Chest 2016, 150, 799–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.; Mathur, S.; Hargreave, F.; Boulet, L.P.; Xie, F.; Young, J.; Jeffrey Wilkins, H.; Henkel, T.; Nair, P. Reslizumab for poorly controlled, eosinophilic asthma: A randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011, 184, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.J.; Velez, M.I.; Rogers, L. Reslizumab in the management of poorly controlled asthma: The data so far. J. Asthma Allergy 2016, 9, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Sahota, J.; Robinson, D.S. Update on new biologics for intractable eosinophilic asthma: Impact of reslizumab. Drug Des. Devel. Ther. 2018, 12, 1173–1181. [Google Scholar] [CrossRef] [Green Version]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’Acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2. [Google Scholar] [CrossRef]
- Laviolette, M.; Gossage, D.L.; Gauvreau, G.; Leigh, R.; Olivenstein, R.; Katial, R.; Busse, W.W.; Wenzel, S.; Wu, Y.; Datta, V.; et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J. Allergy Clin. Immunol. 2013, 132, 1086–1096. [Google Scholar] [CrossRef] [Green Version]
- Nowak, R.M.; Parker, J.M.; Silverman, R.A.; Rowe, B.H.; Smithline, H.; Khan, F.; Fiening, J.P.; Kim, K.; Molfino, N.A. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am. J. Emerg. Med. 2015, 33, 14–20. [Google Scholar] [CrossRef]
- Pham, T.H.; Damera, G.; Newbold, P.; Ranade, K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir. Med. 2016, 111, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Katial, R.; Gossage, D.; Sari, S.; Wang, B.; Kolbeck, R.; Coyle, A.J.; Koike, M.; Spitalny, G.L.; Kiener, P.A.; et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor α antibody, in a phase I study of subjects with mild asthma. J. Allergy Clin. Immunol. 2010, 125, 1237–1244. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Goldman, M.; Hirsch, I.; Zangrilli, J.G.; Newbold, P.; Xu, X. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: Subanalyses of the Phase III SIROCCO and CALIMA studies. Curr. Med. Res. Opin. 2017, 33, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of enhanced response with benralizumab for patients with severe asthma: Pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Wechsler, M.E.; Mark FitzGerald, J.; Menzies-Gow, A.; Wu, Y.; Hirsch, I.; Goldman, M.; Newbold, P.; Zangrilli, J.G. Baseline Patient Factor Impact on the Clinical Efficacy of Benralizumab for Severe Asthma. Eur. Respir. J. 2018, 52, 1800936. [Google Scholar] [CrossRef] [Green Version]
- Chipps, B.E.; Newbold, P.; Hirsch, I.; Trudo, F.; Goldman, M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann. Allergy Asthma Immunol. 2018, 120, 504–511.e4. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Bruni, A.; Terracciano, R.; Pelaia, G. Benralizumab in the treatment of severe asthma: Design, development and potential place in therapy. Drug Des. Devel. Ther. 2018, 12, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, G.T.; FitzGerald, J.M.; Bleecker, E.R.; Laviolette, M.; Bernstein, D.; LaForce, C.; Mansfield, L.; Barker, P.; Wu, Y.; Jison, M.; et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2017, 5, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Calzetta, L.; Rinaldi, B.; Cazzola, M. Pharmacokinetic/pharmacodynamic drug evaluation of benralizumab for the treatment of asthma. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Bleecker, E.R.; FitzGerald, J.M.; Ferguson, G.T.; Barker, P.; Sproule, S.; Olsson, R.F.; Martin, U.J.; Goldman, M.; Yañez, A.; et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir. Med. 2019, 7, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.L.M.; Rothenberg, M.E. 2021 Year in Review: Spotlight on Eosinophils. J. Allergy Clin. Immunol. 2021, 149, 517–524. [Google Scholar] [CrossRef]
- Singh, D.; Fuhr, R.; Bird, N.P.; Mole, S.; Hardes, K.; Man, Y.L.; Cahn, A.; Yancey, S.W.; Pouliquen, I.J. A Phase 1 study of the long-acting anti-IL-5 monoclonal antibody GSK3511294 in patients with asthma. Br. J. Clin. Pharmacol. 2022, 88, 702. [Google Scholar] [CrossRef]
- Bochner, B.S. “siglec”ting the allergic response for therapeutic targeting. Glycobiology 2016, 26, 546–552. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.A.; Chang, A.T.; Youngblood, B.A.; Bochner, B.S. Eosinophil and mast cell Siglecs: From biology to drug target. J. Leukoc. Biol. 2020, 108, 73–81. [Google Scholar] [CrossRef]
- Anesi, S.D.; Tauber, J.; Nguyen, Q.D.; Chang, P.; Berdy, G.J.; Lin, C.C.; Chu, D.S.; Levine, H.T.; Fernandez, A.D.; Roy, N.; et al. Lirentelimab for severe and chronic forms of allergic conjunctivitis. J. Allergy Clin. Immunol. 2022, 150, 631–639. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti–Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Walsh, G.M. Eosinophil apoptosis and clearance in asthma. J. Cell Death 2013, 6, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, E.A.; Jackson, D.J.; Heffler, E.; Mathur, S.K.; Bredenoord, A.J.; Pavord, I.D.; Akuthota, P.; Roufosse, F.; Rothenberg, M.E. Eosinophil Knockout Humans: Uncovering the Role of Eosinophils Through Eosinophil-Directed Biological Therapies. Annu. Rev. Immunol. 2021, 39, 719–757. [Google Scholar] [CrossRef]
- Prin, L.; Capron, M.; Tonnel, A.B.; Bletry, O.; Capron, A. Heterogeneity of human peripheral blood eosinophils: Variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int. Arch. Allergy Immunol. 1983, 72, 336–346. [Google Scholar] [CrossRef]
- Kroegel, C.; Liu, M.C.; Hubbard, W.C.; Lichtenstein, L.M.; Bochner, B.S. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: Surface phenotype, density heterogeneity, and prostanoid production. J. Allergy Clin. Immunol. 1994, 93, 725–734. [Google Scholar] [CrossRef]
- Kuo, H.P.; Yu, T.R.; Yu, C.T. Hypodense eosinophil number relates to clinical severity, airway hyperresponsiveness and response to inhaled corticosteroids in asthmatic subjects. Eur. Respir. J. 1994, 7, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Abdala Valencia, H.; Loffredo, L.F.; Misharin, A.V.; Berdnikovs, S. Phenotypic plasticity and targeting of Siglec-FhighCD11clow eosinophils to the airway in a murine model of asthma. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 267–271. [Google Scholar] [CrossRef]
- Percopo, C.M.; Brenner, T.A.; Ma, M.; Kraemer, L.S.; Hakeem, R.M.A.; Lee, J.J.; Rosenberg, H.F. SiglecF + Gr1 hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J. Leukoc. Biol. 2017, 101, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Matucci, A.; Nencini, F.; Maggiore, G.; Chiccoli, F.; Accinno, M.; Vivarelli, E.; Bruno, C.; Locatello, L.G.; Palomba, A.; Nucci, E.; et al. High proportion of inflammatory CD62L low eosinophils in blood and nasal polyps of severe asthma patients. Clin. Exp. Allergy 2023, 53, 78–87. [Google Scholar] [CrossRef]
- Yun, Y.; Kanda, A.; Kobayashi, Y.; Van Bui, D.; Suzuki, K.; Sawada, S.; Baba, K.; Yagi, M.; Asako, M.; Okazaki, H.; et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol. Int. 2020, 69, 232–238. [Google Scholar] [CrossRef]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Watanabe, T.; Saitoh, A.; Hirosaki, T.; Araki, Y.; Kikawada, T.; Betsuyaku, T.; Ohara, O.; et al. Dysregulated fatty acid metabolism in nasal polyp-derived eosinophils from patients with chronic rhinosinusitis. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1113–1124. [Google Scholar] [CrossRef]
- Mengelers, H.J.J.; Maikoe, T.; Hooibrink, B.; Kuypers, T.W.; Kreukniet, J.; Lammers, J.W.J.; Koenderman, L. Down modulation of L-Selection expression on eosinophils recovered from bronchoalveolar lavage fluid after allergen provocation. Clin. Exp. Allergy 1993, 23, 196–204. [Google Scholar] [CrossRef]
- Shah, K.; Ignacio, A.; McCoy, K.D.; Harris, N.L. The emerging roles of eosinophils in mucosal homeostasis. Mucosal Immunol. 2020, 13, 574–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farache Trajano, L.; Smart, N. Immunomodulation for optimal cardiac regeneration: Insights from comparative analyses. npj Regen. Med. 2021, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaikhly, T.; Murphy, R.C.; Parker, A.; Lai, Y.; Altman, M.C.; Larmore, M.; Altemeier, W.A.; Frevert, C.W.; Debley, J.S.; Piliponsky, A.M.; et al. Location of eosinophils in the airway wall is critical for specific features of airway hyperresponsiveness and T2 inflammation in asthma. Eur. Respir. J. 2022, 60, 2101865. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Dunnette, S.L.; Reed, C.E.; Ackerman, S.J.; Peters, M.S.; Gleich, G.J. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am. Rev. Respir. Dis. 1985, 132, 981–985. [Google Scholar] [CrossRef]
- Peters, M.S.; Gleich, G.J.; Dunnette, S.L.; Fukuda, T. Ultrastructural study of eosinophils from patients with the hypereosinophilic syndrome: A morphological basis of hypodense eosinophils. Blood 1988, 71, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Januskevicius, A.; Jurkeviciute, E.; Janulaityte, I.; Kalinauskaite-Zukauske, V.; Miliauskas, S.; Malakauskas, K. Blood Eosinophils Subtypes and Their Survivability in Asthma Patients. Cells 2020, 9, 1248. [Google Scholar] [CrossRef]
- Van Hulst, G.; Jorssen, J.; Jacobs, N.; Henket, M.; Louis, R.; Schleich, F.; Bureau, F.; Desmet, C.J. Anti-IL5 mepolizumab minimally influences residual blood eosinophils in severe asthma. Eur. Respir. J. 2022, 59, 2100935. [Google Scholar] [CrossRef]
- Ijaz, T.; Pazdrak, K.; Kalita, M.; Konig, R.; Choudhary, S.; Tian, B.; Boldogh, I.; Brasier, A.R. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ. J. 2014, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Diver, S.; Sridhar, S.; Khalfaoui, L.C.; Russell, R.J.; Emson, C.; Griffiths, J.M.; de Los Reyes, M.; Yin, D.; Colice, G.; Brightling, C.E. Feno differentiates epithelial gene expression clusters: Exploratory analysis from the MESOS randomized controlled trial. J. Allergy Clin. Immunol. 2022, 150, 830–840. [Google Scholar] [CrossRef]
- Denton, E.; Price, D.B.; Tran, T.N.; Canonica, G.W.; Menzies-Gow, A.; FitzGerald, J.M.; Sadatsafavi, M.; Perez de Llano, L.; Christoff, G.; Quinton, A.; et al. Cluster Analysis of Inflammatory Biomarker Expression in the International Severe Asthma Registry. J. Allergy Clin. Immunol. Pract. 2021, 9, 2680–2688.e7. [Google Scholar] [CrossRef]
- Chen, M.; Shepard, K.; Yang, M.; Raut, P.; Pazwash, H.; Holweg, C.T.J.; Choo, E. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin. Exp. Allergy 2021, 51, 546–555. [Google Scholar] [CrossRef]
- Corren, J.; Du, E.; Gubbi, A.; Vanlandingham, R. Variability in Blood Eosinophil Counts in Patients with Eosinophilic Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1224–1231.e9. [Google Scholar] [CrossRef]
- Kroes, J.A.; Zielhuis, S.W.; van Roon, E.N.; ten Brinke, A. Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem. Pharmacol. 2020, 179, 113978. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Matera, M.G.; Laitano, R.; Ritondo, B.L.; Hanania, N.A.; Cazzola, M. Severe Asthma and Biological Therapy: When, Which, and for Whom. Pulm. Ther. 2020, 6, 47–66. [Google Scholar] [CrossRef] [Green Version]
- Eger, K.; Kroes, J.A.; ten Brinke, A.; Bel, E.H. Long-Term Therapy Response to Anti–IL-5 Biologics in Severe Asthma—A Real-Life Evaluation. J. Allergy Clin. Immunol. Pract. 2021, 9, 1194–1200. [Google Scholar] [CrossRef]
- McDowell, P.J.; Diver, S.; Yang, F.; Borg, C.; Busby, J.; Brown, V.; Shrimanker, R.; Cox, C.; Brightling, C.E.; Chaudhuri, R.; et al. The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): A prospective observational study. Lancet Respir. Med. 2021, 9, 1174–1184. [Google Scholar] [CrossRef]
- Nair, P.; O’Byrne, P.M. Medical algorithms: Approach to adult asthma exacerbations. Allergy 2021, 76, 3556–3559. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Bafadhel, M.; Busse, W.W.; Casale, T.B.; Kocks, J.W.H.; Pavord, I.D.; Szefler, S.J.; Woodruff, P.G.; de Giorgio-Miller, A.; Trudo, F.; et al. An expert consensus framework for asthma remission as a treatment goal. J. Allergy Clin. Immunol. 2020, 145, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Menzies-Gow, A.; Szefler, S.J.; Busse, W.W. The Relationship of Asthma Biologics to Remission for Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1090–1098. [Google Scholar] [CrossRef]
- Maglio, A.; Vitale, C.; Pelaia, C.; D’Amato, M.; Ciampo, L.; Sferra, E.; Molino, A.; Pelaia, G.; Vatrella, A. Severe Asthma Remissions Induced by Biologics Targeting IL5/IL5r: Results from a Multicenter Real-Life Study. Int. J. Mol. Sci. 2023, 24, 2455. [Google Scholar] [CrossRef]
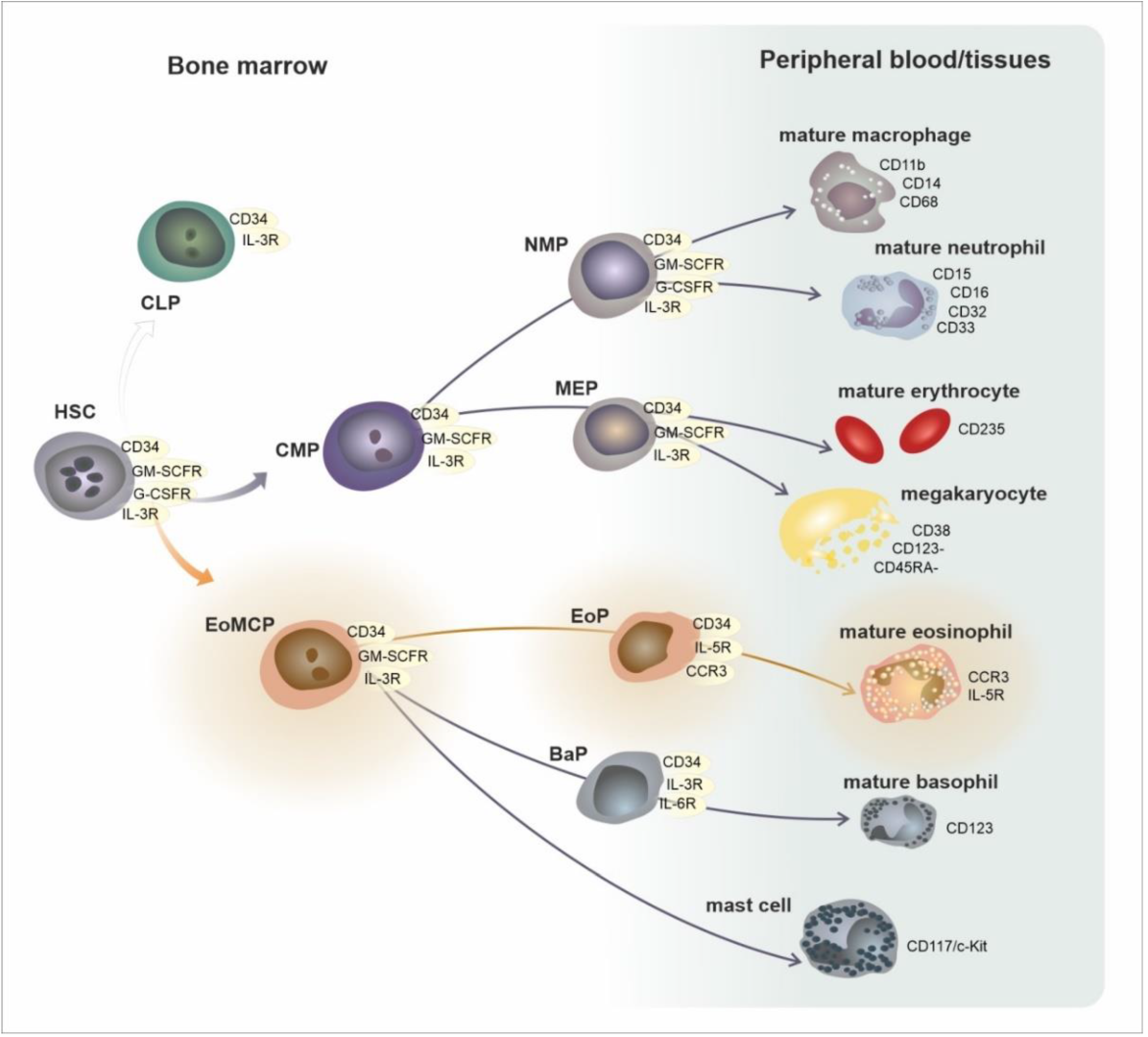
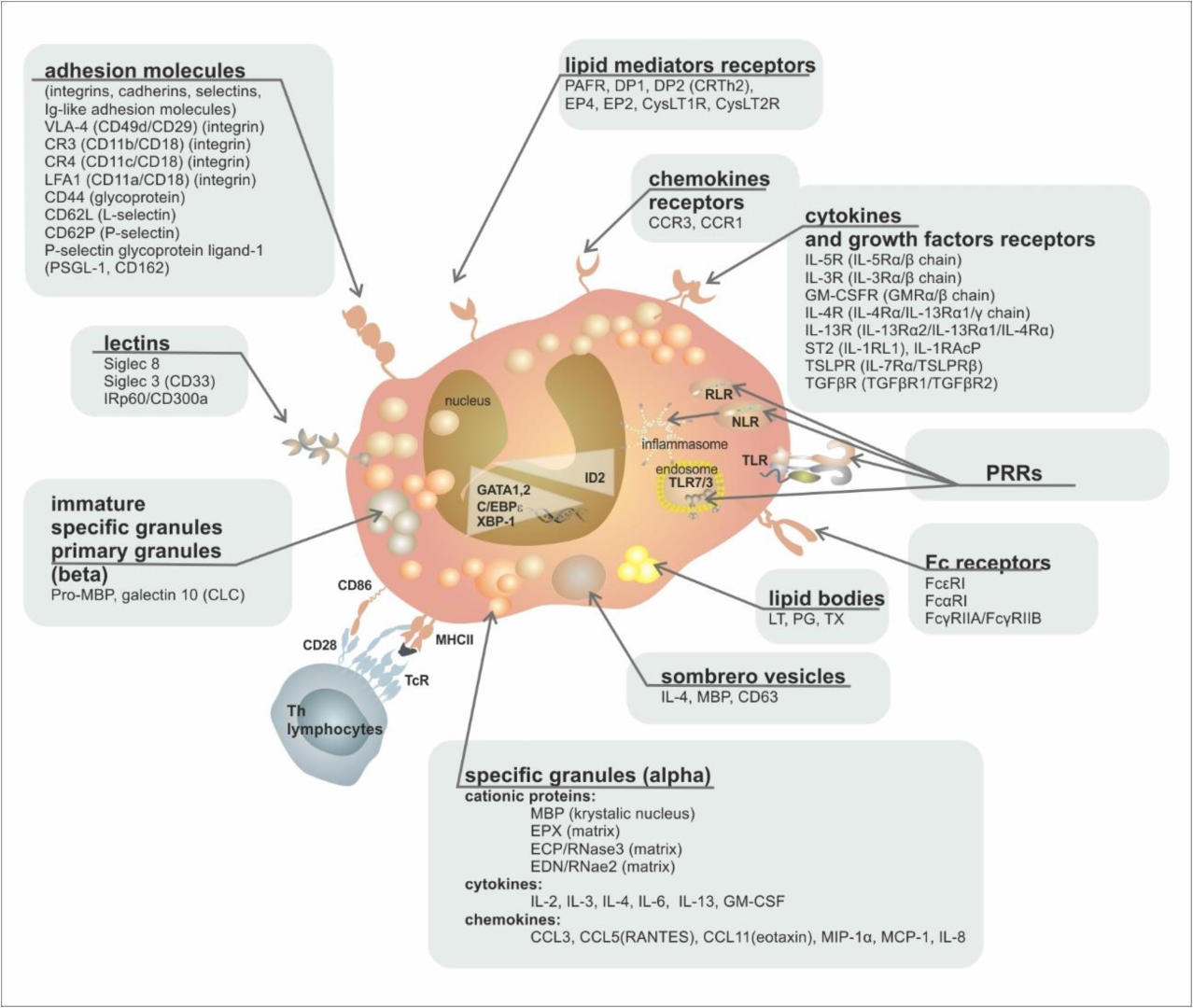
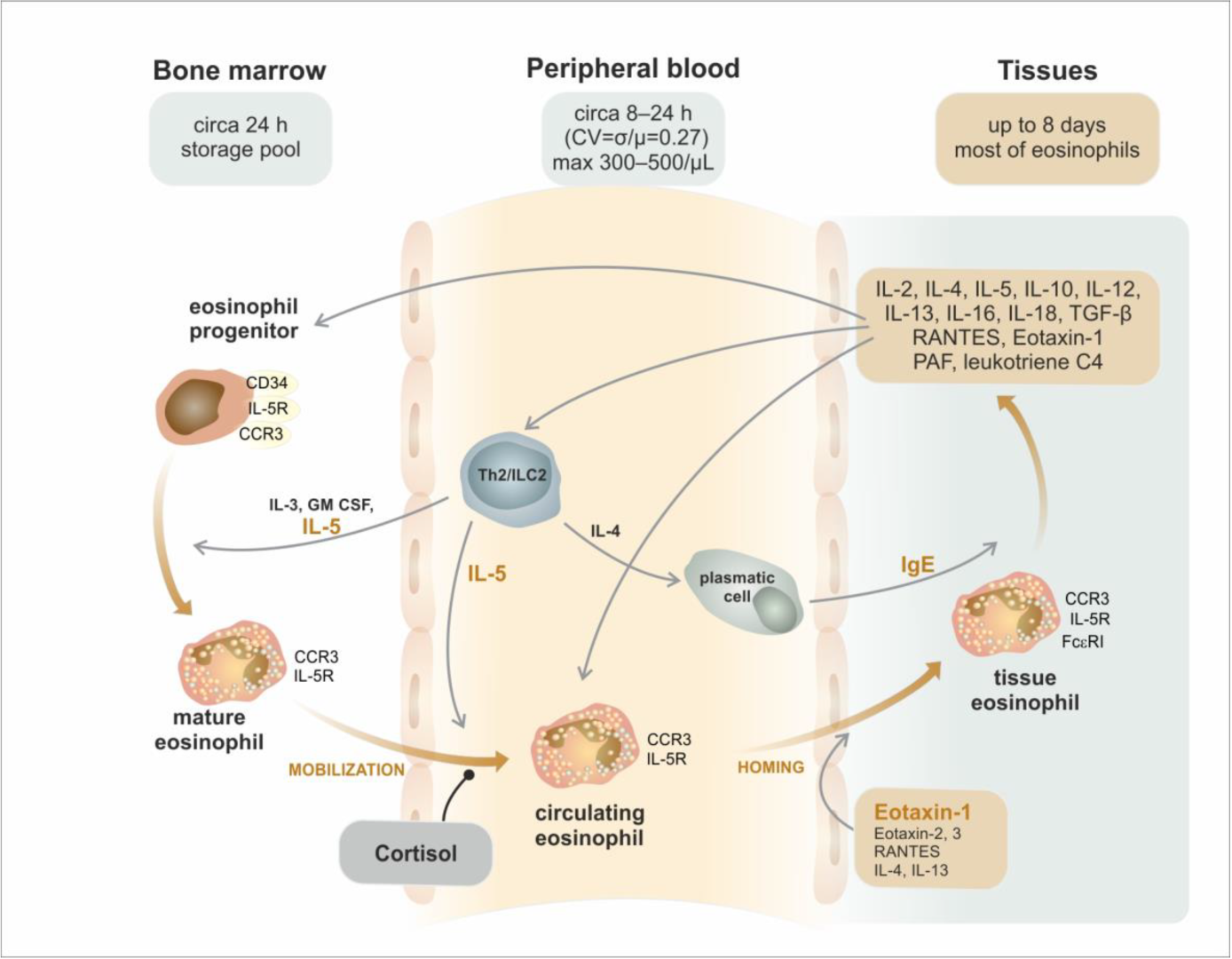



| Area/Subset | Developmental Cell Type | T1/2 | Phenotype |
|---|---|---|---|
| bone marrow | CMP—common myeloid progenitor EoMCP—eosinophil/mast cell progenitor | circa 24 h | CD34, GM-SCFR, IL-3R |
| EoP—eosinophil progenitor | CD34, GM-SCFR, IL-3R, CCR3, IL-5Rα | ||
| mature eosinophil | GM-SCFR, IL-3R, CCR3, IL-5Rα, VLA-4 (CD49d/CD29), PSGL-1 (CD162) | ||
| peripheral blood | mature eosinophil | 8–24 h | GM-SCFR, IL-3R, CCR3, IL-5Rα, VLA-4 (CD49d/CD29), PSGL-1 (CD162) |
| peripheral tissues | mature eosinophil | 8 days | GM-SCFR, IL-3R, CCR3, IL-5Rα, FcεRI, VLA-4 (CD49d/CD29), PSGL-1 (CD162) |
| activated eosinophil | ? | GM-SCFR, IL-3R, CCR3, IL-5Rα, FcεRI, CD63, CD9, CD69 | |
| Mature eosinophil subsets in mice and humans | |||
| mice | hEos—homeostatic eosinophil rEos—resident eosinophil (IL-5 independent) | lungs: 36 h, GIT: 6 days | Siglec-FmedCD62L+ CD101lo, CCR3, IL-5R, F4/80, CD11c− |
| iEos—inflammatory eosinophil (IL-5 dependent) | ? | Siglec-FhiCD62L−CD101hi, CCR3, IL-5R, CD11clow | |
| humans | hEos—homeostatic eosinophil rEos—resident eosinophil (IL-5 independent) | ? | Siglec-8+CD62L+IL-3Rlow |
| iEos—inflammatory eosinophil (IL-5 dependent) | ? | Siglec-8+CD62LlowIL-3Rhigh | |
| Group of Receptors | Receptor | Ligand | Function |
|---|---|---|---|
| cytokine and growth factor receptors | IL-5R (IL-5Rα/β chain)/(CD125/CD131) | IL-5 | proliferation, growth, bone marrow escape, survival |
| IL-3R (IL-3Rα/β chain)/(CD123/CD131) | IL-3 | growth, survival | |
| GM-CSFR (GMRα/β chain)/(CD116/CD131) | GM-CSF | growth, survival | |
| IL-4R (IL-4Rα/IL-13Rα1/γ chain) | IL-4 | activation | |
| IL-13R (IL-13Rα2/IL-13Rα1/IL-4Rα) | IL-13 | activation | |
| ST2 (IL-1RL1) | IL-33 | growth, survival | |
| TSLPR (IL-7Rα/TSLPRβ) | TSLP | growth, survival | |
| TGFβR (TGFβR1/TGFβR2) | TGFβ | inhibition of survival | |
| chemokine receptors | CCR3 | Eotaxin 1, 2, 3 (CCL11/CCL24/CCL26), MCP-3, MCP-4 | chemotaxis |
| CCR1 | MIP-1α (CCL3), RANTES (CCL5) | chemotaxis | |
| lipid mediator receptors | PAFR | PAF | activation |
| DP2 (CRTh2) | PgD2 | chemotaxis | |
| DP1 | PgD1 | chemotaxis | |
| EP4 | PgE2 | activation/inactivation | |
| EP2 | PgE2 | activation/inactivation | |
| CysLT1R | LTD4, LTC4, LTE4 | activation | |
| CysLT2R | LTD4, LTC4, LTE4 | activation | |
| Pattern Recognition Receptors (PRRs) | TLR1, 2, 3, 4, 5, 6, 7, 9, 10 (Toll-Like Receptors) | PAMPs | activation, degranulation |
| NLR1, 2 (NOD-Like Receptors) | PAMPs, DAMPs | activation | |
| RLR (RIG-Like Receptors) | dsRNA | activation | |
| PAR-2 | protease-activated receptor | activation | |
| RAGE (Receptor for Advanced Glycation Products) | Advanced Glycation Products | activation | |
| Fc receptors | FcεRI | IgE | nonactivation |
| FcαRI | IgA | activation | |
| FcγRIIA/FcγRIIB | IgG | activation/inactivation | |
| MHC | MHCII (+CD80, +CD86, +CD40) | TCR + CD4 | antigen presentation |
| adhesion molecules (integrins, cadherins, selectins, Ig-like adhesion molecules) | VLA-4 (CD49d/CD29) (integrin) | VCAM, fibronectin | activation, adhesion |
| CR3 (CD11b/CD18) (integrin) | iC3b | activation, adhesion | |
| CR4 (CD11c/CD18) (integrin) | iC3b | activation, adhesion | |
| LFA1 (CD11a/CD18) (integrin) | ICAM-1, ICAM-2 | activation, adhesion | |
| CD44 (glycoprotein) | hyaluronic acid | adhesion, homing | |
| CD62L (L-selectin) | CD34, GlyCAM-1, MadCAM-1 | adhesion, homing | |
| CD62P (P-selectin) | P-selectin glycoprotein ligand-1 (PSGL-1) | activation, adhesion | |
| PSGL-1 (P-selectin glycoprotein ligand, CD162) | P-selectin (CD62P) | activation, adhesion | |
| CD34 (fosfoglykoprotein) | L-selectin | adhesion, migration | |
| lectins | Siglec-8 | Sialyl–Lewis X (CD15s) | apoptosis induction |
| Siglec-3 (CD33) | sialyl acid | apoptosis induction | |
| IRp60/CD300a | sialyl acid | inhibition of growth signals (IL-3/IL-5/GMCSF) |
| Antimicrobial Immunity | Antigen Recognition by Eosinophils | Innate Immune Reaction Mediated by Eosinophils | Eosinophil-Dependent Adapted Immune Reaction |
|---|---|---|---|
| antiparasitic | IgA and IgG bonded to the parasite surface/FcαRI, FcγRII | cytotoxic proteins (MBP, EPX, ECP, EDN) | parasite antigen presentation |
| T-cell modulation response toward the Th2 subset | |||
| stimulation of IgM production | |||
| antifungal | mould aspartate proteases/PAR-2 (Protease Activated Receptor 2) | cytotoxic proteins (MBP, EDN) | unclear |
| β-glucan/CR3 (CD11b) | chemokine production (MIP-1α, MCP-1, IL-8) | ||
| antibacterial | PAMPs PRRs (TLR/Toll-like receptors/, NLR/NOD-like receptors/, RAGE/Receptor for Advanced Glycation Endproducts/) | phagocytosis and cytolysis (MBP, ECP) | bacterial antigen presentation IgM production stimulation |
| bactericidal effect (MBP, ECP, EPX, production of ROS) | |||
| extracellular traps (mitochondrial DNA, cytotoxic proteins) | |||
| antiviral | PAMPs | EDN and ECP—RNAase activity | viral antigen presentation |
| PRR (TLR7/3, RLR/RIG-I-like receptor/, NLR/NOD-like receptors/) | iNOS induction, NO synthesis | T-cell response modulation toward the Th1 subset | |
| IL-6 production |
| The Cut-Off Level of AEC | Frequency in Asthma | Clinical Interpretation |
|---|---|---|
| >150/μL | 69% | T2-high phenotype according to the GINA recommendations, prediction of the clinical response to anti-IL-4R therapy |
| >300/μL | 37% | prediction of the clinical response to antieosinophil treatment (anti-IL-5 (R)) |
| probable sputum eosinophilia | ||
| not significantly predictive concerning anti-IgE and anti-TSLP therapy | ||
| >400/μL | 16% | high risk of loss of control of asthma |
| Feature | Mepolizumab (anti IL-5), IgG1 kappa | Reslizumab (anti IL-5), IgG4 kappa | Benralizumab (anti Il-5Rα), IgG1 kappa | |
|---|---|---|---|---|
| laboratory | baseline AEC | ≥150/μL at baseline or ≥300/μL last year | ≥400/μL | not limited initially, posthoc ≥ 300/μL vs. <300/μL |
| allergy, baseline IgE | no limitation | no limitation | no limitation | |
| baseline FeNO | no limitation | no limitation | no limitation | |
| clinical | severe asthma exacerbation rate | ≥2 | ≥1 | ≥2 |
| inhaled corticosteroids | high doses | medium to high doses (≥440 μg fluticasone/day) | high doses | |
| oral corticosteroids | no limitation | limited ≤ 10 mg prednisone/day | no limitation | |
| FEV1 (%predicted) | <80% (<90% for age <18 yrs) at screening | no limitation | <80% (<90% for age <18 yrs) at screening and randomisation | |
| ACQ score | no limitation | ACQ-7 ≥ 1.5 at screening and randomisation | ACQ-6 ≥ 1.5 at screening | |
| GINA treatment step | 4–5 | 3–5 | 4–5 | |
| dosing | dosing interval | 4 weeks | 4 weeks | 4/8 weeks |
| dose | 100 mg (300 mg EGPA) | 3 mg/kg iv for 20–50 min | 30 mg | |
| route of application | sc | iv | sc | |
| home-use | yes | no | yes | |
| approved | other indications | EGPA, HES, CRSwNP | none | none |
| Mepolizumab | Reslizumab | Benralizumab | |
|---|---|---|---|
| Dosing/indication | 100 mg/4 weeks sc | 3 mg/kg/4 weeks iv | 30 mg/(4)8 weeks sc |
| (asthma, CRSwNP, EGPA, HES) | (asthma) | (asthma) | |
| 300 mg/4 weeks sc (EGPA) | |||
| 300 mg sc/750 mg iv/4 weeks (HES) | |||
| The onset of the clin. effect | 24 h.—max. in 4 weeks | 24 h.—max.? | 24 h. max. |
| Eosinophils BM | ↓ 70% (elevation of progenitors) | ↓ ? | ↓ ~100% (incl. progenitors) |
| Eosinophils PB | ↓ ~76–88% (according to dosing) | ↓ ~77–92% | ↓ ~100% |
| Eosinophils in sputum | ↓ 50% (progenitors?) | ↓ circa 56% (vs placebo) | ↓ 82–96% |
| Eosinophil receptors | ↑ mIL-5Rα?, ↓ IL-3Rα, ↑CRTH2 | IL-5Rα? | ↓ IL-5Rα mRNA |
| CCR3 no change | |||
| Other cells | No effect on basophils, mast cells?, no effect on T-ly or the elevation of T-ly IL-5+ | No effect on T-ly | ↓ basophils, mast cells? ILC2? |
| Other cytokines | ↑ IL-5, CCL-13, CCL-17, CCL-22, | ↑ IL-5, no change in IL-3 | IL-5?, IL-3?, ↑ eotaxin 1 and 2, IL-6 |
| eotaxin 1 (CCL-11), IL-3? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novosad, J.; Krčmová, I.; Souček, O.; Drahošová, M.; Sedlák, V.; Kulířová, M.; Králíčková, P. Subsets of Eosinophils in Asthma, a Challenge for Precise Treatment. Int. J. Mol. Sci. 2023, 24, 5716. https://doi.org/10.3390/ijms24065716
Novosad J, Krčmová I, Souček O, Drahošová M, Sedlák V, Kulířová M, Králíčková P. Subsets of Eosinophils in Asthma, a Challenge for Precise Treatment. International Journal of Molecular Sciences. 2023; 24(6):5716. https://doi.org/10.3390/ijms24065716
Chicago/Turabian StyleNovosad, Jakub, Irena Krčmová, Ondřej Souček, Marcela Drahošová, Vratislav Sedlák, Martina Kulířová, and Pavlína Králíčková. 2023. "Subsets of Eosinophils in Asthma, a Challenge for Precise Treatment" International Journal of Molecular Sciences 24, no. 6: 5716. https://doi.org/10.3390/ijms24065716
APA StyleNovosad, J., Krčmová, I., Souček, O., Drahošová, M., Sedlák, V., Kulířová, M., & Králíčková, P. (2023). Subsets of Eosinophils in Asthma, a Challenge for Precise Treatment. International Journal of Molecular Sciences, 24(6), 5716. https://doi.org/10.3390/ijms24065716





