A Single Nucleotide Variation of CRS2 Affected the Establishment of Photosynthetic System in Rice
Abstract
1. Introduction
2. Results
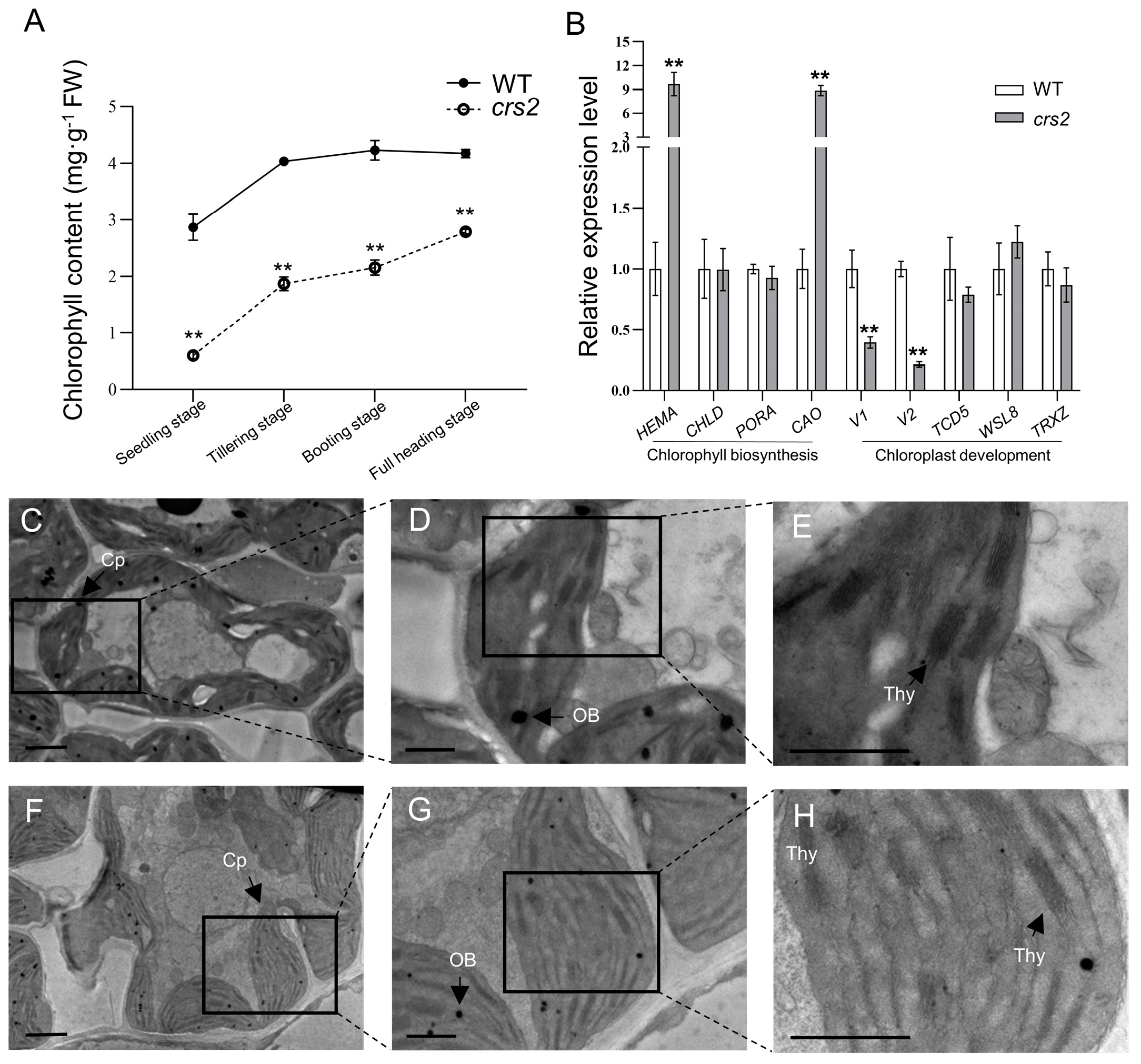
2.1. Phenotype of Mutant crs2
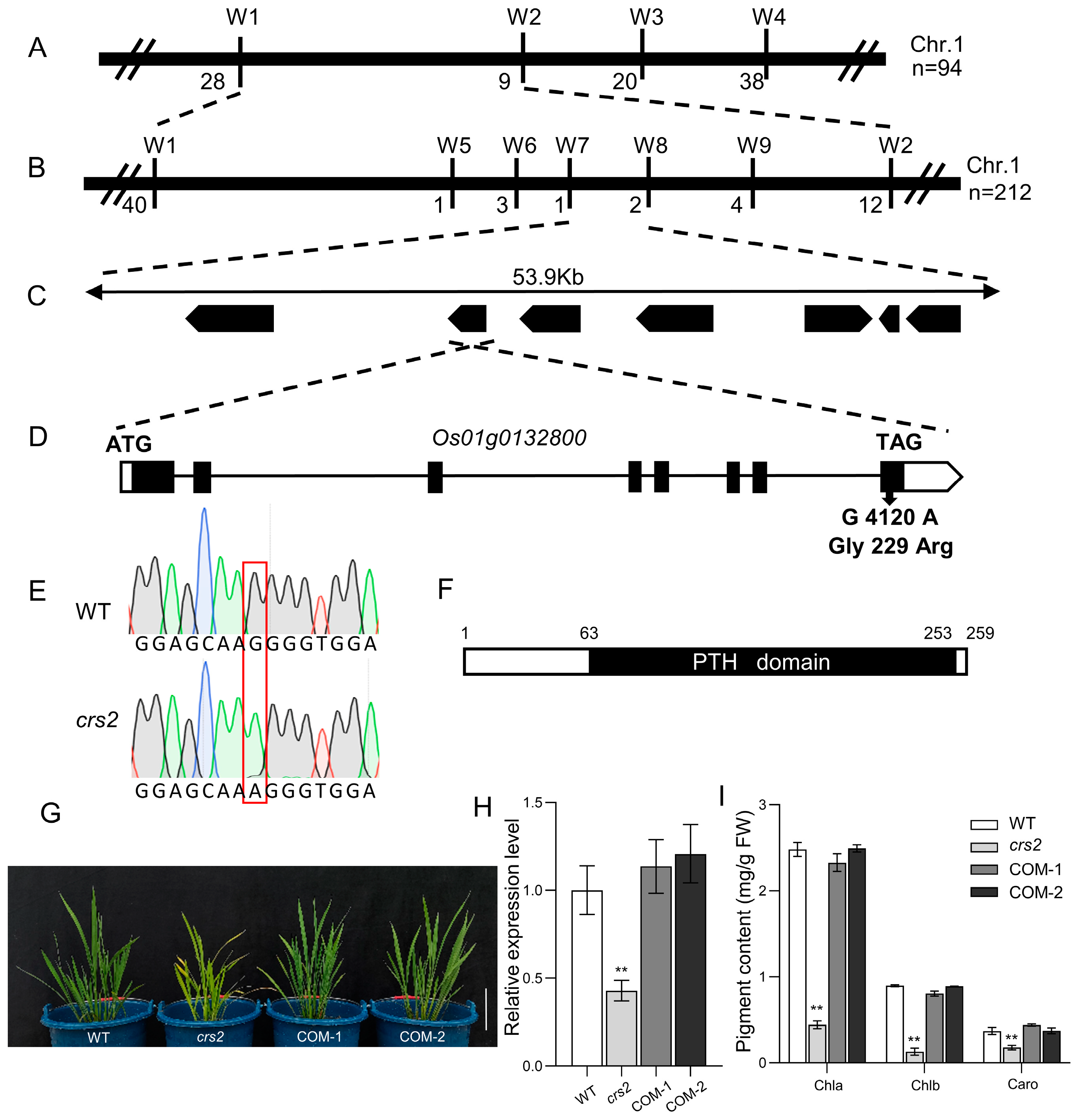
2.2. Map-Based Cloning of CRS2 and Complementation Analysis
2.3. Expression Pattern and Subcellular Localization of CRS2
2.4. Abnormal Chloroplast Proteins in crs2
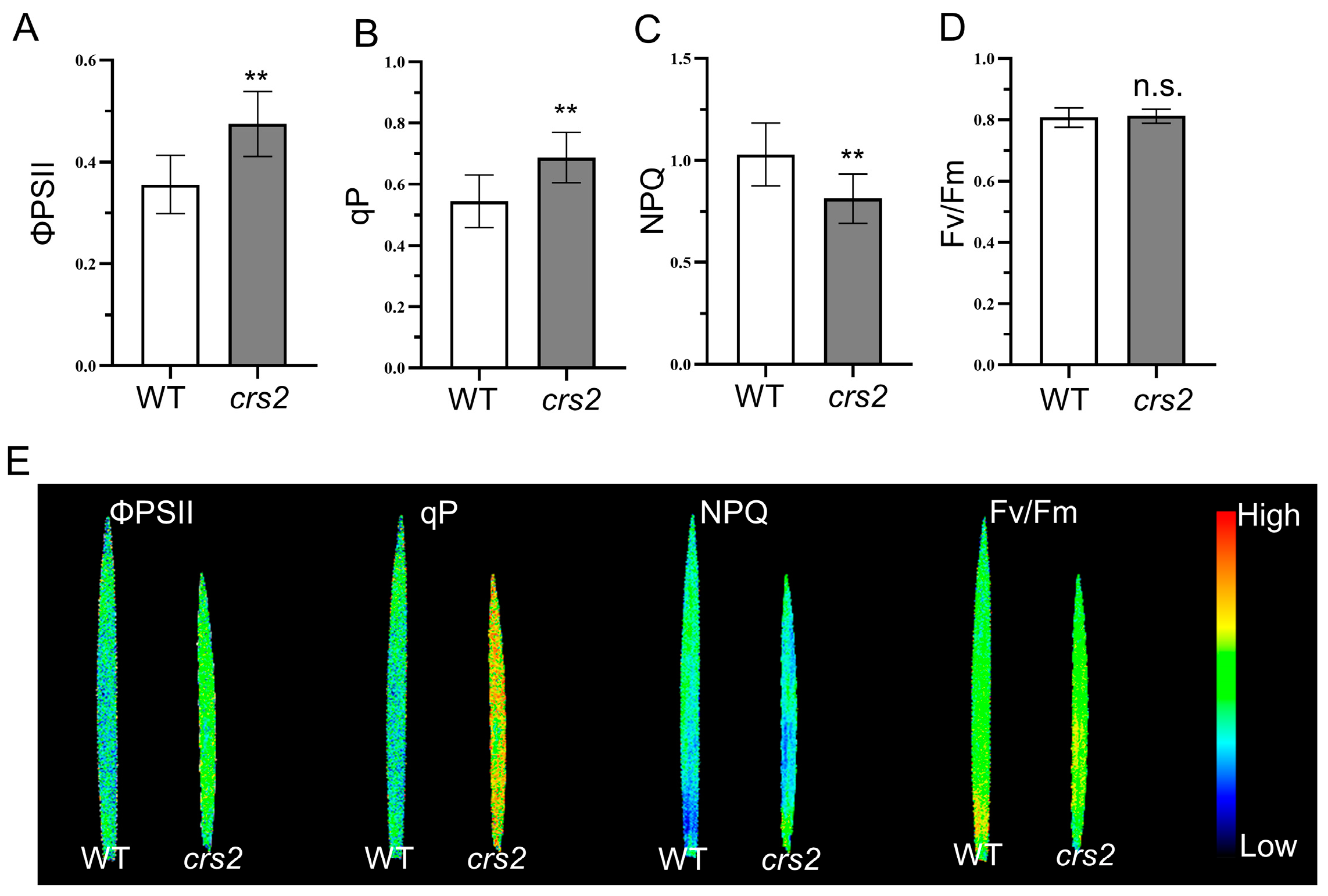
2.5. Analysis of Chlorophyll Fluorescence
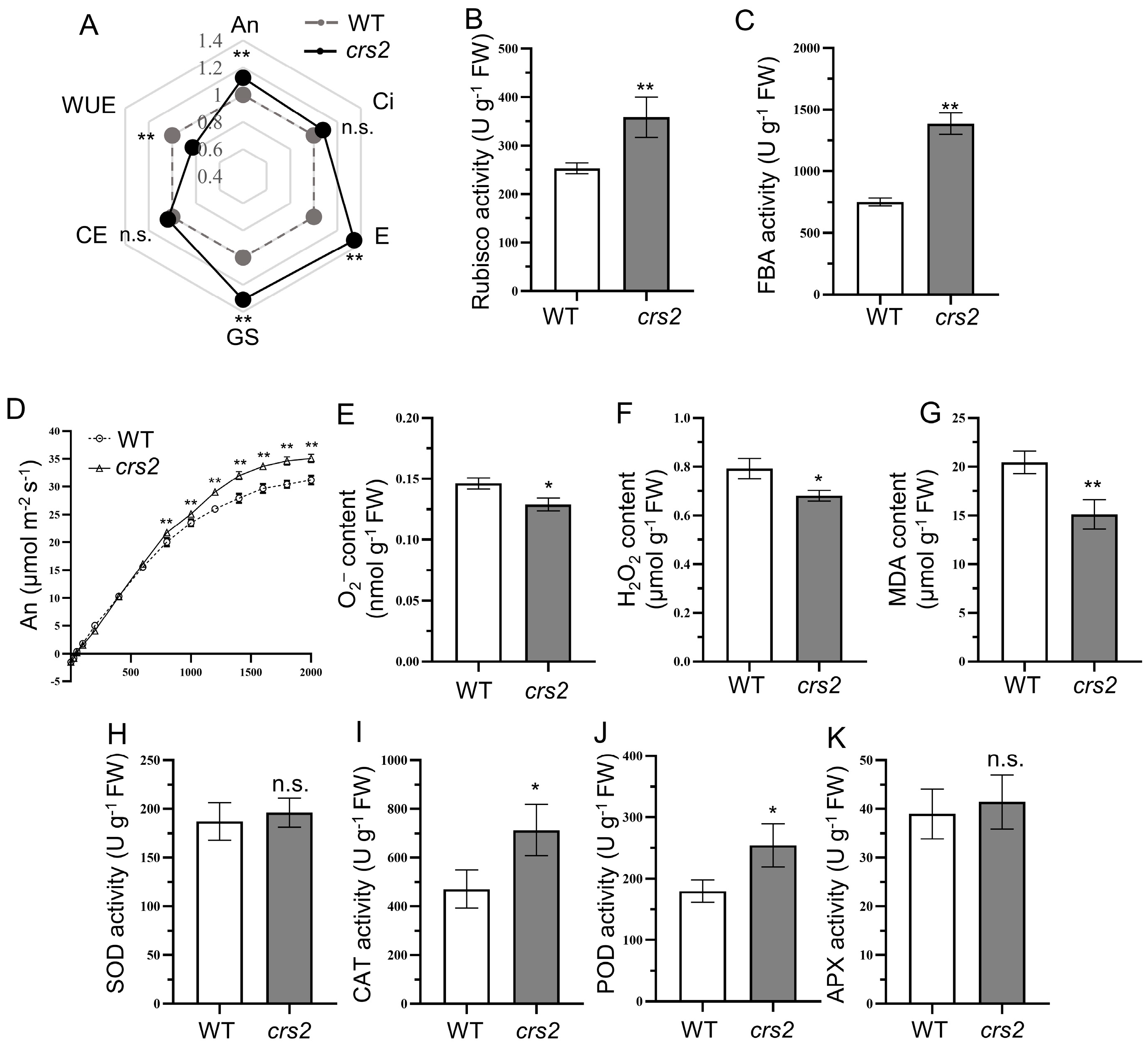
2.6. High Light Adaptability Comparison between WT and crs2
3. Discussion
3.1. The G229R Mutation in CRS2 Is Responsible for the Decreased Chlorophyll Content
3.2. The crs2 Mutant Shows Higher Photosynthetic Efficiency
3.3. High Protective Enzyme Activity Is the Guarantee of crs2 to Cope with the Intensity of the High Light
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurement of Chlorophyll Content
4.3. Transmission Electron Microscopy (TEM)
4.4. Determination of ROS-Related Physiological Indices
4.5. Measurement of Photosynthetic Gas Exchange and Chlorophyll Fluorescence Parameters
4.6. Map-Based Cloning and Complementation of CRS2
4.7. RNA Extraction and Quantitative Real-Time PCR (qRT–PCR) Analysis
4.8. Histochemical β-Glucuronidase (GUS) Assays
4.9. Subcellular Localization of the CRS2 Protein
4.10. Protein Extraction and Western Blotting
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoschke, R.; Bock, R. Chloroplast Translation: Structural and Functional Organization, Operational Control, and Regulation. Plant Cell 2018, 30, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nakamura, Y.; Kaneko, T.; Asamizu, E.; Tabata, S. Complete Structure of the Chloroplast Genome of Arabidopsis thaliana. DNA Res. 1999, 6, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Varani, G.; Nagai, K. RNA Recognition by RNP Proteins during RNA Processing. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 407–445. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.; Xing, Y.; Tan, J.; Chen, X.; Zhang, J.; Peng, H.; Xie, Q.; Zhang, Z. Albino Leaf 2 Is Involved in the Splicing of Chloroplast Group I and II Introns in Rice. J. Exp. Bot. 2016, 67, 5339–5347. [Google Scholar] [CrossRef]
- Muthusamy, M.; Kim, J.-H.; Kim, J.A.; Lee, S.-I. Plant RNA Binding Proteins as Critical Modulators in Drought, High Salinity, Heat, and Cold Stress Responses: An Updated Overview. Int. J. Mol. Sci. 2021, 22, 6731. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, W.; Wen, K.; Chen, G.; Sun, J.; Tian, Y.; Tang, W.; Yu, J.; An, H.; Wu, T.; et al. OsPPR6, a Pentatricopeptide Repeat Protein Involved in Editing and Splicing Chloroplast RNA, Is Required for Chloroplast Biogenesis in Rice. Plant Mol. Biol. 2017, 95, 345–357. [Google Scholar] [CrossRef]
- Yusupov, M.M.; Yusupova, G.Z.; Baucom, A.; Lieberman, K.; Earnest, T.N.; Cate, J.H.; Noller, H.F. Crystal Structure of the Ribosome at 5.5 A Resolution. Science 2001, 292, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Subramanian, A.R. The Plastid Ribosomal Proteins. Identification of All the Proteins in the 50 S Subunit of an Organelle Ribosome (Chloroplast). J. Biol. Chem. 2000, 275, 28466–28482. [Google Scholar] [CrossRef]
- Rogalski, M.; Ruf, S.; Bock, R. Tobacco Plastid Ribosomal Protein S18 Is Essential for Cell Survival. Nucleic Acids Res. 2006, 34, 4537–4545. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.; Zhang, Y.; Huang, S.; Liu, Z.; Fei, D.; Feng, H. A Missense Mutation of Plastid RPS4 Is Associated with Chlorophyll Deficiency in Chinese Cabbage (Brassica campestris ssp. pekinensis). BMC Plant Biol. 2018, 18, 130. [Google Scholar] [CrossRef]
- Lin, D.; Jiang, Q.; Zheng, K.; Chen, S.; Zhou, H.; Gong, X.; Xu, J.; Teng, S.; Dong, Y. Mutation of the Rice ASL2 Gene Encoding Plastid Ribosomal Protein L21 Causes Chloroplast Developmental Defects and Seedling Death. Plant Biol. Stuttg. Ger. 2015, 17, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Zoschke, R.; Watkins, K.P.; Barkan, A. A Rapid Ribosome Profiling Method Elucidates Chloroplast Ribosome Behavior in Vivo. Plant Cell 2013, 25, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, J.; Bayraktar, O.A.; Prikryl, J.; Barkan, A. Site-Specific Binding of a PPR Protein Defines and Stabilizes 5′ and 3′ MRNA Termini in Chloroplasts. EMBO J. 2009, 28, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Okuda, K.; Peng, L.; Shikanai, T. PROTON GRADIENT REGULATION 3 Recognizes Multiple Targets with Limited Similarity and Mediates Translation and RNA Stabilization in Plastids. Plant J. Cell Mol. Biol. 2011, 67, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tiller, N.; Weingartner, M.; Thiele, W.; Maximova, E.; Schöttler, M.A.; Bock, R. The Plastid-Specific Ribosomal Proteins of Arabidopsis Thaliana Can Be Divided into Non-Essential Proteins and Genuine Ribosomal Proteins. Plant J. Cell Mol. Biol. 2012, 69, 302–316. [Google Scholar] [CrossRef]
- van Rooijen, R.; Kruijer, W.; Boesten, R.; van Eeuwijk, F.A.; Harbinson, J.; Aarts, M.G.M. Natural Variation of YELLOW SEEDLING1 Affects Photosynthetic Acclimation of Arabidopsis thaliana. Nat. Commun. 2017, 8, 1421. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kalisz, A.; Sękara, A.; Pokluda, R.; Jezdinský, A.; Neugebauerová, J.; Slezák, K.A.; Kunicki, E. Sequential Response of Sage Antioxidant Metabolism to Chilling Treatment. Molecules 2019, 24, 4087. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Su, X.; Ge, P.; Zhou, Y.; Hao, Y.; Shu, H.; Gao, C.; Cheng, S.; Zhu, G.; et al. Early Response of Radish to Heat Stress by Strand-Specific Transcriptome and MiRNA Analysis. Int. J. Mol. Sci. 2019, 20, 3321. [Google Scholar] [CrossRef]
- Li, X.; Liao, M.; Huang, J.; Xu, Z.; Lin, Z.; Ye, N.; Zhang, Z.; Peng, X. Glycolate Oxidase-Dependent H2O2 Production Regulates IAA Biosynthesis in Rice. BMC Plant Biol. 2021, 21, 326. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.; Hussain, S.; Khan, I.A.; Saeed, M.; Saud, S.; Hassan, S.; Adnan, M.; Amanullah; et al. Suppressing Photorespiration for the Improvement in Photosynthesis and Crop Yields: A Review on the Role of S-Allantoin as a Nitrogen Source. J. Environ. Manag. 2019, 237, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.D.; Barkan, A. Recruitment of a Peptidyl-TRNA Hydrolase as a Facilitator of Group II Intron Splicing in Chloroplasts. EMBO J. 2001, 20, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shen, L.; Wang, Z.; Hu, G.; Ren, D.; Hu, J.; Zhu, L.; Gao, Z.; Zhang, G.; Guo, L.; et al. OsCAF1, a CRM Domain Containing Protein, Influences Chloroplast Development. Int. J. Mol. Sci. 2019, 20, 4386. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Shen, L.; Ren, D.; Hu, J.; Zhu, L.; Zhang, G.; Guo, L.; Zeng, D.; Qian, Q. OsCRS2 Encoding a Peptidyl-tRNA Hydrolase Protein Is Essential for Chloroplast Development in Rice. Plant Growth Regul. 2020, 92, 535–545. [Google Scholar] [CrossRef]
- Kühlbrandt, W.; Wang, D.N. Three-Dimensional Structure of Plant Light-Harvesting Complex Determined by Electron Crystallography. Nature 1991, 350, 130–134. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, J.; Wang, Y.; Yang, Q.; Chen, T.; Chen, Y.; Chi, D.; Xia, G.; Siddique, K.H.M.; Wang, T. Photosynthesis, Chlorophyll Fluorescence, and Yield of Peanut in Response to Biochar Application. Front. Plant Sci. 2021, 12, 650432. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, J.; Zeng, F.; Wang, Q.; Zhu, L.; Li, H.; Guo, N.; Chen, H. Photorespiration Regulates Carbon-Nitrogen Metabolism by Magnesium Chelatase D Subunit in Rice. J. Agric. Food Chem. 2021, 69, 112–125. [Google Scholar] [CrossRef]
- Herrera, A.; Fernández, M.D.; Taisma, M.A. Effects of Drought on CAM and Water Relations in Plants of Peperomia carnevalii. Ann. Bot. 2000, 86, 511–517. [Google Scholar] [CrossRef]
- Xue, G.-P.; Drenth, J.; McIntyre, C.L. TaHsfA6f Is a Transcriptional Activator That Regulates a Suite of Heat Stress Protection Genes in Wheat (Triticum aestivum L.) Including Previously Unknown Hsf Targets. J. Exp. Bot. 2015, 66, 1025–1039. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Zhang, L.; Hu, Z.; Liu, J.; Hua, W.; Xu, S.; Liu, J. Genome-Wide Identification and Characterization of FBA Gene Family in Polyploid Crop Brassica Napus. Int. J. Mol. Sci. 2019, 20, 5749. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning Photosynthesis to Sustainably Meet Global Food and Bioenergy Demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Solar Energy Conversion Efficiencies in Photosynthesis: Minimizing the Chlorophyll Antennae to Maximize Efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Ko, S.-S.; Jhong, C.-M.; Shih, M.-C. Blue Light Acclimation Reduces the Photoinhibition of Phalaenopsis aphrodite (Moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167. [Google Scholar] [CrossRef]
- Rao, Y.; Xu, N.; Li, S.; Hu, J.; Jiao, R.; Hu, P.; Lin, H.; Lu, C.; Lin, X.; Dai, Z.; et al. PE-1, Encoding Heme Oxygenase 1, Impacts Heading Date and Chloroplast Development in Rice (Oryza sativa L.). J. Agric. Food Chem. 2019, 67, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Sun, Q.; Deng, D.; Liu, H.; Chen, S.; Yin, Z. Genetic Determinants Controlling Maize Rubisco Activase Gene Expression and a Comparison with Rice Counterparts. BMC Plant Biol. 2019, 19, 351. [Google Scholar] [CrossRef]
- Sukhov, V.; Surova, L.; Sherstneva, O.; Katicheva, L.; Vodeneev, V. Variation Potential Influence on Photosynthetic Cyclic Electron Flow in Pea. Front. Plant Sci. 2014, 5, 766. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, T.; Amombo, E.; Wang, G.; Xie, Y.; Fu, J. The Alleviation of Heat Damage to Photosystem II and Enzymatic Antioxidants by Exogenous Spermidine in Tall Fescue. Front. Plant Sci. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and Biochemical Responses in Two Ornamental Shrubs to Drought Stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Feng, B.H.; Li, G.Y.; Islam, M.; Fu, W.M.; Zhou, Y.Q.; Chen, T.T.; Tao, L.X.; Fu, G.F. Strengthened Antioxidant Capacity Improves Photosynthesis by Regulating Stomatal Aperture and Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Activity. Plant Sci. 2020, 290, 110245. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, Q.; Fan, M.; Zhang, X.; Feng, P.; Zhu, L.; Wu, J.; Cheng, X.; Wang, J. A Single Nucleotide Variation of CRS2 Affected the Establishment of Photosynthetic System in Rice. Int. J. Mol. Sci. 2023, 24, 5796. https://doi.org/10.3390/ijms24065796
Chen H, Wang Q, Fan M, Zhang X, Feng P, Zhu L, Wu J, Cheng X, Wang J. A Single Nucleotide Variation of CRS2 Affected the Establishment of Photosynthetic System in Rice. International Journal of Molecular Sciences. 2023; 24(6):5796. https://doi.org/10.3390/ijms24065796
Chicago/Turabian StyleChen, Hongwei, Qi Wang, Mingqian Fan, Xijuan Zhang, Pulin Feng, Lin Zhu, Jiayi Wu, Xiaoyi Cheng, and Jiayu Wang. 2023. "A Single Nucleotide Variation of CRS2 Affected the Establishment of Photosynthetic System in Rice" International Journal of Molecular Sciences 24, no. 6: 5796. https://doi.org/10.3390/ijms24065796
APA StyleChen, H., Wang, Q., Fan, M., Zhang, X., Feng, P., Zhu, L., Wu, J., Cheng, X., & Wang, J. (2023). A Single Nucleotide Variation of CRS2 Affected the Establishment of Photosynthetic System in Rice. International Journal of Molecular Sciences, 24(6), 5796. https://doi.org/10.3390/ijms24065796




