Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins
Abstract
1. Introduction
2. Results
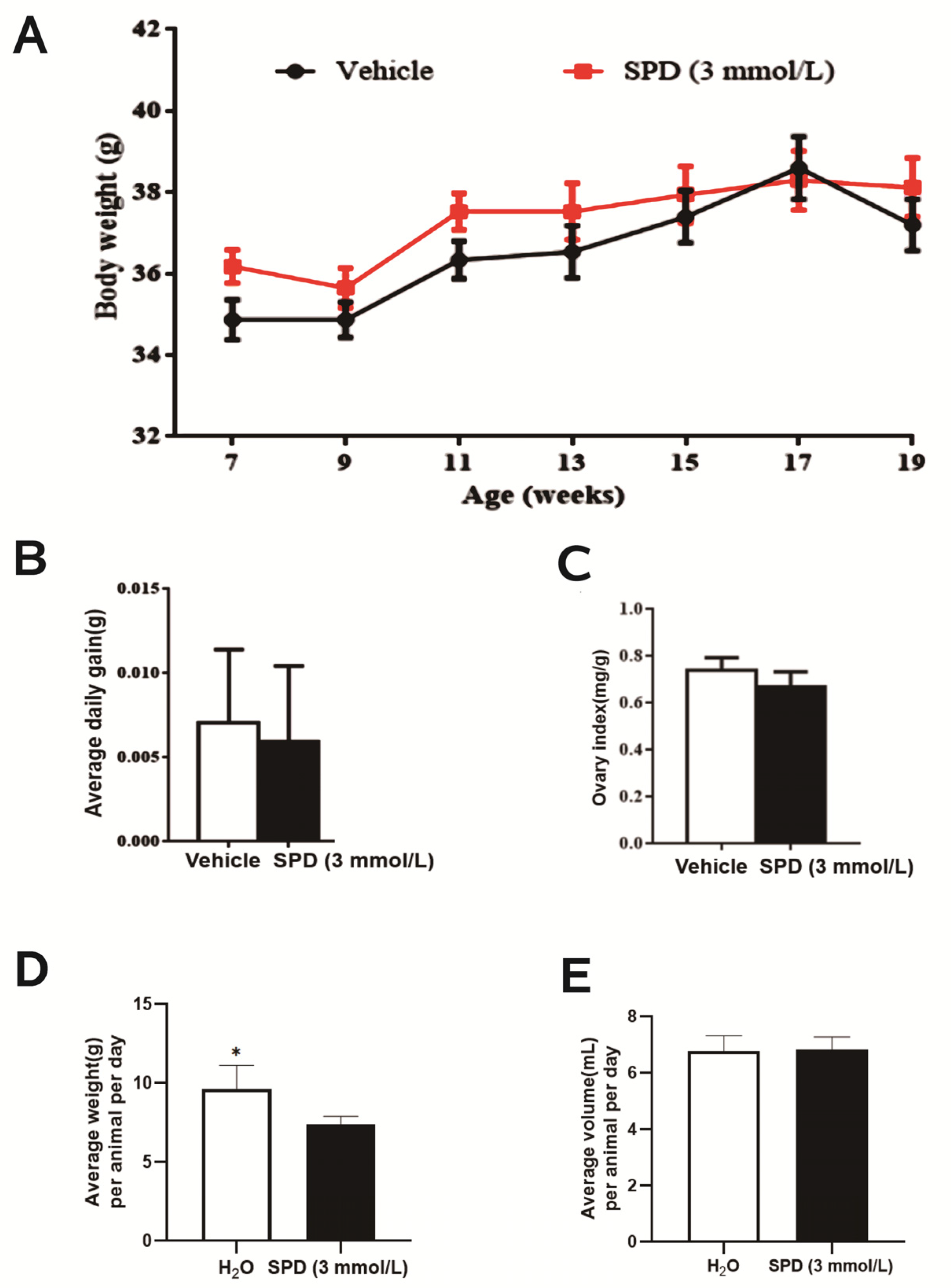
2.1. Effects of Spermidine on Water Intake, Body Weight and Ovarian Index in Mice
2.2. Effects of Spermidine on Ovarian Histomorphology and Follicular Development in Mice
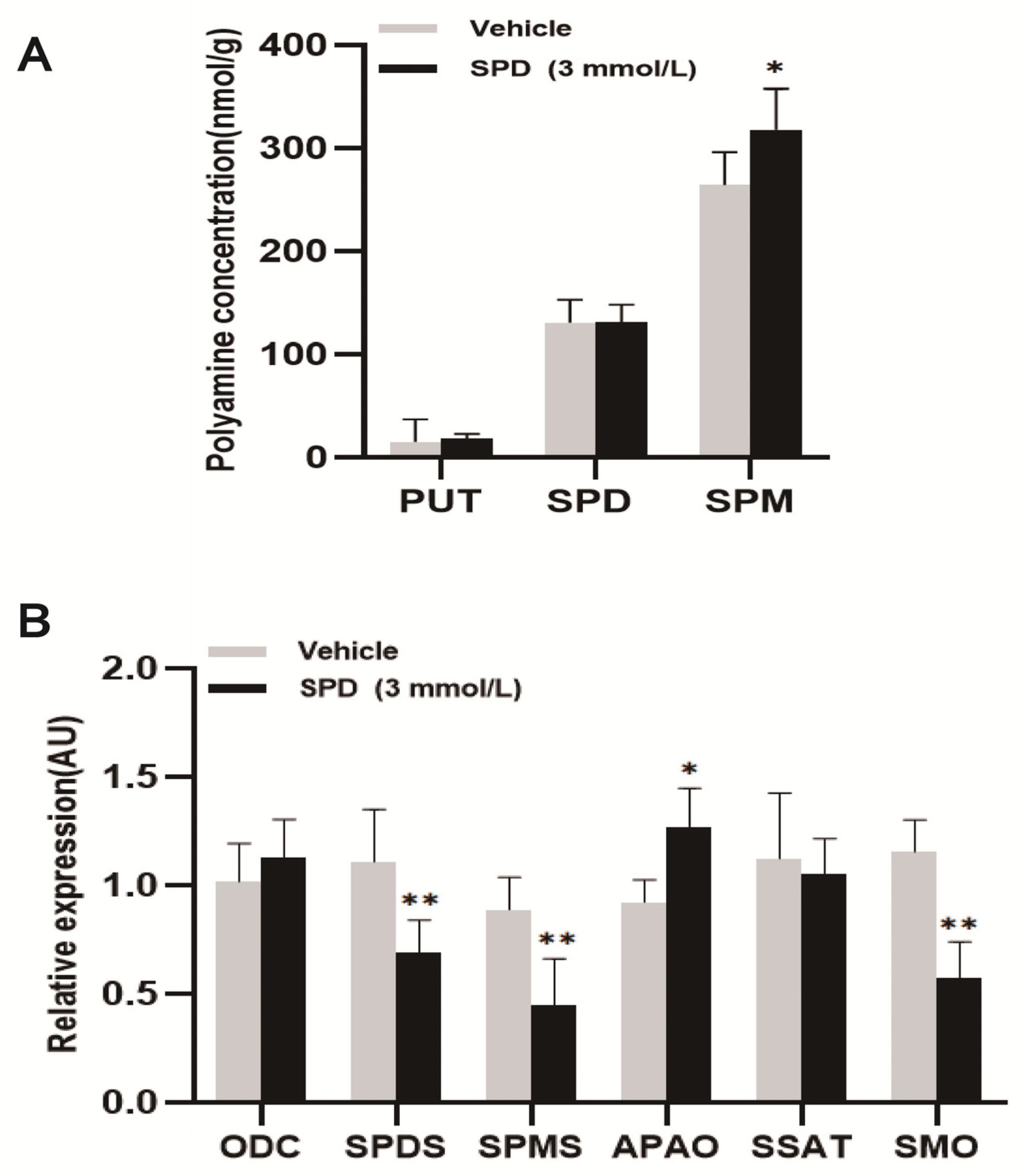
2.3. Effects of Spermidine on Polyamine Content and Expression of Key Metabolic Genes in Mouse Ovaries
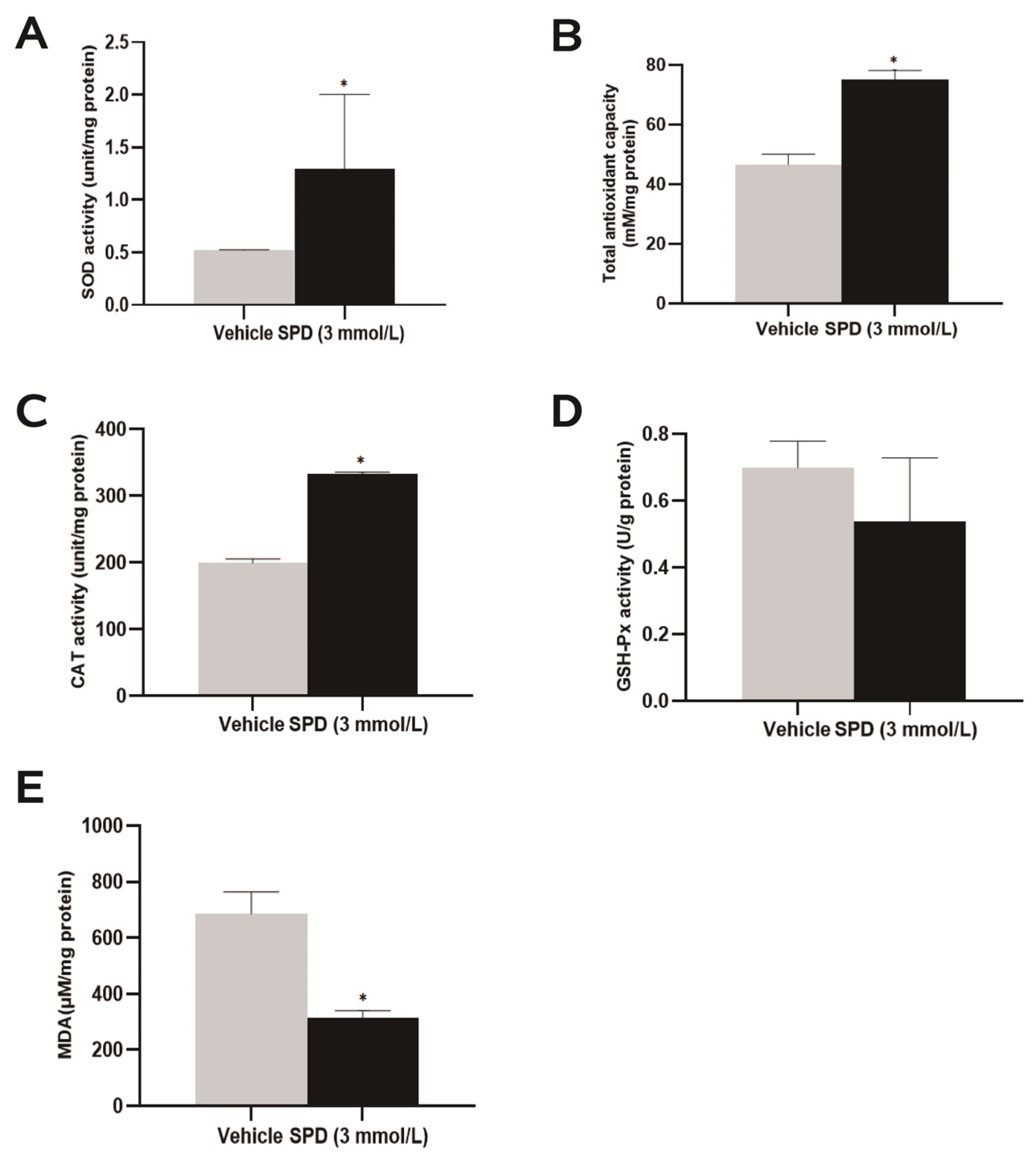
2.4. Spermidine Activates Antioxidant Enzyme Activity to Protect the Ovary
2.5. Spermidine Induces Ovarian Autophagy in Mice
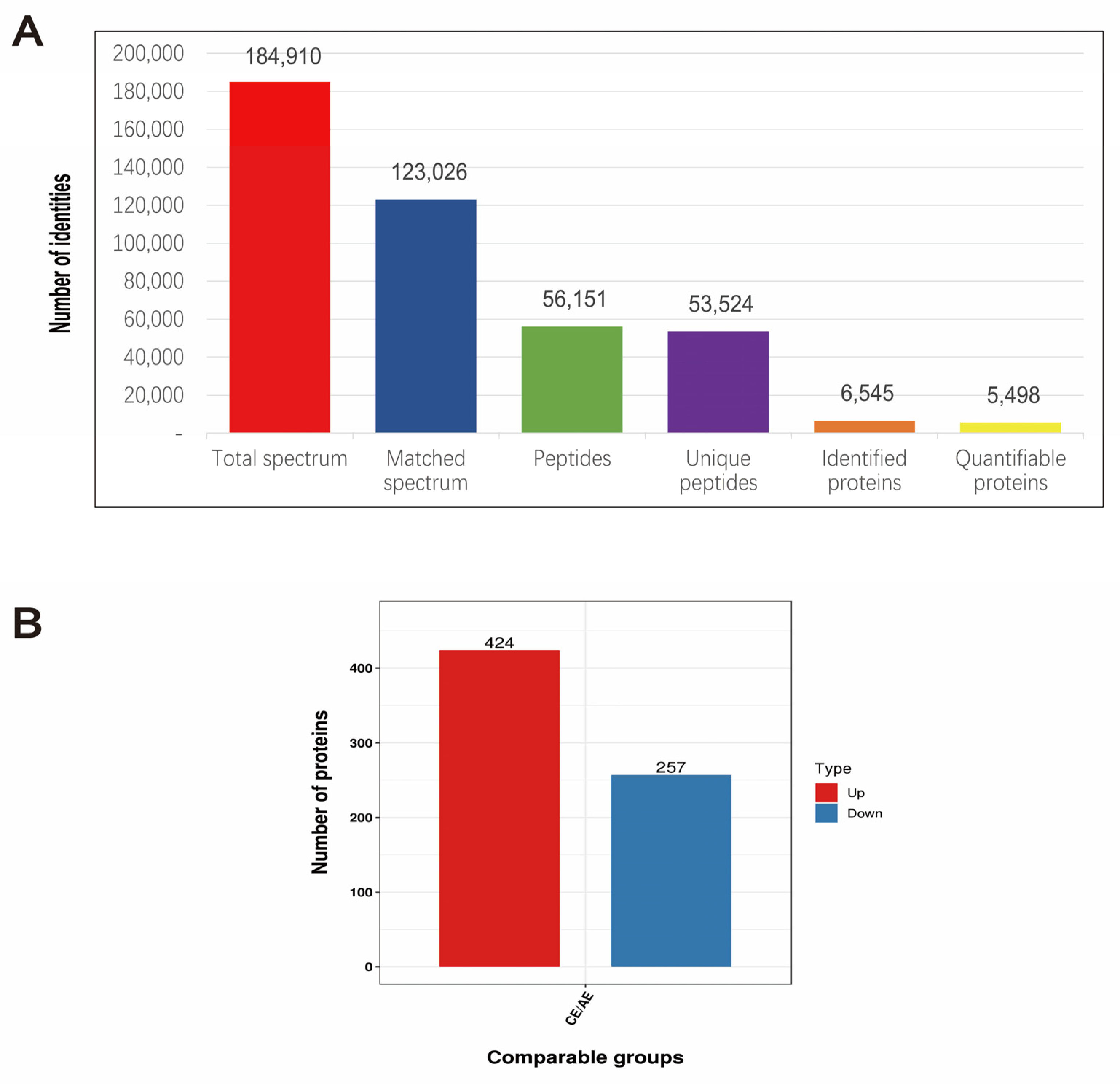
2.6. Protein Sample Consistency Test and Protein Identification
2.7. Screening of Differentially Expressed Proteins
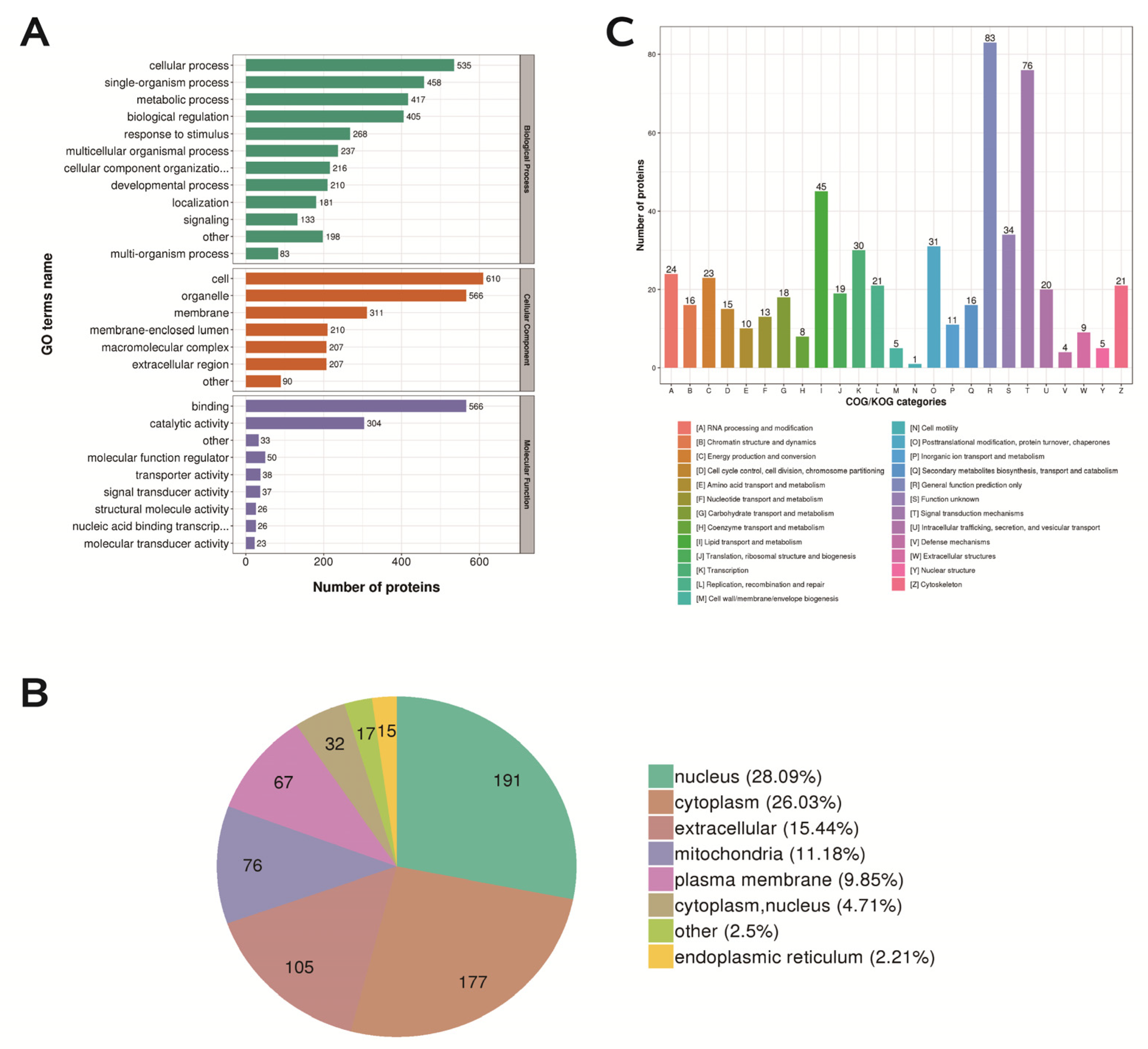
2.8. Functional Classification, Subcellular Structure Localization Classification, and COG/KOG Functional Classification of Differentially Expressed Proteins
2.9. Functional Enrichment Analysis of Differentially Expressed Proteins
2.10. Protein Domain Enrichment
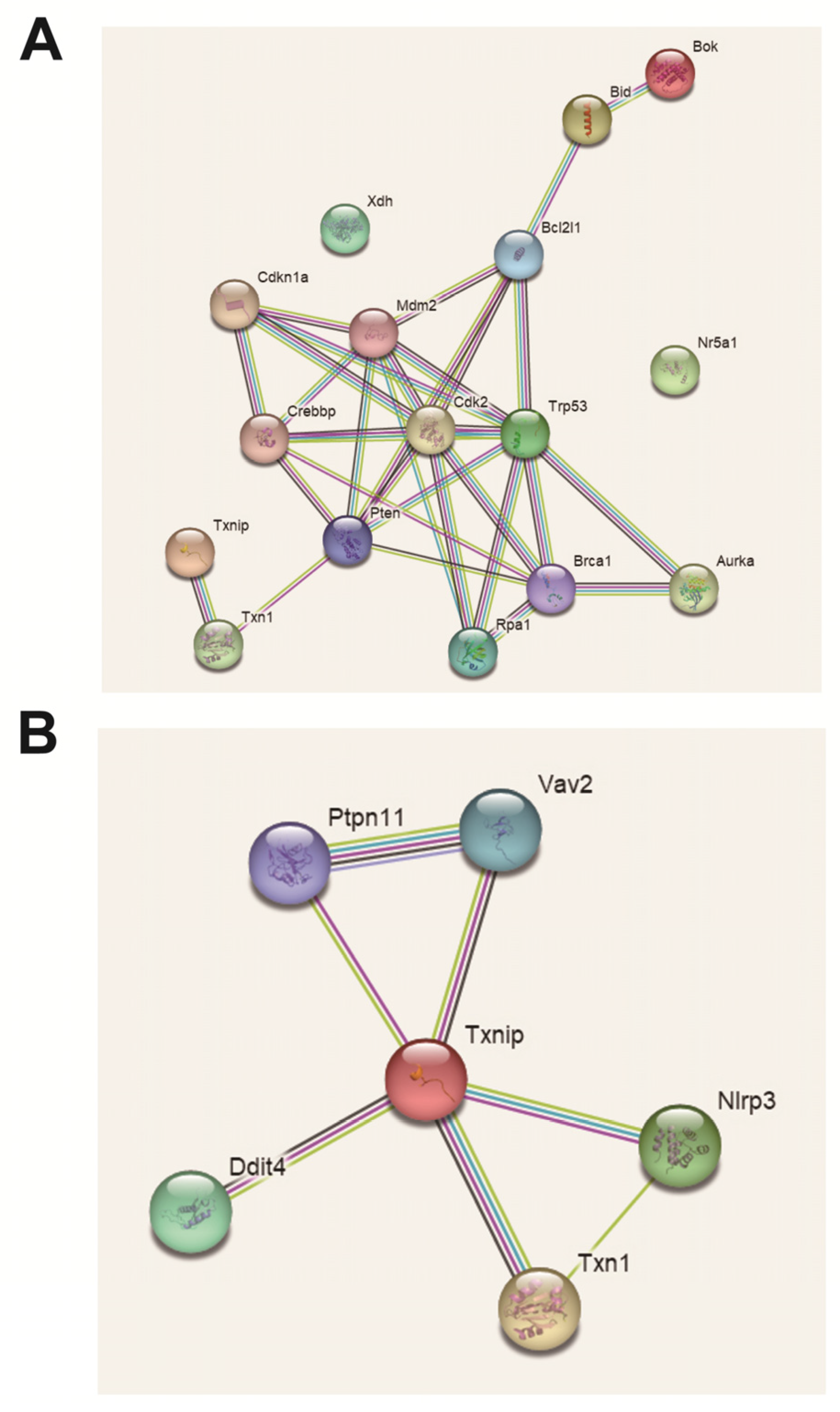
2.11. Analysis of Protein Interaction Network
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Animals and Sample Collection
5.2. Main Reagents and Kits
5.3. Analysis Software
5.4. Morphological Detection of Ovarian Tissue
5.5. Immunohistochemical Detection of Autophagy-Related Proteins in Ovarian Tissue
5.6. Antioxidant Index and Lipid Peroxidation Levels Detection
5.7. Determination of Polyamine Content in Mouse Ovarian Tissue by High-Performance Liquid Chromatography
5.8. Real-Time Fluorescence Quantitative PCR Detection
5.9. Western Blot Detection of Protein Expression
5.10. Protein Extraction and Consistency Test of Repeated Samples
5.11. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
5.12. Database Search and Bioinformatics Analysis
5.13. Differential Protein Screening
5.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P.; Sachdeva, S.N. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. J. Cell. Physiol. 2022, 237, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [PubMed]
- Zhou, J.; Peng, X.; Mei, S. Autophagy in Ovarian Follicular Development and Atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L.; Hsueh, A.J. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 1991, 129, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zong, D.K.; Tong, G.Q.; Li, L. Apoptotic mechanism of premature ovarian failure and rescue effect of Traditional Chinese Medicine: A review. J. Tradit. Chin. Med. 2021, 41, 492–498. [Google Scholar]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S.; Reis, F.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells–A review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Misra, H.P. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol. Cell. Biochem. 2004, 262, 127–133. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, C.A.; Pegg, A.E. Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts, and the sensitivity of fibroblasts to 1,3-bis-(2-chloroethyl)-N-nitrosourea. Biochem. J. 2000, 351 Pt 2, 439–447. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Park, M.H. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N(1)-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 2015, 468, 435–447. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Zhang, Y.; Lin, X.; Song, Y.; Xue, Z.; Qian, H.; Wang, S.; Wan, G.; Zheng, X.; et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017, 8, e2738. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Spermidine ameliorates 3-nitropropionic acid (3-NP)-induced striatal toxicity: Possible role of oxidative stress, neuroinflammation, and neurotransmitters. Physiol. Behav. 2016, 155, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Xiang, R.; Hui, H.E.; Kang, B.; Jiang, D.; Rong, M.A.; Liu, Y.J.C.J.o.A.N. Effects of Polyamine Regulation on Animal Reproduction and Its Mechanism. Chin. J. Anim. Nutr. 2014, 26, 3251–3255. [Google Scholar]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. USA 2003, 100, 2261–2265. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Chen, W.; Poy, G.; Cam, M.; Stiles, D.; Tabor, H. Microarray studies on the genes responsive to the addition of spermidine or spermine to a Saccharomyces cerevisiae spermidine synthase mutant. Yeast 2009, 26, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Kensler, T.W.; Casero, R.A., Jr. Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: Effect of the polyamine metabolite acrolein. Biochem. Biophys. Res. Commun. 2003, 305, 662–670. [Google Scholar] [CrossRef]
- Yang, H.; Lee, S.E.; Kim, G.D.; Park, H.R.; Park, Y.S. Hemeoxygenase-1 mediates an adaptive response to spermidine-induced cell death in human endothelial cells. Oxid. Med. Cell. Longev. 2013, 2013, 238734. [Google Scholar] [CrossRef]
- Yadav, M.; Parle, M.; Jindal, D.K.; Sharma, N. Potential effect of spermidine on GABA, dopamine, acetylcholinesterase, oxidative stress and proinflammatory cytokines to diminish ketamine-induced psychotic symptoms in rats. Biomed. Pharmacother. 2018, 98, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sava, I.G.; Battaglia, V.; Rossi, C.A.; Salvi, M.; Toninello, A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic. Biol. Med. 2006, 41, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Marino, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Benit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Duerrschmidt, N.; Zabirnyk, O.; Nowicki, M.; Ricken, A.; Hmeidan, F.A.; Blumenauer, V.; Borlak, J.; Spanel-Borowski, K. Lectin-like oxidized low-density lipoprotein receptor-1-mediated autophagy in human granulosa cells as an alternative of programmed cell death. Endocrinology 2006, 147, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Serke, H.; Vilser, C.; Nowicki, M.; Hmeidan, F.A.; Blumenauer, V.; Hummitzsch, K.; Losche, A.; Spanel-Borowski, K. Granulosa cell subtypes respond by autophagy or cell death to oxLDL-dependent activation of the oxidized lipoprotein receptor 1 and toll-like 4 receptor. Autophagy 2009, 5, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Vilser, C.; Hueller, H.; Nowicki, M.; Hmeidan, F.A.; Blumenauer, V.; Spanel-Borowski, K. The variable expression of lectin-like oxidized low-density lipoprotein receptor (LOX-1) and signs of autophagy and apoptosis in freshly harvested human granulosa cells depend on gonadotropin dose, age, and body weight. Fertil. Steril. 2010, 93, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil. Steril. 2011, 95, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Dong, A.; Niu, X.; Dong, A.; Lee, S.; Ma, C.; Wang, L. Effects of cadmium on oxidative stress activities in plasma of freshwater turtle Chinemys reevesii. Environ. Sci. Pollut. Res. 2018, 25, 8027–8034. [Google Scholar] [CrossRef] [PubMed]
- Stier, A.; Reichert, S.; Massemin, S.; Bize, P.; Criscuolo, F. Constraint and cost of oxidative stress on reproduction: Correlative evidence in laboratory mice and review of the literature. Front. Zool. 2012, 9, 37. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Zeng, J.; Liao, B.; Peng, X.; Li, T.; Li, J.; Tan, Q.; Li, X.; Yang, Y.; et al. Proteomics analysis of potential serum biomarkers for insulin resistance in patients with polycystic ovary syndrome. Int. J. Mol. Med. 2020, 45, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Grapov, D.D.; Wanichthanarak, K.; DeFelice, B.C.; Salemi, M.R.; Rom, W.N.; Gandara, D.R.; Phinney, B.S.; Fiehn, O.; Pass, H.; et al. Integrated Metabolomics and Proteomics Highlight Altered Nicotinamide- and Polyamine Pathways in Lung Adenocarcinoma. Carcinogenesis 2017, 38, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Freitag, K.; Sterczyk, N.; Wendlinger, S.; Obermayer, B.; Schulz, J.; Farztdinov, V.; Mulleder, M.; Ralser, M.; Houtman, J.; Fleck, L.; et al. Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer’s disease mouse model. J. Neuroinflamm. 2022, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A., Jr. Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Pegg, A.E. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J. Biol. Chem. 2018, 293, 18681–18692. [Google Scholar] [CrossRef]
- Jiang, D.; Mo, G.; Jiang, Y.; Kang, B. Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus. Open Life Sci. 2021, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.H.; Yan, J.L.; Wang, Q.J.; Chen, H.C.; Ma, X.Z.; Yin, J.; Gao, L.P. Spermidine ameliorates the neuronal aging by improving the mitochondrial function in vitro. Exp. Gerontol. 2018, 108, 77–86. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Al-Johny, B.O.; Al-Ghamdi, K.M.S.; Al-Zahrani, H.A.A.; Anwar, Y.; Firoz, A.; Al-Kenani, N.; Almatry, M.A.A. Endophytic bacteria isolated from Solanum nigrum L. alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by sodA gene. Ecotoxicol. Environ. Saf. 2019, 174, 197–207. [Google Scholar] [CrossRef]
- Reddy, R.D.; Yao, J.K. Free radical pathology in schizophrenia: A review. Prostaglandins Leukot. Essent. Fat. Acids 1996, 55, 33–43. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Shi, J.; Yang, X.; Wang, Z. Hepatic antioxidative responses to PCDPSs and estimated short-term biotoxicity in freshwater fish. Aquat. Toxicol. 2012, 120–121, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41 Pt 2, 1819–1828. [Google Scholar] [CrossRef]
- Jacob, R.A.; Burri, B.J. Oxidative damage and defense. Am. J. Clin. Nutr. 1996, 63, 985s–990s. [Google Scholar] [CrossRef] [PubMed]
- Baghcheghi, Y.; Beheshti, F.; Shafei, M.N.; Salmani, H.; Sadeghnia, H.R.; Soukhtanloo, M.; Anaeigoudari, A.; Hosseini, M. The effects of vitamin E on brain derived neurotrophic factor, tissues oxidative damage and learning and memory of juvenile hypothyroid rats. Metab. Brain Dis. 2018, 33, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.P.; Yang, X.M.; Duan, Z.H.; Shang, J.H.; Luo, P.; Xiao, W.; Zhang, D.Y.; Liu, H.Z. Squid ink polysaccharide prevents autophagy and oxidative stress affected by cyclophosphamide in Leydig cells of mice: A pilot study. Iran. J. Basic Med. Sci. 2017, 20, 1194–1199. [Google Scholar] [PubMed]
- Jantaro, S.; Baebprasert, W.; Piyamawadee, C.; Sodsuay, O.; Incharoensakdi, A. Exogenous spermidine alleviates UV-induced growth inhibition of Synechocystis sp. PCC 6803 via reduction of hydrogen peroxide and malonaldehyde levels. Appl. Biochem. Biotechnol. 2014, 173, 1145–1156. [Google Scholar] [CrossRef]
- Yoon, S.P.; Kim, J. Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity. Anat. Cell Biol. 2018, 51, 189–199. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.Y.; Zhu, W.L.; Liu, X.Q.; Liu, H.F.; Cai, W.W.; Huang, S.Q.; et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401–6414. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Garg, G.; Singh, A.K.; Verma, A.K.; Bissoyi, A.; Rizvi, S.I. Spermidine, a caloric restriction mimetic, provides neuroprotection against normal and D-galactose-induced oxidative stress and apoptosis through activation of autophagy in male rats during aging. Biogerontology 2021, 22, 35–47. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic Control of Longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef]
- Macedo, J.C.; Vaz, S.; Logarinho, E. Mitotic Dysfunction Associated with Aging Hallmarks. Adv. Exp. Med. Biol. 2017, 1002, 153–188. [Google Scholar]
- Qi, Y.; Qiu, Q.; Gu, X.; Tian, Y.; Zhang, Y. ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts. Sci. Rep. 2016, 6, 24700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, Z.G.; Chen, Y.; Chen, J.; Khor, S.; Li, J.; He, Z.; Wang, Q.; Zhang, H.; Xu, K.; et al. Spermidine promotes nucleus pulposus autophagy as a protective mechanism against apoptosis and ameliorates disc degeneration. J. Cell. Mol. Med. 2018, 22, 3086–3096. [Google Scholar] [CrossRef]
- Ye, B.; Fan, C.; Shen, Y.; Wang, Q.; Hu, H.; Xiang, M. The Antioxidative Role of Autophagy in Hearing Loss. Front. Neurosci. 2018, 12, 1010. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Kaushik, M.; Niranjan, R.; Richards, J.S.; Ebright, B.; Venkatasubbu, G.D. Zinc Oxide nanoparticles induce oxidative and proteotoxic stress in ovarian cancer cells and trigger apoptosis Independent of p53-mutation status. Appl. Surf. Sci. 2019, 487, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.O.; Seo, S.K.; Kim, Y.S.; Woo, S.H.; Lee, K.H.; Yi, J.Y.; Lee, S.J.; Choe, T.B.; Lee, J.H.; An, S.; et al. TXNIP potentiates Redd1-induced mTOR suppression through stabilization of Redd1. Oncogene 2011, 30, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, J.; Chutkow, W.A.; Lee, S.; Kim, J.B.; Yan, J.; Tian, R.; Lindsey, M.L.; Feener, E.P.; Seidman, C.E.; Seidman, J.G.; et al. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J. Clin. Invest. 2012, 122, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, R.; Li, B.; Guo, Y.; Yin, T.; Xia, Y.; Zhang, F.; Lian, K.; Liu, Y.; Wang, H.; et al. TXNIP/Redd1 signalling and excessive autophagy: A novel mechanism of myocardial ischaemia/reperfusion injury in mice. Cardiovasc. Res. 2020, 116, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Dennis, M.; Song, X.; Vadysirisack, D.D.; Salunke, D.; Nash, Z.; Yang, Z.; Liesa, M.; Yoshioka, J.; Matsuzawa, S.; et al. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat. Commun. 2015, 6, 7014. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, K.; Das, A.; Li, G.; Song, Y.; Luo, R.; Cheung, J.P.Y.; Zhang, T.; Li, S.; Yang, C. The REDD1/TXNIP Complex Accelerates Oxidative Stress-Induced Apoptosis of Nucleus Pulposus Cells through the Mitochondrial Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 7397516. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Li, H.; Zhao, X.; Liu, B.; Lu, L. TXNIP positively regulates the autophagy and apoptosis in the rat muller cell of diabetic retinopathy. Life Sci. 2021, 267, 118988. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Kelly, D.J.; Tan, C.Y.; Gill, A.; Cheng, D.; Braet, F.; Park, J.S.; Sue, C.M.; Pollock, C.A.; et al. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep. 2016, 6, 29196. [Google Scholar] [CrossRef] [PubMed]
- Zhenzhen, L.; Wenting, L.; Jianmin, Z.; Guangru, Z.; Disheng, L.; Zhiyu, Z.; Feng, C.; Yajing, S.; Yingxiang, H.; Jipeng, L.; et al. miR-146a-5p/TXNIP axis attenuates intestinal ischemia-reperfusion injury by inhibiting autophagy via the PRKAA/mTOR signaling pathway. Biochem. Pharmacol. 2022, 197, 114839. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.Z.; An, Q.M.; Wu, Y.X.; Zhao, Y. Screening up-regulated genes related to bovine follicular development based on Illumina sequencing. Henan Agric. Sci. J. 2020, 49, 7. [Google Scholar]
- Salhab, M.; Dhorne-Pollet, S.; Auclair, S.; Guyader-Joly, C.; Brisard, D.; Dalbies-Tran, R.; Dupont, J.; Ponsart, C.; Mermillod, P.; Uzbekova, S. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol. Reprod. Dev. 2013, 80, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Terlecki-Zaniewicz, L.; Lämmermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmüllner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 2018, 10, 1103–1132. [Google Scholar] [CrossRef] [PubMed]
- Kulus, M.; Józkowiak, M.; Kulus, J.; Popis, M.; Antosik, P. “Cell cycle process”, “cell division” and “cell proliferation” belong to ontology groups highly regulated during long–term culture of porcine oviductal epithelial cells. Med. J. Cell Biol. 2019, 7, 15–24. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.S.; Kim, E.Y.; Ko, J.J.; Yoon, T.K.; Lee, W.S.; Lee, K.A. Thioredoxin-interacting protein regulates glucose metabolism and affects cytoplasmic streaming in mouse oocytes. PLoS ONE 2013, 8, e70708. [Google Scholar] [CrossRef] [PubMed]
- Kulus, M.; Kranc, W.; Sujka-Kordowska, P.; Celichowski, P.; Konwerska, A.; Jankowski, M.; Jeseta, M.; Skowronski, M.T.; Piotrowska-Kempisty, H.; Bukowska, D.; et al. Transcriptomic analysis of expression of genes regulating cell cycle progression in porcine ovarian granulosa cells during short-term in vitro primary culture. Histochem. Cell Biol. 2020, 153, 397–412. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, L.; Chen, W.; Zhang, Y.; Hua, R.; Wang, W.; Zhang, T.; Wu, H. The protective effects of pretreatment with resveratrol in cyclophosphamide-induced rat ovarian granulosa cell injury: In vitro study. Reprod. Toxicol. 2020, 95, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Cheung, C.K.; Wang, Y.; Tsang, B.K. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front. Biosci. 2003, 8, 222–237. [Google Scholar]





| Serial Number | Protein Name | Expression Regulation | Fold Change |
|---|---|---|---|
| O35425 | Bok | Up | 1.546 |
| P70444 | Bid | Up | 1.4635 |
| P33242 | Nr5a1 | Up | 1.955 |
| Q00519 | XDH | Down | 0.5005 |
| P70399 | p53 | Up | 1.445 |
| Q8BG60 | TXNIP | Down | 0.5075 |
| Bioinformatics Analysis Methods | Tool | Version and URL |
|---|---|---|
| Mass spectrum data analysis | MaxQuant | v.1.5.2.8 http://www.maxquant.org/ (accessed on 29 May 2021) |
| Motif analyze | MoMo | v.5.0.2 http://meme-suite.org/tools/momo (accessed on 29 May 2021) |
| GO Notes | InterProScan | v.5.14-53.0 http://www.ebi.ac.uk/interpro/ (accessed on 29 May 2021) |
| Domain Notes | InterProScan | v.5.14-53.0 http://www.ebi.ac.uk/interpro/ (accessed on 29 May 2021) |
| (KEGG Notes | KAAS | v.2.0 http://www.genome.jp/kaas-bin/kaas_main (accessed on 29 May 2021) |
| KEGG Mapper | V2.5 http://www.kegg.jp/kegg/mapper.html (accessed on 29 May 2021) | |
| Subcellular localization | Wolfpsort | v.0.2 http://www.genscript.com/psort/wolf_psort.html (accessed on 29 May 2021) |
| CELLO | v.2.5 http://cello.life.nctu.edu.tw/ | |
| Enrichment analysis | Perl module | v.1.31 https://metacpan.org/pod/Text::NSP::Measures::2D::Fisher (accessed on 29 May 2021) |
| Cluster heatmap | R Package pheatmap | v.2.0.3 https://cran.r-project.org/web/packages/cluster/ (accessed on 29 May 2021) |
| Protein interaction | Blast | v.2.2.26 http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 29 May 2021) |
| R package network D3 | v.0.4 https://cran.r-project.org/web/packages/networkD3/ (accessed on 29 May 2021) |
| Gene | Primer Sequence (5’-3’) | Gene Accession Number | Tm (°C) | Amplified Fragment (Bp) |
|---|---|---|---|---|
| β-actin | F: GGGTCAGAAGGACTCCTATG R: GTAACAATGCCATGTTCAAT | XM_015141809.2 | 57.0 | 90 |
| ODC | F: TTGACTGCCACATCCTTG R: GCTCTGCTATCGTTACACT | XM_021201619.1 | 58.0 | 199 |
| SPDS | F: ACCAGCTCATGAAGACAGCACTCA R: TGCTACACAGCATGAAGCCGATCT | XM_021160349.1 | 60.0 | 189 |
| SPMS | F: TTCGGGTGACTCAGTTCCTGCTAA R: AACGGAGACCCTCCTTCAGCAAAT | XM_009214.4 | 60.0 | 199 |
| APAO | F: AGTCTTCACATGTGCTCTGTGGGT R: TGGCAATTGTGGGTTTCCTGTCAC | XM_021167504.1 | 59.0 | 131 |
| SSAT | F: TGCCGGTGTAGACAATGACAACCT R: TAAAGCTTTGGAATGGGTGCTCGC | XM_021153071.1 | 59.0 | 114 |
| SMO | F: TVTGCACAGAGATGCTTCGACAGT R: TTGAGCCCACCTGTGTGTAGGAAT | XM_021184579.1 | 59.0 | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Guo, Y.; Niu, C.; Long, S.; Jiang, Y.; Wang, Z.; Wang, X.; Sun, Q.; Ling, W.; An, X.; et al. Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. Int. J. Mol. Sci. 2023, 24, 5793. https://doi.org/10.3390/ijms24065793
Jiang D, Guo Y, Niu C, Long S, Jiang Y, Wang Z, Wang X, Sun Q, Ling W, An X, et al. Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. International Journal of Molecular Sciences. 2023; 24(6):5793. https://doi.org/10.3390/ijms24065793
Chicago/Turabian StyleJiang, Dongmei, Yongni Guo, Chunyang Niu, Shiyun Long, Yilong Jiang, Zelong Wang, Xin Wang, Qian Sun, Weikang Ling, Xiaoguang An, and et al. 2023. "Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins" International Journal of Molecular Sciences 24, no. 6: 5793. https://doi.org/10.3390/ijms24065793
APA StyleJiang, D., Guo, Y., Niu, C., Long, S., Jiang, Y., Wang, Z., Wang, X., Sun, Q., Ling, W., An, X., Ji, C., Zhao, H., & Kang, B. (2023). Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. International Journal of Molecular Sciences, 24(6), 5793. https://doi.org/10.3390/ijms24065793






