Novel Rigidochromic and Anti-Kasha Dual Emission Fluorophores Based on D-π-A Dyads as the Promising Materials for Potential Applications Ranging from Optoelectronics and Optical Sensing to Biophotonics and Medicine
Abstract
1. Introduction
2. Results and Discussion
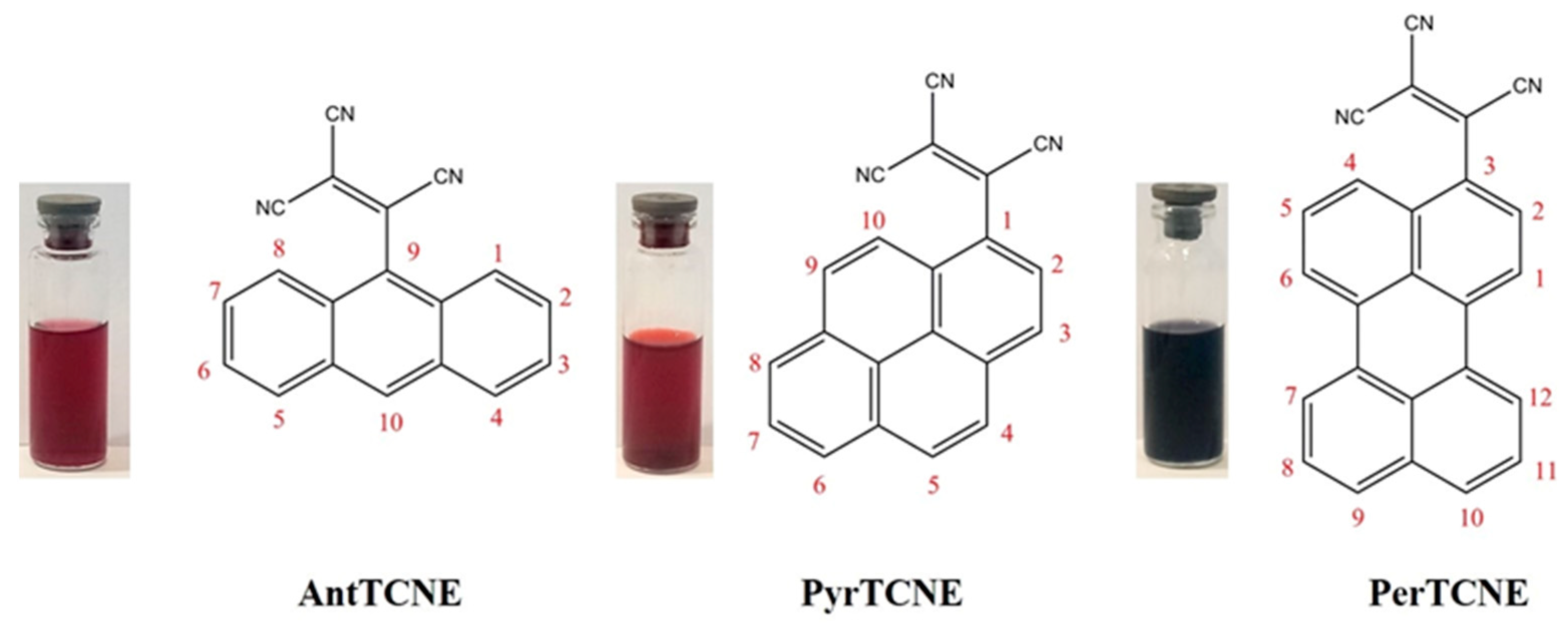
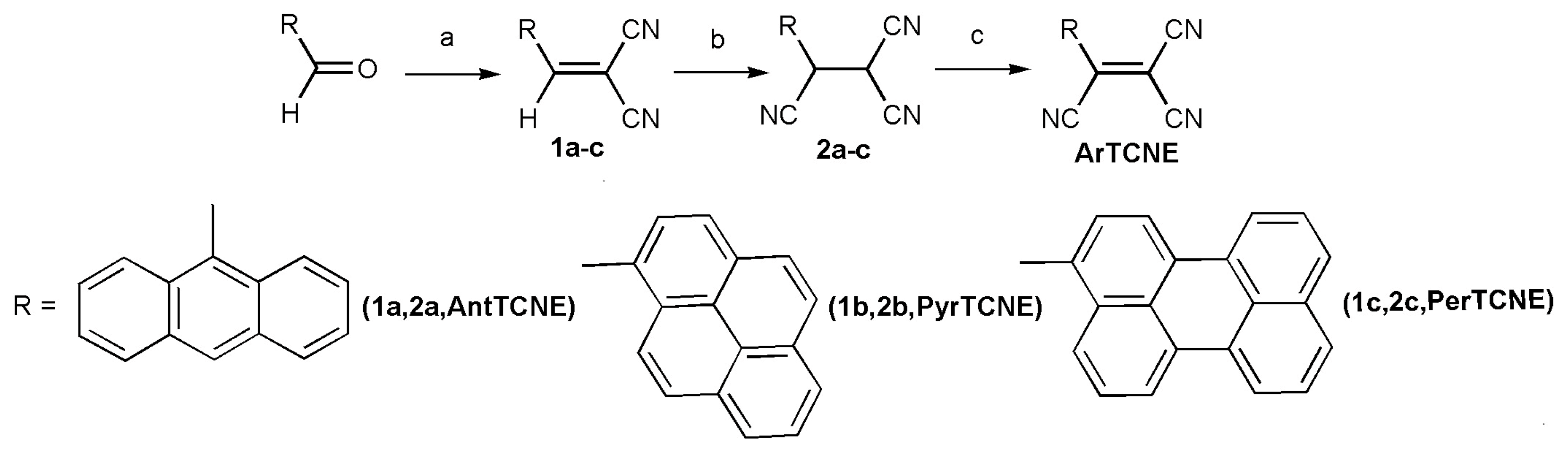
2.1. Synthesis and Characterization
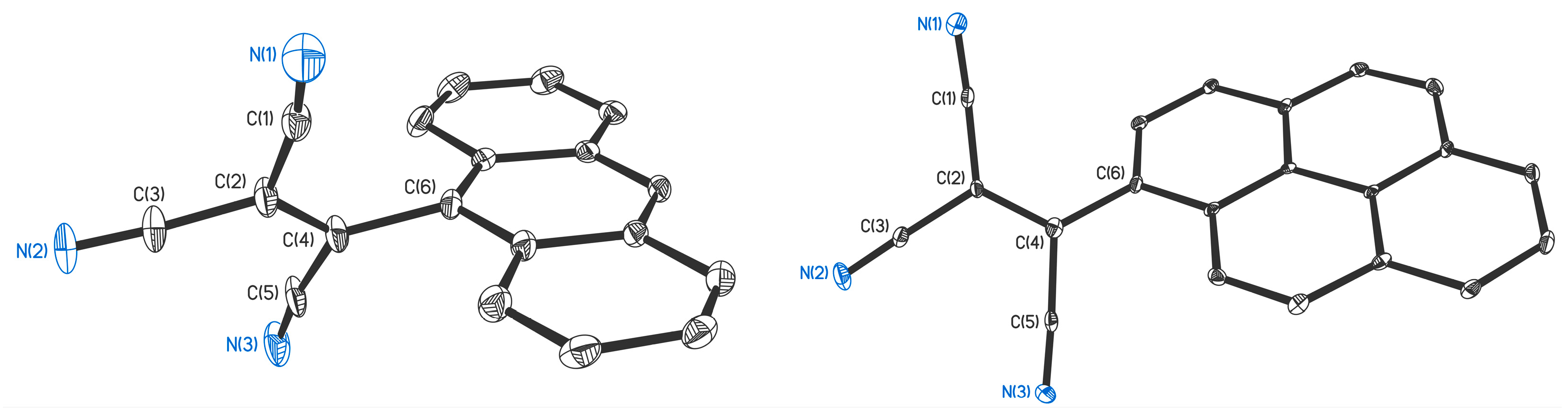
2.2. Structural and Thermal Properties
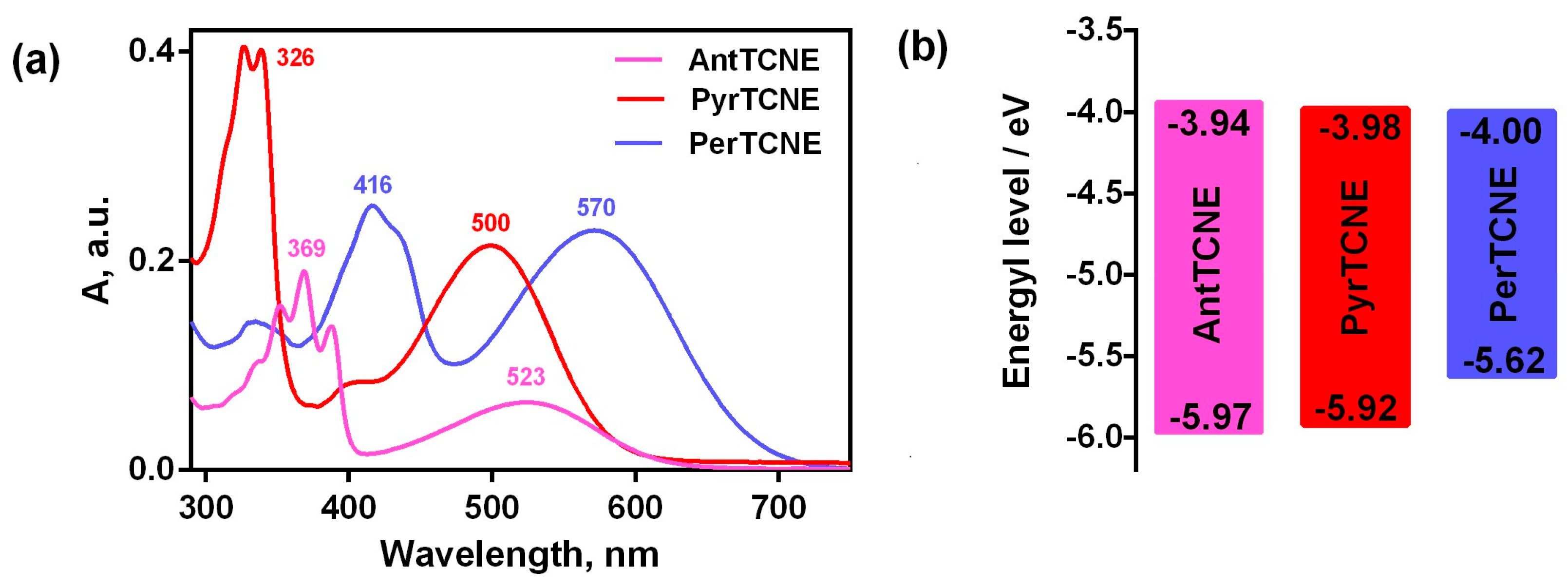
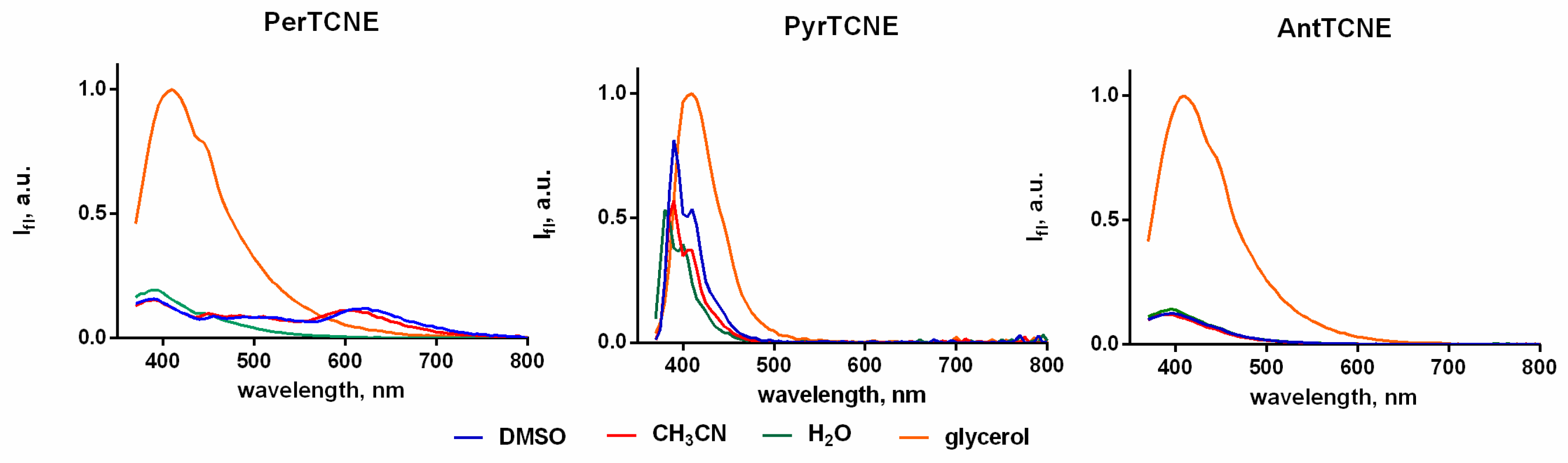
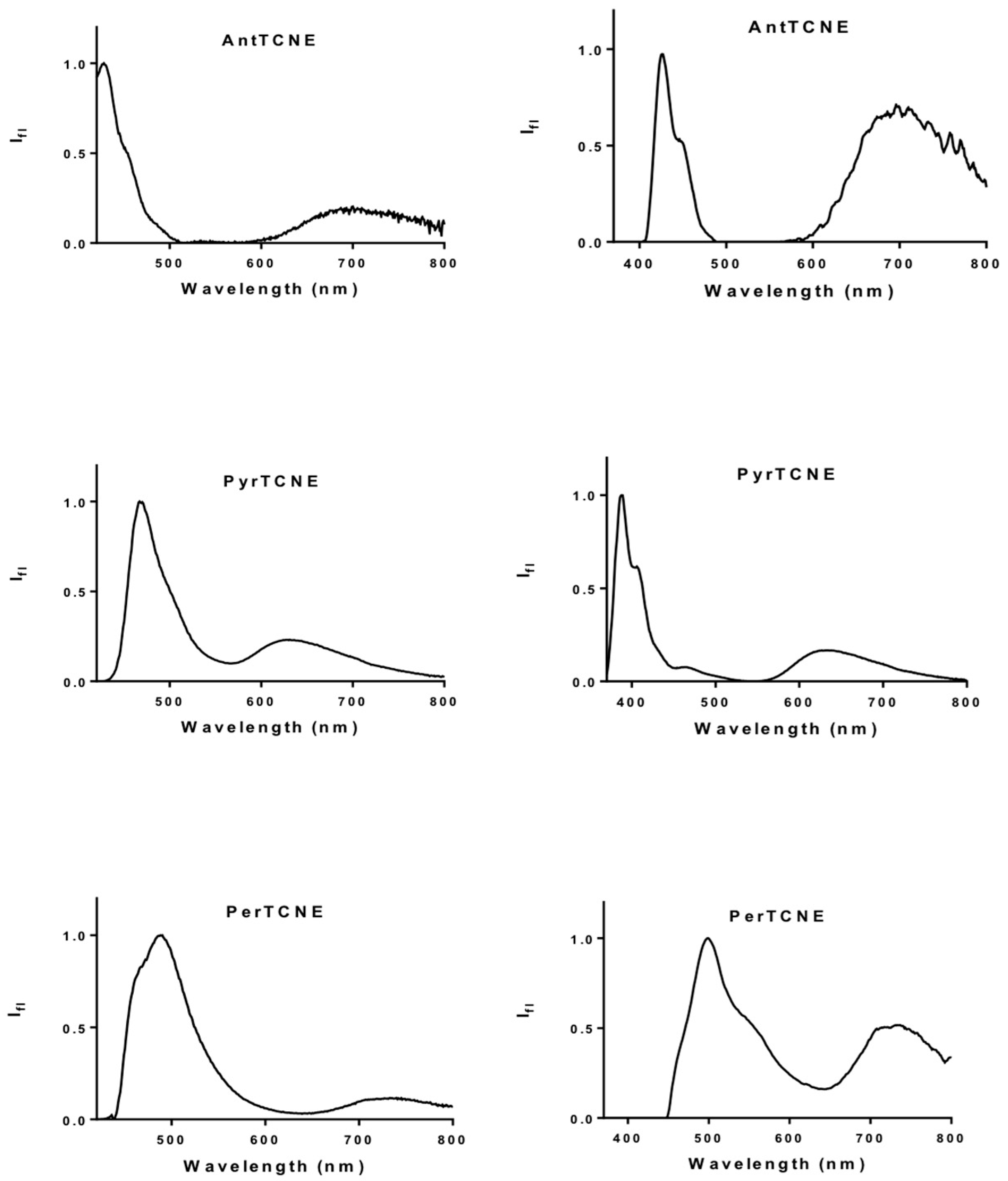
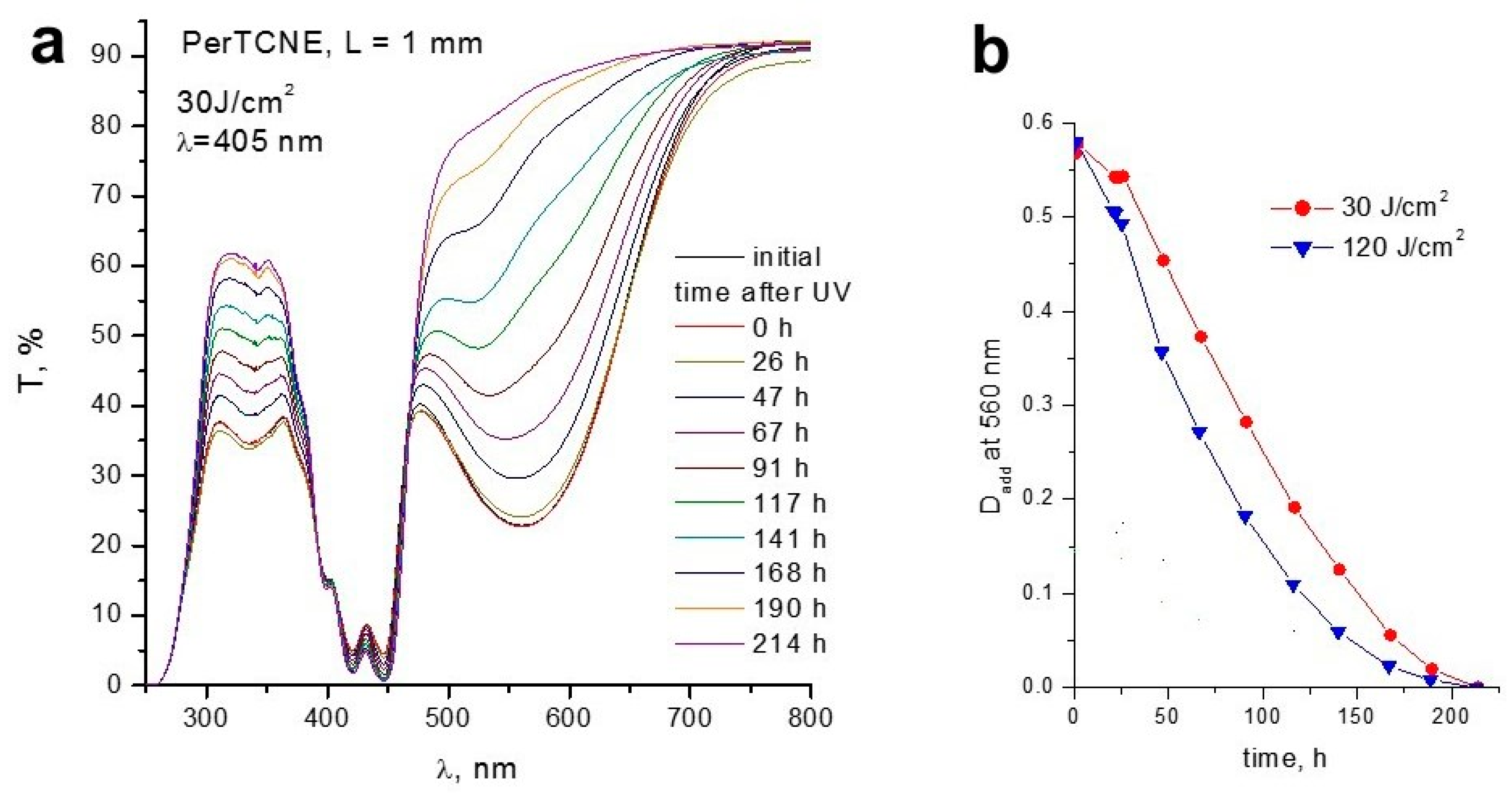
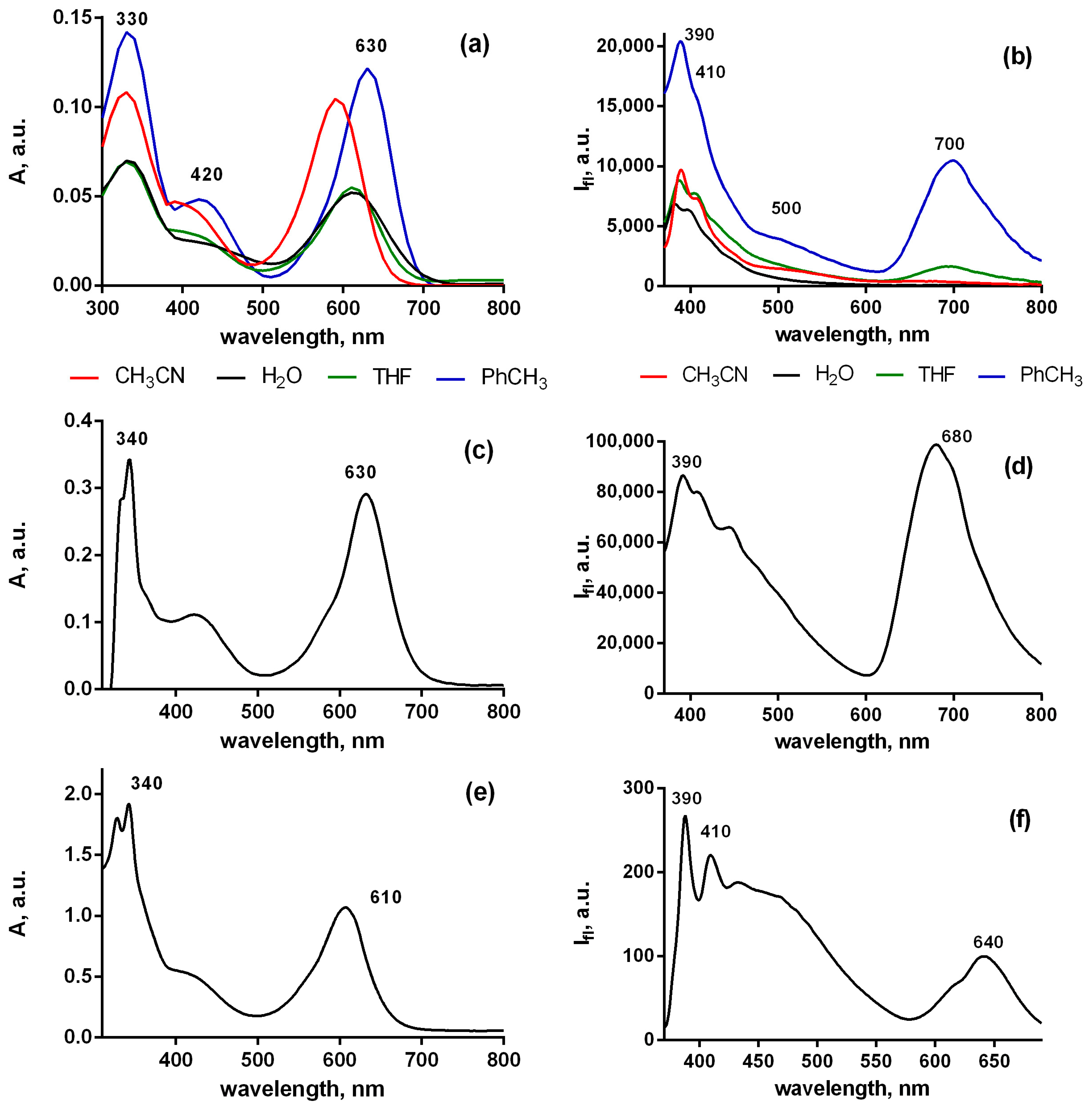
2.3. Photophysical Properties
Two-Photon Absorption Investigations
2.4. Electrochemical Investigation
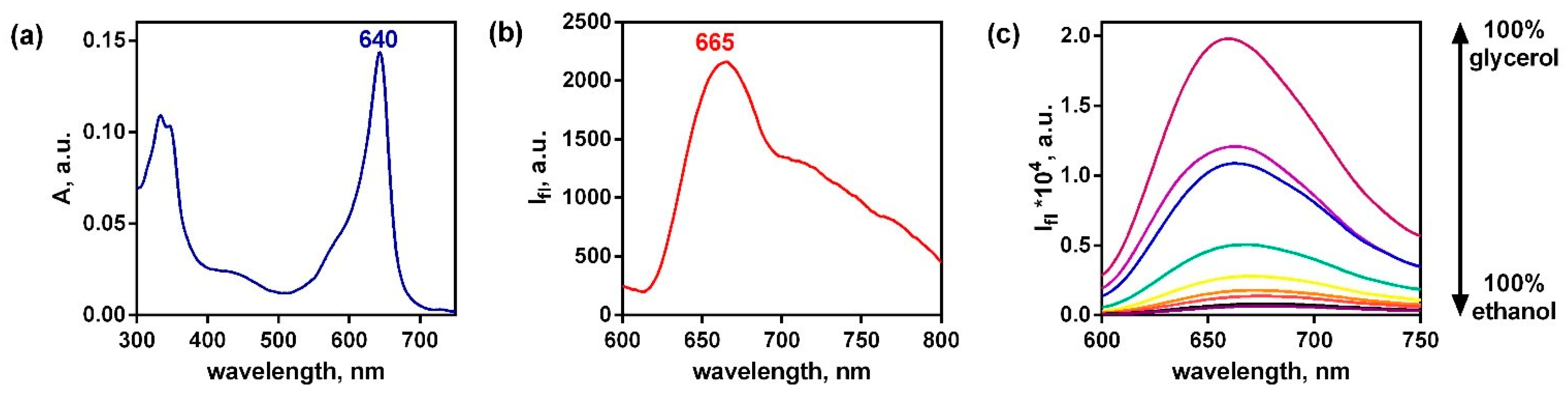
2.5. Template Assembling of the New Cyanoporphyrazine Frameworks Using AntTCNE, PyrTCNE and PerTCNE as the Structural Units
3. Materials and Methods
3.1. Reagents and Equipment
3.2. Powder X-ray Diffraction
3.3. Single Crystal X-ray Crystallography
3.4. Computational Details
3.5. Synthetic Details
3.5.1. Synthesis of 1a–c
3.5.2. Synthesis of 2a–c
3.5.3. Synthesis of AntTCNE, PyrTCNE, PerTCNE
3.5.4. Synthesis of 3,8,13,18-Tetra(pyrene-1-yl)-2,7,12,17-Tetracyanoporphyrazine (Pyr4CN4Pz)
3.6. Cell lines and Culturing Conditions
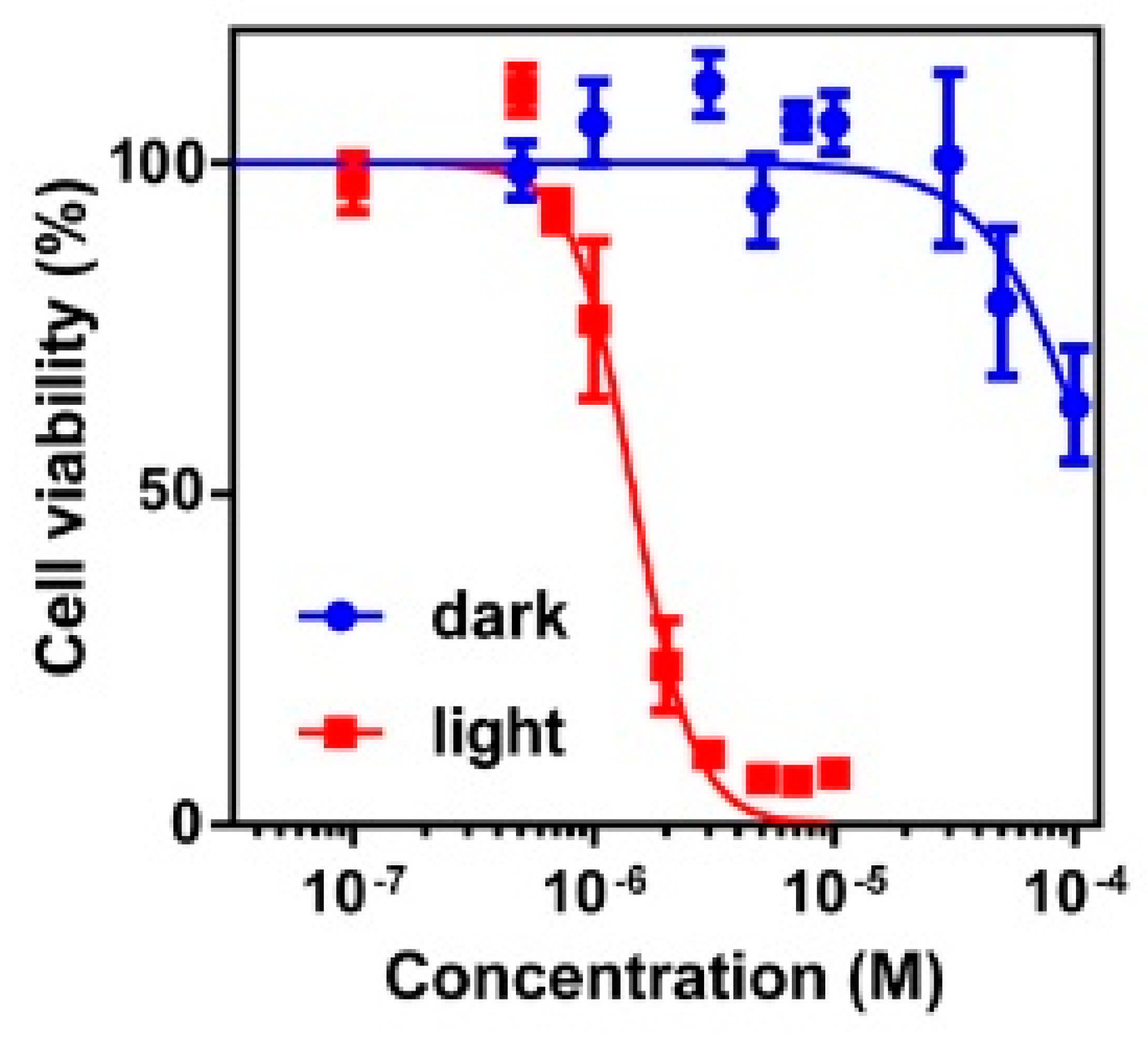
3.7. Study of Photodynamic Activity of Pyr4CN4Pz In Vitro
3.8. Estimation of Singlet Oxygen Production by Pyr4CN4Pz
3.9. Electrochemistry Experiment
3.9.1. Irradiation of Solutions with Laser Diode at 405 nm
3.9.2. Measurement of the Temporal Dynamics of Changes in Absorption
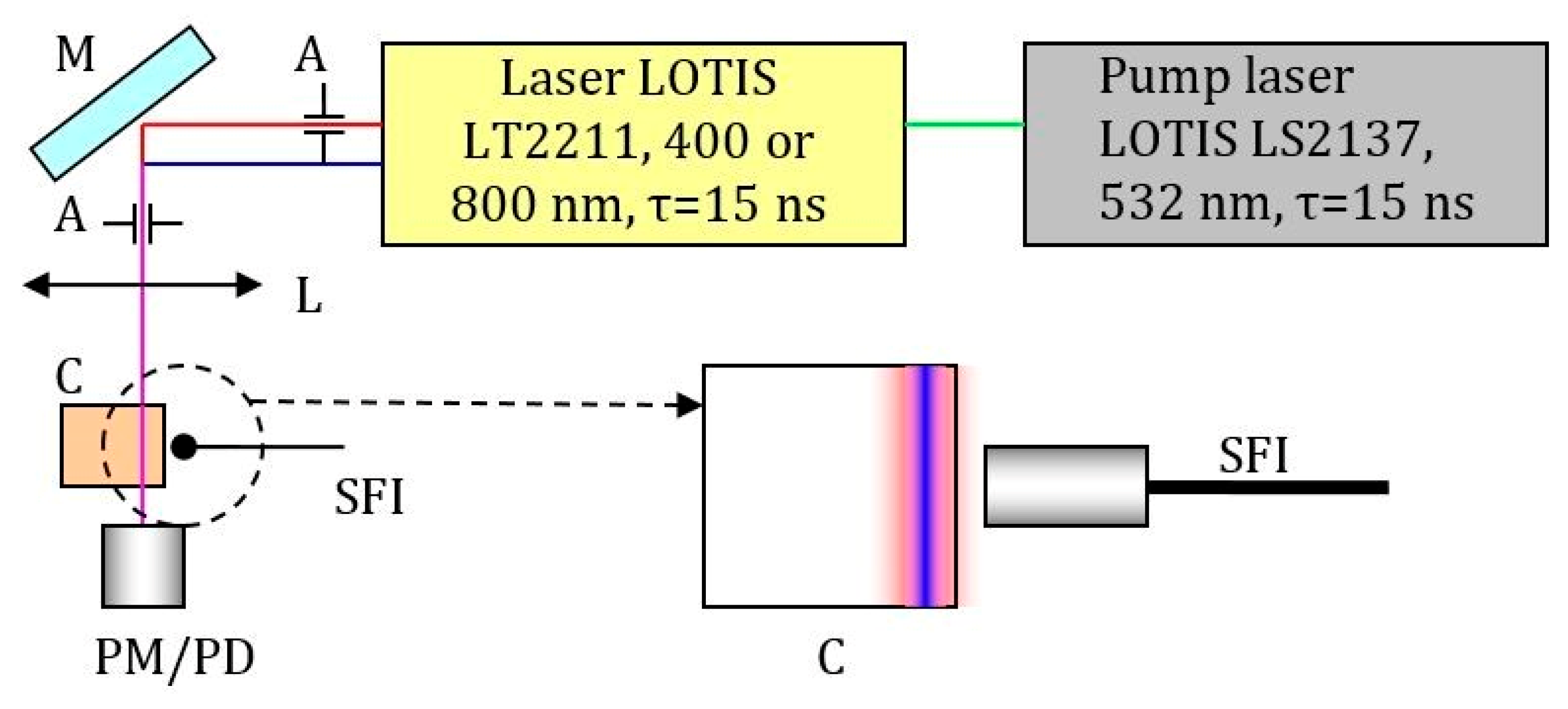
3.9.3. Setup for Two-Photon Absorption Measurement by Comparing the Fluorescence of Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Harvey, M.D.; Pace, J.T.; Yee, G.T. A room temperature ferrimagnet, vanadium[pentafluorophenyltricyanoethylene]2. Polyhedron 2007, 26, 2037–2041. [Google Scholar] [CrossRef]
- Harvey, M.D.; Crawford, T.D.; Yee, G.T. Room-Temperature and Near-Room-Temperature Molecule-Based Magnets. Inorg. Chem. 2008, 47, 5649–5655. [Google Scholar] [CrossRef]
- Kaul, B.B.; Yee, G.T. Two new acceptor building blocks for ‘high Tc’ coordination polymer magnets. Inorg. Chim. Acta 2001, 326, 9–12. [Google Scholar] [CrossRef]
- Amshumali, M.K.; Harvey, M.D.; Yee, G.T. Room temperature and near-room temperature coordination polymer magnets. Synth. Met. 2014, 188, 53–56. [Google Scholar] [CrossRef]
- Klapshina, L.G.; Grigoryev, I.S.; Douglas, W.E.; Trifonov, A.A.; Gudilenkov, I.D.; Semenov, V.V.; Bushuk, B.A.; Bushuk, S.B. Metal template assembly of highly functionalized octacyanoporphyrazine framework from TCNE structural units. Chem. Commun. 2007, 1942–1944. [Google Scholar] [CrossRef] [PubMed]
- Klapshina, L.G.; Douglas, W.E.; Grigoryev, I.S.; Korytin, A.I.; Lavrentiev, S.A.; Lopatin, M.A.; Lukyanov, A.Y.; Semenov, V.V.; Gerbier, P.; Treushnikov, V.M. Novel metal-template assembled highly-functionalized cyanoporphyrazine ytterbium and vanadium complexes for potential photonic and optoelectronic applications. J. Mater. Chem. 2009, 19, 3668–3676. [Google Scholar] [CrossRef]
- Klapshina, L.G.; Douglas, W.E.; Grigoryev, I.S.; Ladilina, E.Y.; Shirmanova, M.V.; Mysyagin, S.A.; Balalaeva, I.V.; Zagaynova, E.V. Novel PEG-organized biocompatible fluorescent nanoparticles doped with an ytterbium cyanoporphyrazine complex for biophotonic applications. Chem. Commun. 2010, 46, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Shilyagina, N.Y.; Peskova, N.N.; Lermontova, S.A.; Brilkina, A.A.; Vodeneev, V.A.; Yakimansky, A.V.; Klapshina, L.G.; Balalaeva, I.V. Effective delivery of porphyrazine photosensitizers to cancer cells by polymer brush nanocontainers. J. Biophotonics 2017, 10, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.A.; Turubanova, V.D.; Mitroshina, E.V.; Alzeibak, R.; Peskova, N.N.; Lermontova, S.A.; Klapshina, L.G.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Effect of novel porphyrazine photosensitizers on normal and tumor brain cells. J. Biophotonics 2020, 13, e201960077. [Google Scholar] [CrossRef] [PubMed]
- Yuzhakova, D.V.; Lermontova, S.A.; Grigoryev, I.S.; Muravieva, M.S.; Gavrina, A.I.; Shirmanova, M.V.; Balalaeva, I.V.; Klapshina, L.G.; Zagaynova, E.V. In vivo multimodal tumor imaging and photodynamic therapy with novel theranostic agents based on the porphyrazine framework-chelated gadolinium (III) cation. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3120–3130. [Google Scholar] [CrossRef]
- Lermontova, S.; Grigoryev, I.; Peskova, N.; Ladilina, E.; Lyubova, T.; Plekhanov, V.; Grishin, I.; Balalaeva, I.; Klapshina, L. Cyano-Aryl Porphyrazine Pigments with Polycyclic Substituents as the Promising Agents for Photodynamic Therapy and Potential Sensors of Local Viscosity. Macroheterocycles 2019, 12, 268–275. [Google Scholar] [CrossRef]
- Shirmanova, M.V.; Balalaeva, I.V.; Lekanova, N.; Mysiagin, S.A.; Brilkina, A.A.; Klapshina, L.G.; Zagaĭnova, E.V. Development of a new photosensitizer on the basis of ytterbium porphyrazine complex. Biofizika 2011, 56, 1117–1124. [Google Scholar]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Shimolina, L.E.; Izquierdo, M.A.; López-Duarte, I.; Bull, J.A.; Shirmanova, M.V.; Klapshina, L.G.; Zagaynova, E.V.; Kuimova, M.K. Imaging tumor microscopic viscosity in vivo using molecular rotors. Sci. Rep. 2017, 7, 41097. [Google Scholar] [CrossRef]
- Izquierdo, M.A.; Vyšniauskas, A.; Lermontova, S.A.; Grigoryev, I.S.; Shilyagina, N.Y.; Balalaeva, I.V.; Klapshina, L.G.; Kuimova, M.K. Dual use of porphyrazines as sensitizers and viscosity markers in photodynamic therapy. J. Mater. Chem. B 2015, 3, 1089–1096. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Türkmen, G.; Erten-Ela, S.; Icli, S. Highly soluble perylene dyes: Synthesis, photophysical and electrochemical characterizations. Dye. Pigment. 2009, 83, 297–303. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Chi, Y.; Yu, H.; Li, Y.; Jiang, T.; Wei, X.; Shi, J. Self-assembly, optical and electrical properties of perylene diimide dyes bearing unsymmetrical substituents at bay position. Sci. Rep. 2018, 8, 8208. [Google Scholar] [CrossRef]
- Sharma, G.D.; Suresh, P.; Mikroyannidis, J.A.; Stylianakis, M.M. Efficient bulk heterojunction devices based on phenylenevinylene small molecule and perylene–pyrene bisimide. J. Mater. Chem. 2010, 20, 561–567. [Google Scholar] [CrossRef]
- Li, H.; Earmme, T.; Subramaniyan, S.; Jenekhe, S.A. Bis(Naphthalene Imide)diphenylanthrazolines: A New Class of Electron Acceptors for Efficient Nonfullerene Organic Solar Cells and Applicable to Multiple Donor Polymers. Adv. Energy Mater. 2015, 5, 1402041. [Google Scholar] [CrossRef]
- Rao, P.S.; More, V.G.; Jangale, A.D.; Bhosale, S.V.; Bhosale, R.S.; Puyad, A.L.; Chen, J.-Y.; Li, J.-L.; Bhosale, S.V.; Gupta, A.; et al. A series of V-shaped small molecule non-fullerene electron acceptors for efficient bulk-heterojunction devices. Dye. Pigment. 2019, 171, 107677. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Sisto, T.J.; Zhong, Y.; Zhang, B.; Trinh, M.T.; Miyata, K.; Zhong, X.; Zhu, X.Y.; Steigerwald, M.L.; Ng, F.; Nuckolls, C. Long, Atomically Precise Donor–Acceptor Cove-Edge Nanoribbons as Electron Acceptors. J. Am. Chem. Soc. 2017, 139, 5648–5651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, C.; Jiang, K.; Zhao, J.; Li, Y.; Zhang, L.; Li, Z.; Lai, J.Y.L.; Hu, H.; Ma, T.; et al. A Tetraphenylethylene Core-Based 3D Structure Small Molecular Acceptor Enabling Efficient Non-Fullerene Organic Solar Cells. Adv. Mater. 2015, 27, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xu, X.; Yan, H.; Wu, W.; Li, Z.; Peng, Q. Pronounced Effects of a Triazine Core on Photovoltaic Performance–Efficient Organic Solar Cells Enabled by a PDI Trimer-Based Small Molecular Acceptor. Adv. Mater. 2017, 29, 1605115. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, J.; Zhang, Z.; Peng, H.; Zhang, Z.-G.; Xu, S.; Liu, Y.; Li, Y.; Zou, Y. Thieno[3,2-b]pyrrolo-Fused Pentacyclic Benzotriazole-Based Acceptor for Efficient Organic Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 31985–31992. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Xiao, Y.; Xiao, T.; Zhu, R.; Yan, C.; Fu, Y.; Lu, G.; Lu, X.; Marder, S.R.; et al. Effect of Isomerization on High-Performance Nonfullerene Electron Acceptors. J. Am. Chem. Soc. 2018, 140, 9140–9147. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Weng, K.; Ye, L.; Zhu, L.; Xu, J.; Zhou, J.; Feng, X.; Lu, G.; Tan, S.; Liu, F.; Sun, Y. Optimized active layer morphology toward efficient and polymer batch insensitive organic solar cells. Nat. Commun. 2020, 11, 2855. [Google Scholar] [CrossRef]
- Hin Lee, H.K.; Barbé, J.; Tsoi, W.C. Chapter Ten—Organic and perovskite photovoltaics for indoor applications. In Solar Cells and Light Management; Enrichi, F., Righini, G.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–388. [Google Scholar] [CrossRef]
- Matiko, J.W.; Grabham, N.J.; Beeby, S.P.; Tudor, M.J. Review of the application of energy harvesting in buildings. Meas. Sci. Technol. 2014, 25, 012002. [Google Scholar] [CrossRef]
- Arai, R.; Furukawa, S.; Hidaka, Y.; Komiyama, H.; Yasuda, T. High-Performance Organic Energy-Harvesting Devices and Modules for Self-Sustainable Power Generation under Ambient Indoor Lighting Environments. ACS Appl. Mater. Interfaces 2019, 11, 9259–9264. [Google Scholar] [CrossRef] [PubMed]
- Whitby, R.; Ben-Tal, Y.; MacMillan, R.; Janssens, S.; Raymond, S.; Clarke, D.; Jin, J.; Kay, A.; Simpson, M.C. Photoinitiators for two-photon polymerisation: Effect of branching and viscosity on polymerisation thresholds. RSC Adv. 2017, 7, 13232–13239. [Google Scholar] [CrossRef]
- Jhun, B.H.; Jeong, D.Y.; Nah, S.; Park, S.Y.; You, Y. Novel anti-Kasha fluorophores exhibiting dual emission with thermally activated delayed fluorescence through detouring triplet manifolds. J. Mater. Chem. C 2021, 9, 7083–7093. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.-A.; Hung, W.-Y.; Tang, W.-F.; Hsu, Y.-H.; Chen, C.-L.; Meng, F.-Y.; Chou, P.-T. Control of the Reversibility of Excited-State Intramolecular Proton Transfer (ESIPT) Reaction: Host-Polarity Tuning White Organic Light Emitting Diode on a New Thiazolo[5,4-d]thiazole ESIPT System. Chem. Mater. 2016, 28, 8815–8824. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Lehn, J.M. Bright white-light emission from a single organic compound in the solid state. Angew. Chem. Int. Ed. Engl. 2014, 53, 4572–4577. [Google Scholar] [CrossRef]
- Husband, J.T.; Xie, Y.; Wilks, T.R.; Male, L.; Torrent-Sucarrat, M.; Stavros, V.G.; O’Reilly, R.K. Rigidochromism by imide functionalisation of an aminomaleimide fluorophore. Chem. Sci. 2021, 12, 10550–10557. [Google Scholar] [CrossRef]
- Piskorz, J.; Lijewski, S.; Gierszewski, M.; Gorniak, K.; Sobotta, L.; Wicher, B.; Tykarska, E.; Düzgüneş, N.; Konopka, K.; Sikorski, M.; et al. Sulfanyl porphyrazines: Molecular barrel-like self-assembly in crystals, optical properties and in vitro photodynamic activity towards cancer cells. Dye. Pigment. 2017, 136, 898–908. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Liu, Y.; Song, J.; Li, C.; Bo, Z. Perylene diimide based star-shaped small molecular acceptors for high efficiency organic solar cells. J. Mater. Chem. C 2019, 7, 819–825. [Google Scholar] [CrossRef]
- Qian, H.; Cousins, M.E.; Horak, E.H.; Wakefield, A.; Liptak, M.D.; Aprahamian, I. Suppression of Kasha’s rule as a mechanism for fluorescent molecular rotors and aggregation-induced emission. Nat. Chem. 2017, 9, 83–87. [Google Scholar] [CrossRef]
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical properties and photocytotoxicity of free and liposome-entrapped diazepinoporphyrazines on LNCaP cells under normoxic and hypoxic conditions. Eur. J. Med. Chem. 2018, 150, 64–73. [Google Scholar] [CrossRef]
- Kunnil, J.; Sarasanandarajah, S.; Chacko, E.; Reinisch, L. Fluorescence quantum efficiency of dry Bacillus globigii spores. Opt. Express 2005, 13, 8969–8979. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Therrien, B. Chapter 13—Pyrene: The Guest of Honor. In Organic Nanoreactors; Sadjadi, S., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 421–461. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.; Islam, M.; Bredow, T.; Aziz, M. The Effects of Oxidation States, Spin States and Solvents on Molecular Structure, Stability and Spectroscopic Properties of Fe-Catechol Complexes: A Theoretical Study. Adv. Chem. Eng. Sci. 2017, 07, 137–153. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Wu, S.; Yan, Y.; Hou, H.; Huang, Z.; Li, D.; Zhang, X.; Xiao, Y. Polarity-sensitive and membrane-specific probe quantitatively monitoring ferroptosis through fluorescence lifetime imaging. Anal. Chem. 2022, 94, 11238–11247. [Google Scholar] [CrossRef] [PubMed]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci. Rep. 2021, 11, 7205. [Google Scholar] [CrossRef]
- Bagrov, I.V.; Dadeko, A.; Kiselev, V.M.; Muravieva, T.D.; Starodubtsev, A.M. Comparative studies of the photophysical properties of dimegin, photoditazine and radachlorin. J. Tech. Phys. 2019, 126, 162. [Google Scholar]
- Kulinich, A.V.; Ishchenko, A.A.; Bondarev, S.L.; Knyukshto, V.N. Effect of donor and acceptor end-groups on electronic structure and spectral-fluorescent properties of merocyanines in frozen ethanol. J. Photochem. Photobiol. A Chem. 2021, 405, 112932. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principiles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]


| IFl2/IFl1 | Cell Thickness (mm) | P400/P800 | P800 (W) | r (cm) | f (Hz) | I800 (GW/cm2) | β (cm/W) | δ (cm4s/Photon) |
|---|---|---|---|---|---|---|---|---|
| 400 | 1.0 | 0.01 | 0.045 | 0.01 | 10 | ~1 | 4.5 × 10−12 | 1.85 × 10−48 |
| Aryltricyanoethylene | E11/2 Red, V | E21/2 Red, V | EOx, V | EHOMO, eV | ELUMO, eV | Bandgap |
|---|---|---|---|---|---|---|
| AntTCNE | −0.84 | −1.63 | 1.17 | −5.97 | −3.94 | 2.03 |
| PyrTCNE | −0.82 | −1.44 | 1.12 | −5.92 | −3.98 | 1.94 |
| PerTCNE | −0.80 | −1.41 | 0.82 | −5.62 | −4.00 | 1.62 |
| Pyr4CN4Pz | −1.60 | 0.29 | −6.40 | −4.51 | 1.89 |
| Solvent | η (cP) | ε | Φf | |
|---|---|---|---|---|
| LW | SW | |||
| Castor oil | 1079 | 4.7 | 0.360 | 0.480 |
| CH3Ph | 0.59 | 2.4 | 0.066 | 0.129 |
| THF | 0.55 | 7.5 | 0.030 | 0.168 |
| CH3CN | 0.37 | 37.0 | 0.002 | 0.043 |
| Water | 1.00 | 80.0 | 0.007 | 0.398 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lermontova, S.A.; Arsenyev, M.V.; Cherkasov, A.V.; Fukin, G.K.; Afanasyev, A.V.; Yudintsev, A.V.; Grigoryev, I.S.; Ladilina, E.Y.; Lyubova, T.S.; Shilyagina, N.Y.; et al. Novel Rigidochromic and Anti-Kasha Dual Emission Fluorophores Based on D-π-A Dyads as the Promising Materials for Potential Applications Ranging from Optoelectronics and Optical Sensing to Biophotonics and Medicine. Int. J. Mol. Sci. 2023, 24, 5818. https://doi.org/10.3390/ijms24065818
Lermontova SA, Arsenyev MV, Cherkasov AV, Fukin GK, Afanasyev AV, Yudintsev AV, Grigoryev IS, Ladilina EY, Lyubova TS, Shilyagina NY, et al. Novel Rigidochromic and Anti-Kasha Dual Emission Fluorophores Based on D-π-A Dyads as the Promising Materials for Potential Applications Ranging from Optoelectronics and Optical Sensing to Biophotonics and Medicine. International Journal of Molecular Sciences. 2023; 24(6):5818. https://doi.org/10.3390/ijms24065818
Chicago/Turabian StyleLermontova, Svetlana A., Maxim V. Arsenyev, Anton V. Cherkasov, Georgy K. Fukin, Andrey V. Afanasyev, Andrey V. Yudintsev, Ilya S. Grigoryev, Elena Yu. Ladilina, Tatyana S. Lyubova, Natalia Yu. Shilyagina, and et al. 2023. "Novel Rigidochromic and Anti-Kasha Dual Emission Fluorophores Based on D-π-A Dyads as the Promising Materials for Potential Applications Ranging from Optoelectronics and Optical Sensing to Biophotonics and Medicine" International Journal of Molecular Sciences 24, no. 6: 5818. https://doi.org/10.3390/ijms24065818
APA StyleLermontova, S. A., Arsenyev, M. V., Cherkasov, A. V., Fukin, G. K., Afanasyev, A. V., Yudintsev, A. V., Grigoryev, I. S., Ladilina, E. Y., Lyubova, T. S., Shilyagina, N. Y., Balalaeva, I. V., Klapshina, L. G., & Piskunov, A. V. (2023). Novel Rigidochromic and Anti-Kasha Dual Emission Fluorophores Based on D-π-A Dyads as the Promising Materials for Potential Applications Ranging from Optoelectronics and Optical Sensing to Biophotonics and Medicine. International Journal of Molecular Sciences, 24(6), 5818. https://doi.org/10.3390/ijms24065818









