Molecular Mechanisms Underlying TNFα-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle
Abstract
1. Introduction
2. Results
2.1. Dissociated Bronchiolar Cells Exhibited hASM Phenotype
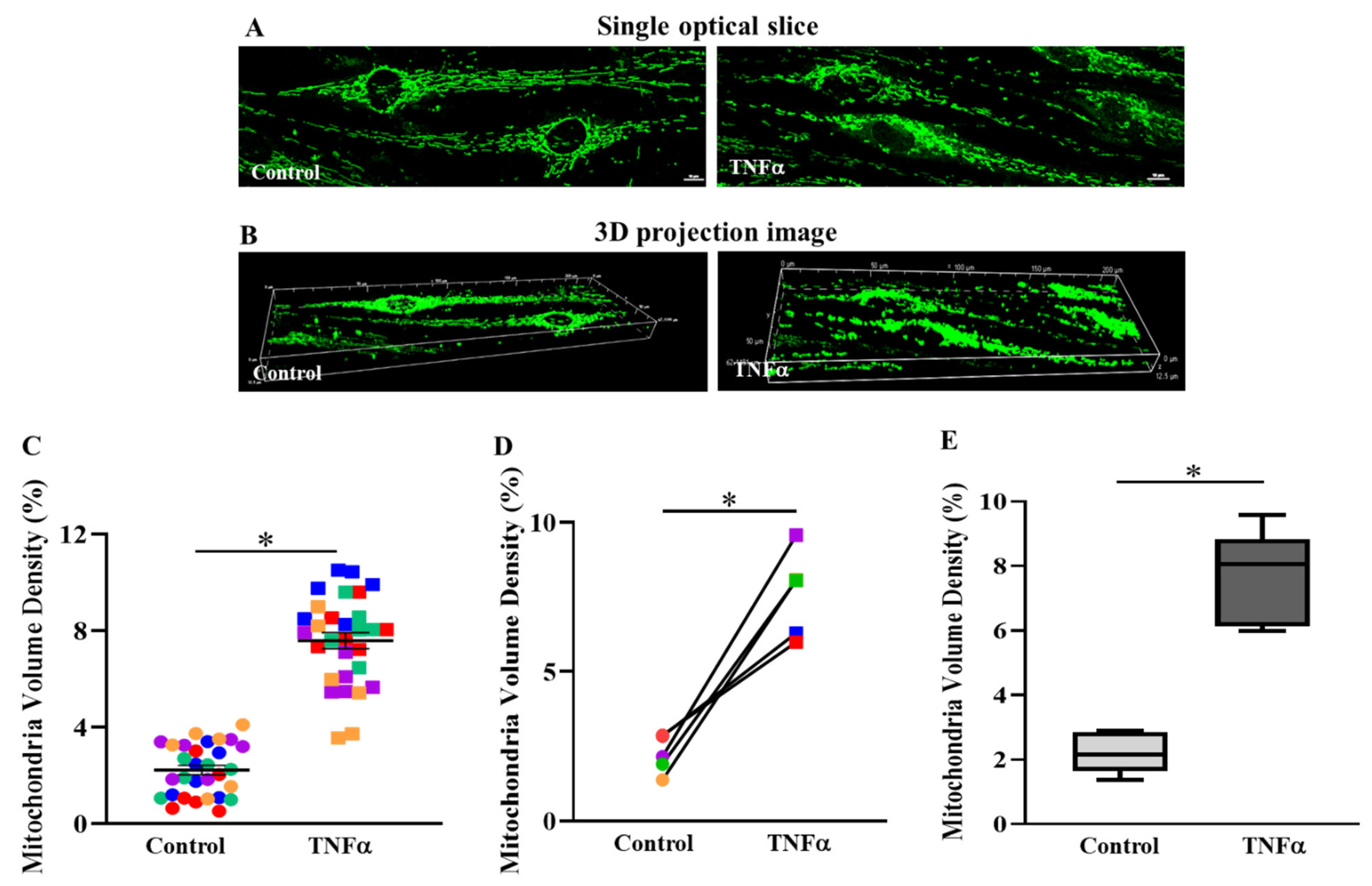
2.2. TNFα Increased Mitochondrial Volume Density in hASM Cells
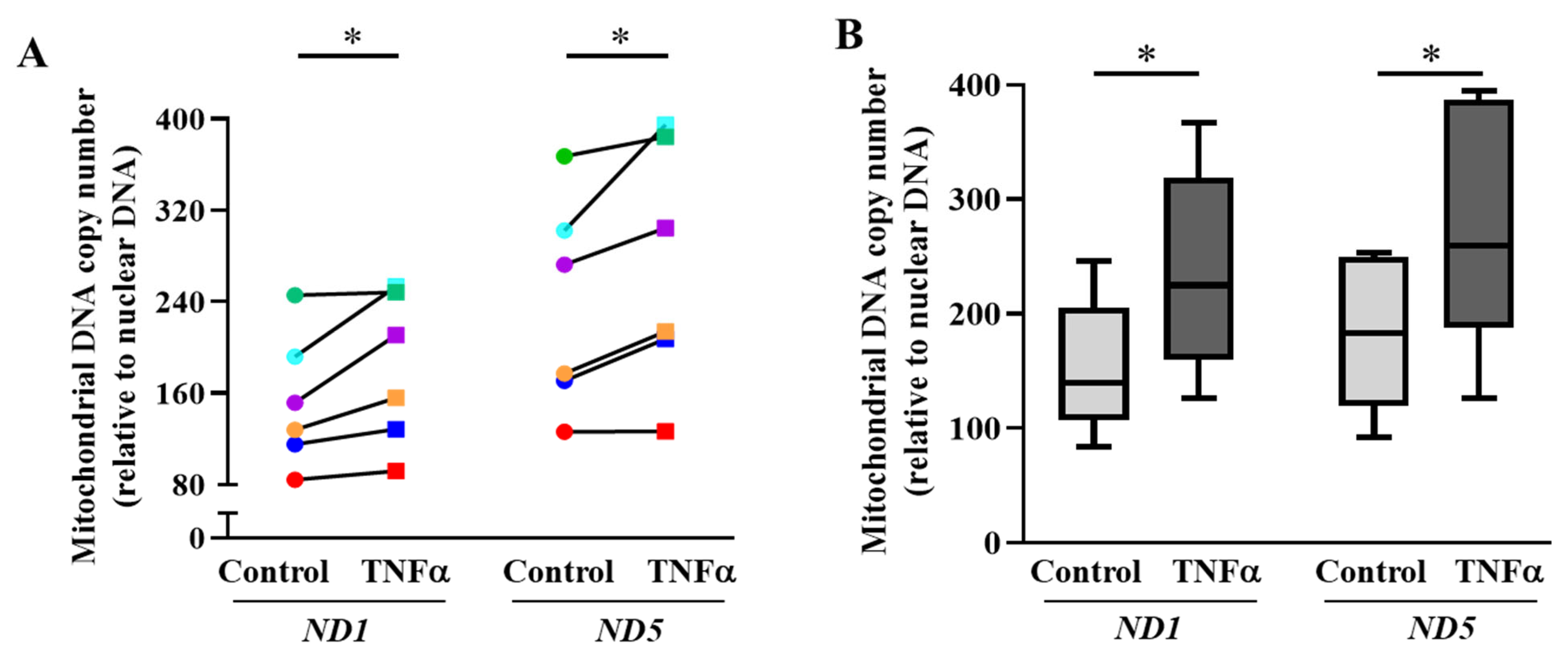
2.3. TNFα Increases mtDNA Copy Number and Mitochondrial Biogenesis in hASM Cells
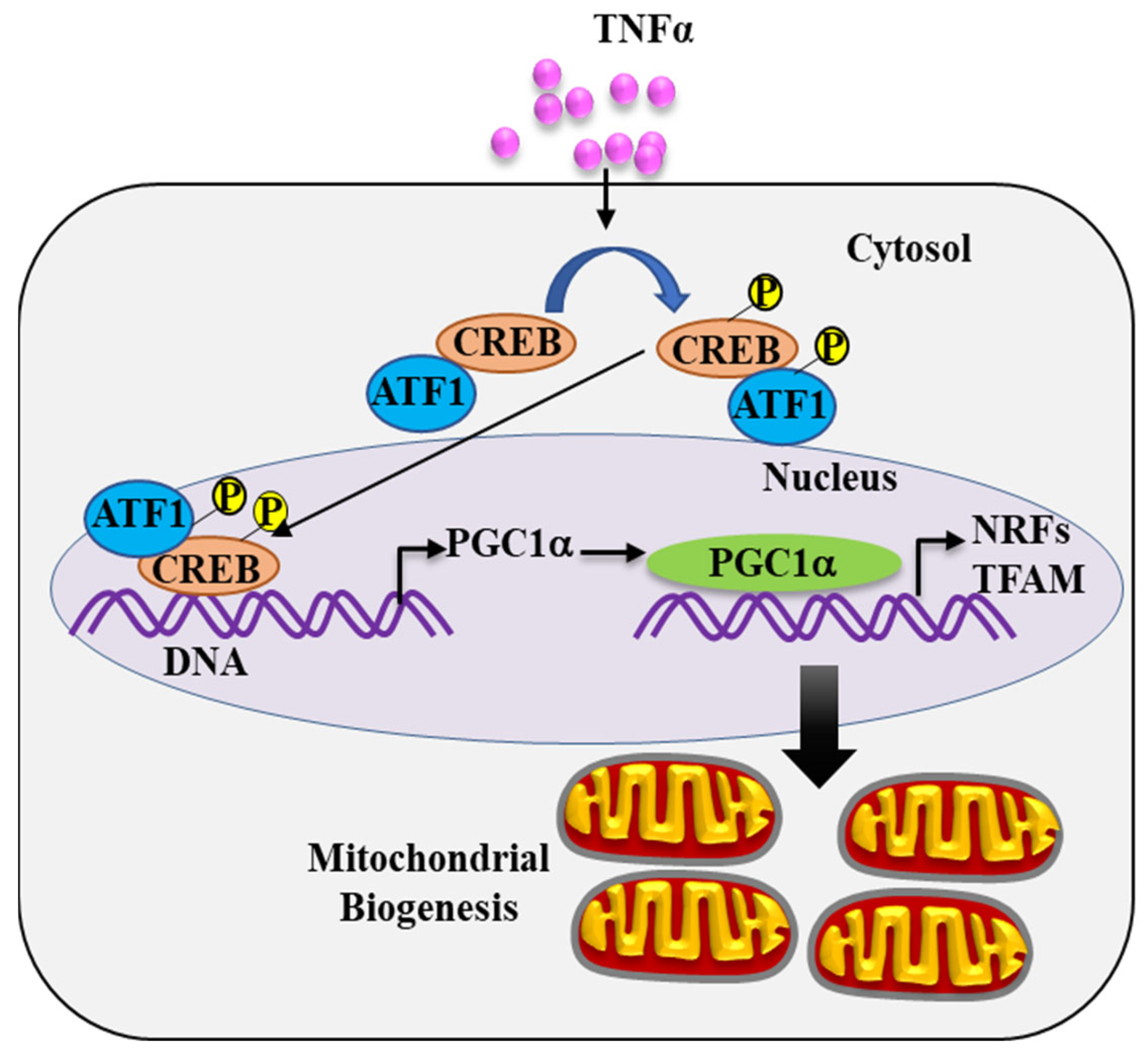
2.4. TNFα-Induced pCREBS133 and pATF1S63 Phosphorylation
2.5. pCREBS133 Transcriptionally Activates PGC1α in TNFα-Treated hASM Cells
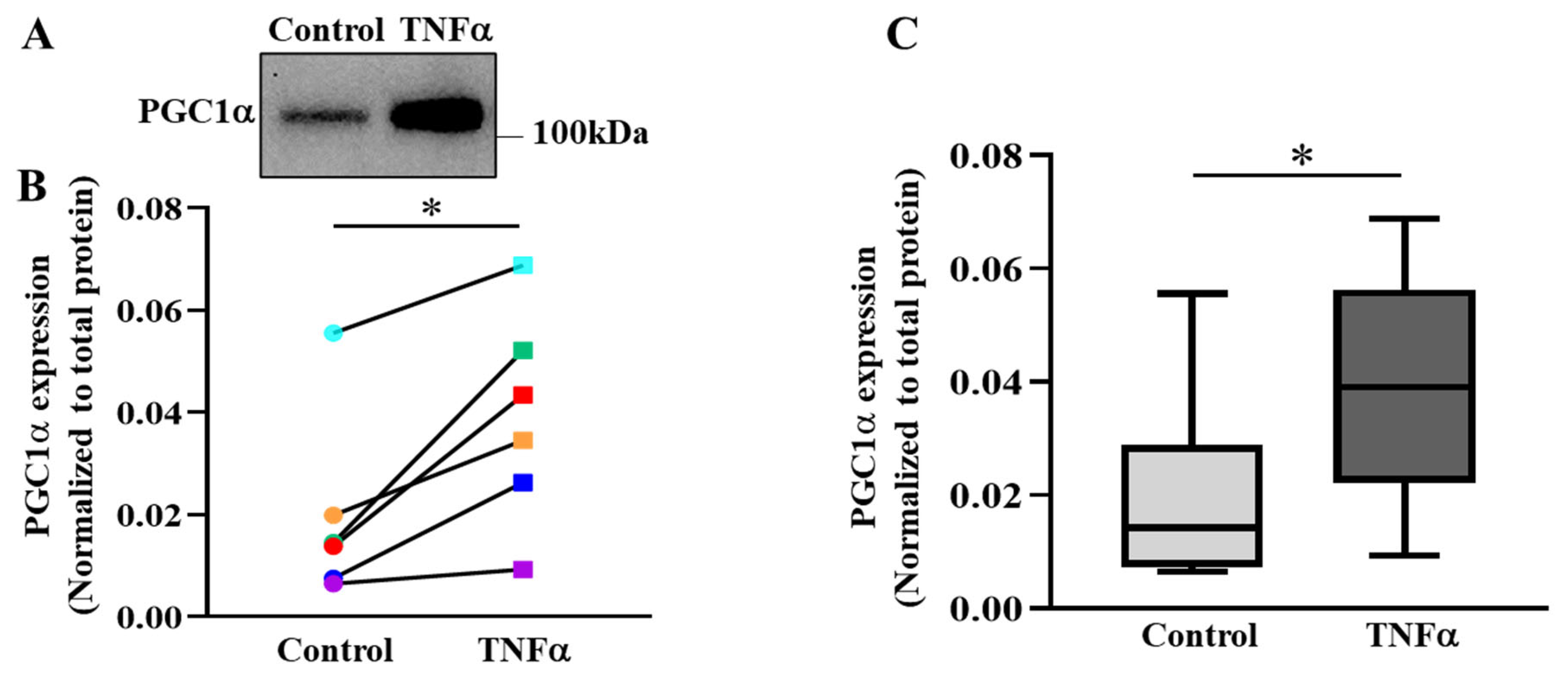
2.6. TNFα Increases the Expression of PGC1α in hASM Cells
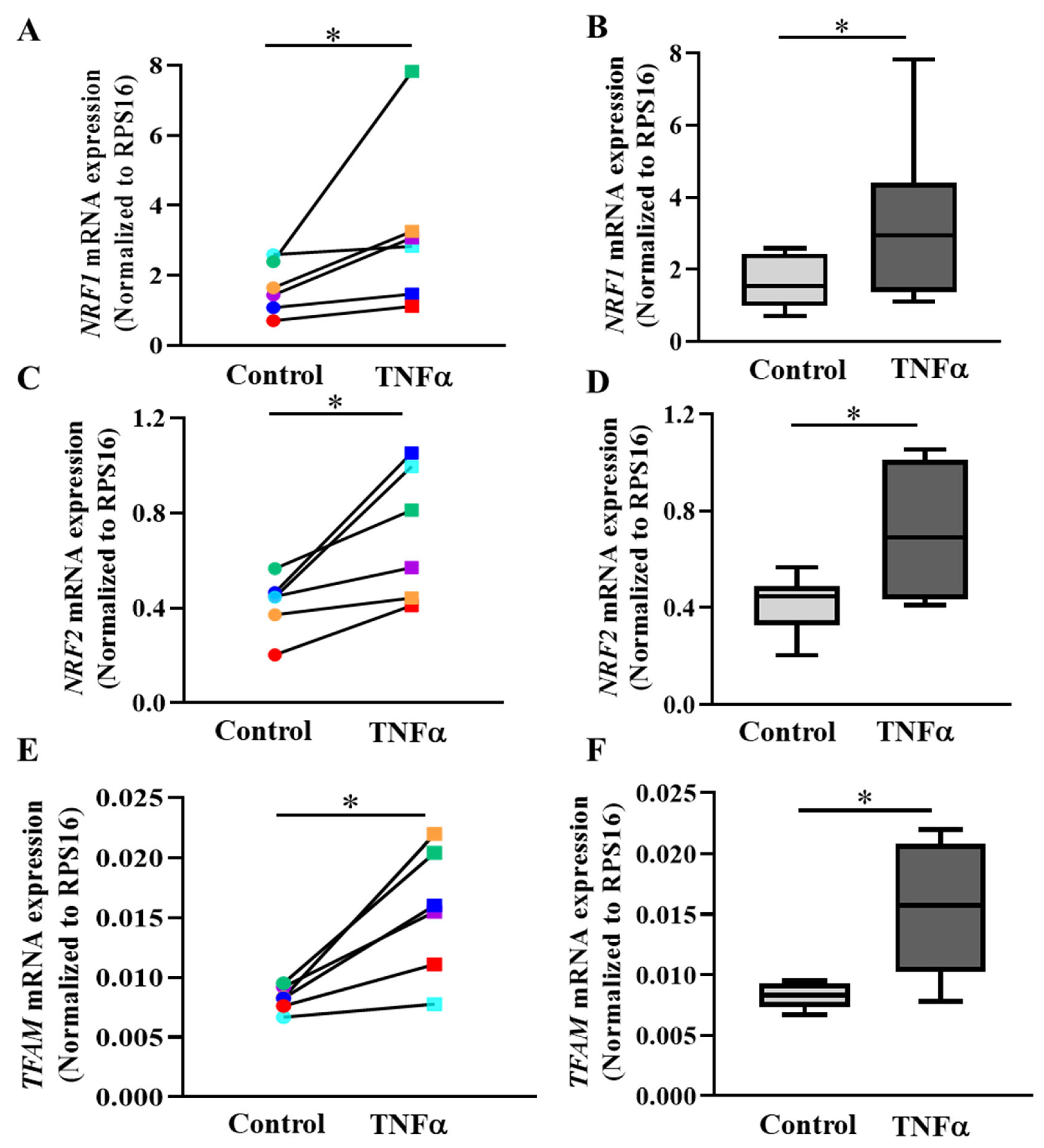
2.7. PGC1α Transcriptionally Activates Expression of Downstream Gene Targets
3. Discussion
3.1. TNFα Increases Mitochondrial Volume Density in hASM Cells
3.2. TNFα Increases Mitochondrial Biogenesis in hASM Cells
3.3. TNFα Increases pCREBS133 and pATF1S63 Phosphorylation in hASM Cells
3.4. pCREBS133 Transcriptionally Activates PGC1α Expression in hASM Cells
3.5. PGC1α Activates Transcription of NRFs and TFAM in hASM Cells
3.6. TNFα Induces an Increase in Metabolic Demand in hASM Cells
3.7. Clinical Significance
3.8. Experimental Limitations and Future Studies
4. Materials and Methods
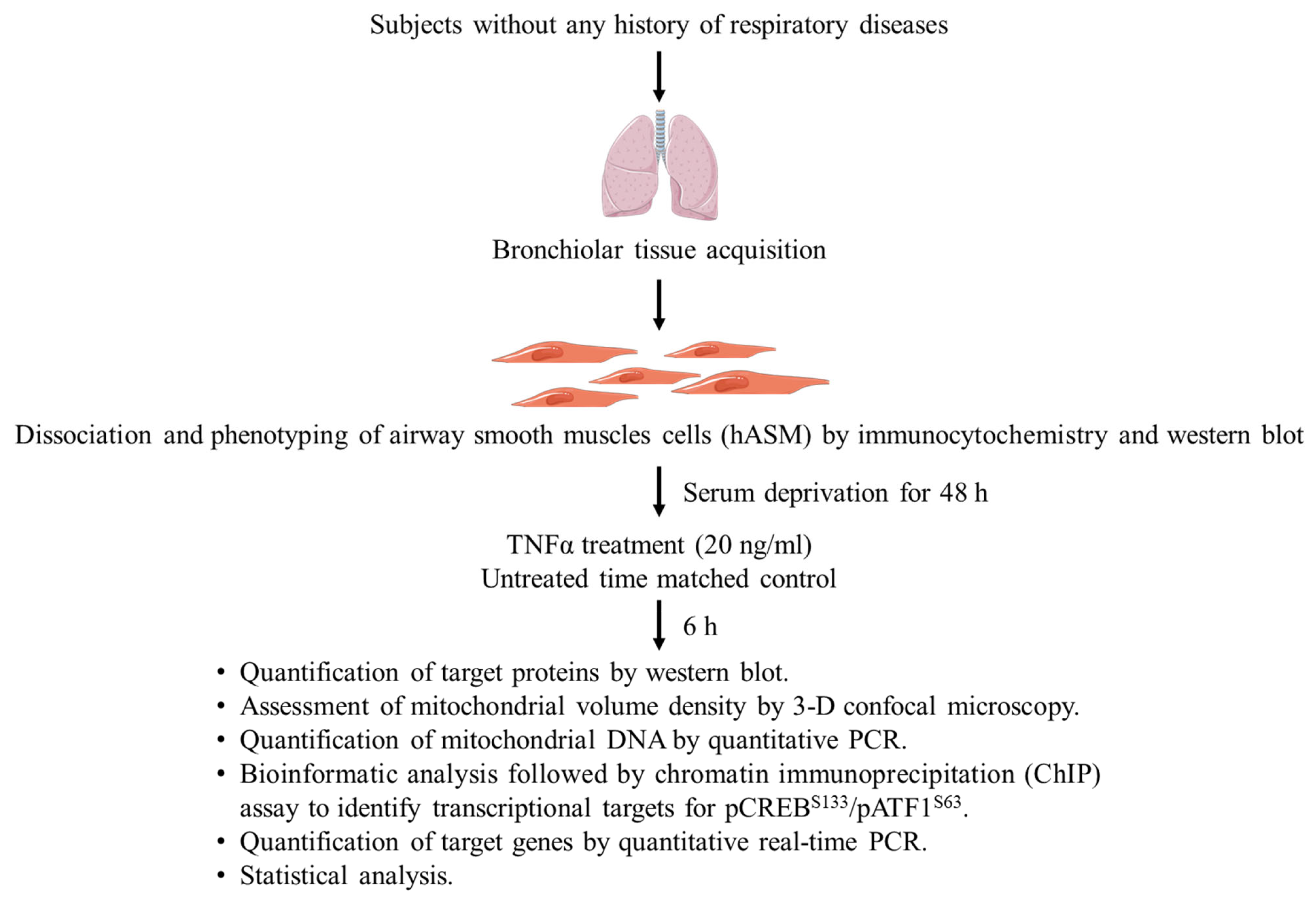
4.1. Dissociation of Cells from Bronchiolar Tissue
4.2. Phenotyping Dissociated hASM Cells
4.3. Experimental Design
4.4. Protein Extraction and Western Blot
4.5. Labeling and Confocal Imaging of Mitochondria in hASM Cells
4.6. Measurement of Mitochondrial Volume Density
4.7. Genomic DNA Extraction and Quantification of mtDNA
4.8. Bioinformatic Analysis for Transcription Factor Binding Site Prediction
4.9. Chromatin Immunoprecipitation Assay (ChIP)
4.10. RNA Extraction, cDNA Preparation and qPCR
4.11. Statistical Analysis
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Busse, W.W.; Calhoun, W.F.; Sedgwick, J.D. Mechanism of airway inflammation in asthma. Am. Rev. Respir. Dis. 1993, 147, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and airway remodeling in severe asthma. Allergy 2022, 77, 3538–3552. [Google Scholar] [CrossRef]
- Angelis, N.; Porpodis, K.; Zarogoulidis, P.; Spyratos, D.; Kioumis, I.; Papaiwannou, A.; Pitsiou, G.; Tsakiridis, K.; Mpakas, A.; Arikas, S.; et al. Airway inflammation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2014, 6 (Suppl. S1), S167–S172. [Google Scholar] [CrossRef]
- Eapen, M.S.; Myers, S.; Walters, E.H.; Sohal, S.S. Airway inflammation in chronic obstructive pulmonary disease (COPD): A true paradox. Expert Rev. Respir. Med. 2017, 11, 827–839. [Google Scholar] [CrossRef]
- Snoeck-Stroband, J.B.; Lapperre, T.S.; Gosman, M.M.; Boezen, H.M.; Timens, W.; ten Hacken, N.H.; Sont, J.K.; Sterk, P.J.; Hiemstra, P.S. Chronic bronchitis sub-phenotype within COPD: Inflammation in sputum and biopsies. Eur. Respir. J. 2008, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, L.; Fossali, T.; Frangipane, V.; Bozzini, S.; Morosini, M.; D’Amato, M.; Lettieri, S.; Urtis, M.; Di Toro, A.; Saracino, L.; et al. Broncho-alveolar inflammation in COVID-19 patients: A correlation with clinical outcome. BMC Pulm. Med. 2020, 20, 301. [Google Scholar] [CrossRef]
- Kaur, G.; Lungarella, G.; Rahman, I. SARS-CoV-2 COVID-19 susceptibility and lung inflammatory storm by smoking and vaping. J. Inflamm. 2020, 17, 21. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Dasgupta, D.; Delmotte, P.; Sieck, G.C. Inflammation-Induced Protein Unfolding in Airway Smooth Muscle Triggers a Homeostatic Response in Mitochondria. Int. J. Mol. Sci. 2020, 22, 363. [Google Scholar] [CrossRef] [PubMed]
- Sieck, G.C.; Dogan, M.; Young-Soo, H.; Osorio Valencia, S.; Delmotte, P. Mechanisms underlying TNFα-induced enhancement of force generation in airway smooth muscle. Physiol. Rep. 2019, 7, e14220. [Google Scholar] [CrossRef]
- Delmotte, P.; Marin Mathieu, N.; Sieck, G.C. TNFα induces mitochondrial fragmentation and biogenesis in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L137–L151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion 2015, 25, 67–75. [Google Scholar] [CrossRef]
- Kozhukhar, N.; Alexeyev, M.F. Limited predictive value of TFAM in mitochondrial biogenesis. Mitochondrion 2019, 49, 156–165. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef]
- Zhang, X.; Odom, D.T.; Koo, S.H.; Conkright, M.D.; Canettieri, G.; Best, J.; Chen, H.; Jenner, R.; Herbolsheimer, E.; Jacobsen, E.; et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 4459–4464. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Martin, K.J.; Arthur, J.S. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem. J. 2014, 458, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Ji, L.L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Kitaoka, Y.; Hayashi, Y.; Munemasa, Y.; Takeda, H.; Kumai, T.; Kobayashi, S.; Ueno, S. Axonal protection by brain-derived neurotrophic factor associated with CREB phosphorylation in tumor necrosis factor-alpha-induced optic nerve degeneration. Acta Neuropathol. 2009, 117, 75–84. [Google Scholar] [CrossRef]
- Gustin, J.A.; Pincheira, R.; Mayo, L.D.; Ozes, O.N.; Kessler, K.M.; Baerwald, M.R.; Korgaonkar, C.K.; Donner, D.B. Tumor necrosis factor activates CRE-binding protein through a p38 MAPK/MSK1 signaling pathway in endothelial cells. Am. J. Physiol. Cell. Physiol. 2004, 286, C547–C555. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Shanware, N.P.; Zhan, L.; Hutchinson, J.A.; Kim, S.H.; Williams, L.M.; Tibbetts, R.S. Conserved and distinct modes of CREB/ATF transcription factor regulation by PP2A/B56gamma and genotoxic stress. PLoS ONE 2010, 5, e12173. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, S.C.; Blendy, J.A.; Rudolph, D.; Monaghan, A.P.; Schmid, W.; Schütz, G. Activating transcription factor 1 and CREB are important for cell survival during early mouse development. Mol. Cell Biol. 2002, 22, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Samten, B.; Townsend, J.C.; Weis, S.E.; Bhoumik, A.; Klucar, P.; Shams, H.; Barnes, P.F. CREB, ATF, and AP-1 transcription factors regulate IFN-gamma secretion by human T cells in response to mycobacterial antigen. J. Immunol. 2008, 181, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D.; Davis, L.A.; Fogarty, M.J.; Sieck, G.C. Mitochondrial Fragmentation and Dysfunction in Type IIx/IIb Diaphragm Muscle Fibers in 24-Month Old Fischer 344 Rats. Front. Physiol. 2021, 12, 727585. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Rana, S.; Mantilla, C.B.; Sieck, G.C. Quantifying mitochondrial volume density in phrenic motor neurons. J. Neurosci. Methods 2021, 353, 109093. [Google Scholar] [CrossRef]
- Chaudhry, A.; Shi, R.; Luciani, D.S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E87–E101. [Google Scholar] [CrossRef]
- Shaywitz, A.J.; Greenberg, M.E. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 1999, 68, 821–861. [Google Scholar] [CrossRef]
- Ortega-Martínez, S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef]
- Xie, H.; Rothstein, T.L. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. J. Immunol. 1995, 154, 1717–1723. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Frank, D.A. CREB in the pathophysiology of cancer: Implications for targeting transcription factors for cancer therapy. Clin. Cancer Res. 2009, 15, 2583–2587. [Google Scholar] [CrossRef]
- Huang, S.R.; Ma, A.Y.; Liu, Y.; Qu, Y. Effects of inflammatory factors including plasma tumor necrosis factor-α in the clinical treatment of acute respiratory distress syndrome. Oncol. Lett. 2017, 13, 5016–5020. [Google Scholar] [CrossRef] [PubMed]
- Brindle, P.; Linke, S.; Montminy, M. Protein-kinase-A-dependent activator in transcription factor CREB reveals new role for CREM repressors. Nature 1993, 364, 821–824. [Google Scholar] [CrossRef]
- Amrani, Y.; Krymskaya, V.; Maki, C.; Panettieri, R.A., Jr. Mechanisms underlying TNF-alpha effects on agonist-mediated calcium homeostasis in human airway smooth muscle cells. Am. J. Physiol. 1997, 273, L1020–L1028. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Han, Y.S.; Delmotte, P.; Sieck, G.C. TNFα enhances force generation in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L994–L1002. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Rhee, J.; Lin, J.; Tarr, P.T.; Spiegelman, B.M. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 7111–7116. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh-Fard, E.; Saft, C.; Akkad, D.A.; Wieczorek, S.; Haghikia, A.; Chan, A.; Epplen, J.T.; Arning, L. PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease. Mol. Neurodegener. 2011, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A.; Suliman, H.B. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J. Biol. Chem. 2006, 281, 324–333. [Google Scholar] [CrossRef]
- Suliman, H.B.; Sweeney, T.E.; Withers, C.M.; Piantadosi, C.A. Co-regulation of nuclear respiratory factor-1 by NFkappaB and CREB links LPS-induced inflammation to mitochondrial biogenesis. J. Cell Sci. 2010, 123, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Mishra, M.; Kowluru, R.A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Blesa, J.R.; Prieto-Ruiz, J.A.; Hernández, J.M.; Hernández-Yago, J. NRF-2 transcription factor is required for human TOMM20 gene expression. Gene 2007, 391, 198–208. [Google Scholar] [CrossRef]
- Kim, M.; Jung, K.; Kim, I.S.; Lee, I.S.; Ko, Y.; Shin, J.E.; Park, K.I. TNF-α induces human neural progenitor cell survival after oxygen-glucose deprivation by activating the NF-κB pathway. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Yap, J.; Chen, X.; Delmotte, P.; Sieck, G.C. TNFα selectively activates the IRE1α/XBP1 endoplasmic reticulum stress pathway in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L483–L493. [Google Scholar] [CrossRef]
- Brown, A.D.; Fogarty, M.J.; Davis, L.A.; Dasgupta, D.; Mantilla, C.B.; Sieck, G.C. Mitochondrial adaptations to inactivity in diaphragm muscle fibers. J. Appl. Physiol. 2022, 133, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D.; Fogarty, M.J.; Sieck, G.C. Mitochondrial morphology and function varies across diaphragm muscle fiber types. Respir Physiol. Neurobiol. 2022, 295, 103780. [Google Scholar] [CrossRef]
- Meylan, P.; Dreos, R.; Ambrosini, G.; Groux, R.; Bucher, P. EPD in 2020: Enhanced data visualization and extension to ncRNA promoters. Nucleic Acids Res. 2020, 48, D65–D69. [Google Scholar] [CrossRef]



| Primary Ab | Manufacturer | Catalog Number | Application | Dilution |
|---|---|---|---|---|
| α-SMA | Abcam, Boston, MA, USA | ab5694 | ICC | 1:500 |
| FSP1/S100a4 | Abcam, Boston, MA, USA | ab124805 | ICC | 1:500 |
| α-SMA | Abcam, Boston, MA, USA | ab5694 | Western blot | 1:1000 |
| PGC1α | Novus Biologicals, Centennial, CO, USA | NBP1-04676 | Western blot | 1:1000 |
| pCREBS133/pATF1S63 | Cell Signaling Technology, Danvers, MA, USA | 9198S | Western blot | 1:1000 |
| Total CREB | Cell Signaling Technology, Danvers, MA, USA | 9104S | Western blot | 1:1000 |
| Total CREB | Cell Signaling Technology, Danvers, MA, USA | 4820S | ChIP | 1:1000 |
| NRF1 | Invitrogen, Carlsbad, CA, USA | MA5-32782 | Western blot | 1:1000 |
| Gene Name | Primer Name | Primer Sequence |
|---|---|---|
| NRF1 | NRF1-F | 5′-GCAACAGTAGCCACATTGGCT-3′ |
| NRF1-R | 5′-GTCGTCTGGATGGTCATCTCAC-3′ | |
| NRF2 | NRF2-F | 5′-CACATCCAGTCAGAAACCAGTGG3′ |
| NRF2-R | 5′-GGAATGTCTGCGCCAAAAGCTG-3′ | |
| PGC1α | PGC1α -ChIP-F | 5′-TGCTTGAAGCCTCCAAAAGT-3′ |
| PGC1α -ChIP-R | 5′-AGTAGGCTGGGCTGTCACTC-3′ | |
| RPS16 | RPS16-F | 5′-GTCTGTGCAGGTCTTCGGACGC-3′ |
| RPS16-R | 5′-GACCATTGCCGCGTTTGCAGTG-3′ | |
| TFAM | TFAM-F | 5′-GTGGTTTTCATCTGTCTTGGCAAG-3′ |
| TFAM-R | 5′-TTCCCTCCAACGCTGGGCAATT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, D.; Mahadev Bhat, S.; Price, A.L.; Delmotte, P.; Sieck, G.C. Molecular Mechanisms Underlying TNFα-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 5788. https://doi.org/10.3390/ijms24065788
Dasgupta D, Mahadev Bhat S, Price AL, Delmotte P, Sieck GC. Molecular Mechanisms Underlying TNFα-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle. International Journal of Molecular Sciences. 2023; 24(6):5788. https://doi.org/10.3390/ijms24065788
Chicago/Turabian StyleDasgupta, Debanjali, Sanjana Mahadev Bhat, Alexis L. Price, Philippe Delmotte, and Gary C. Sieck. 2023. "Molecular Mechanisms Underlying TNFα-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle" International Journal of Molecular Sciences 24, no. 6: 5788. https://doi.org/10.3390/ijms24065788
APA StyleDasgupta, D., Mahadev Bhat, S., Price, A. L., Delmotte, P., & Sieck, G. C. (2023). Molecular Mechanisms Underlying TNFα-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle. International Journal of Molecular Sciences, 24(6), 5788. https://doi.org/10.3390/ijms24065788








