Resmetirom Ameliorates NASH-Model Mice by Suppressing STAT3 and NF-κB Signaling Pathways in an RGS5-Dependent Manner
Abstract
:1. Introduction
2. Results
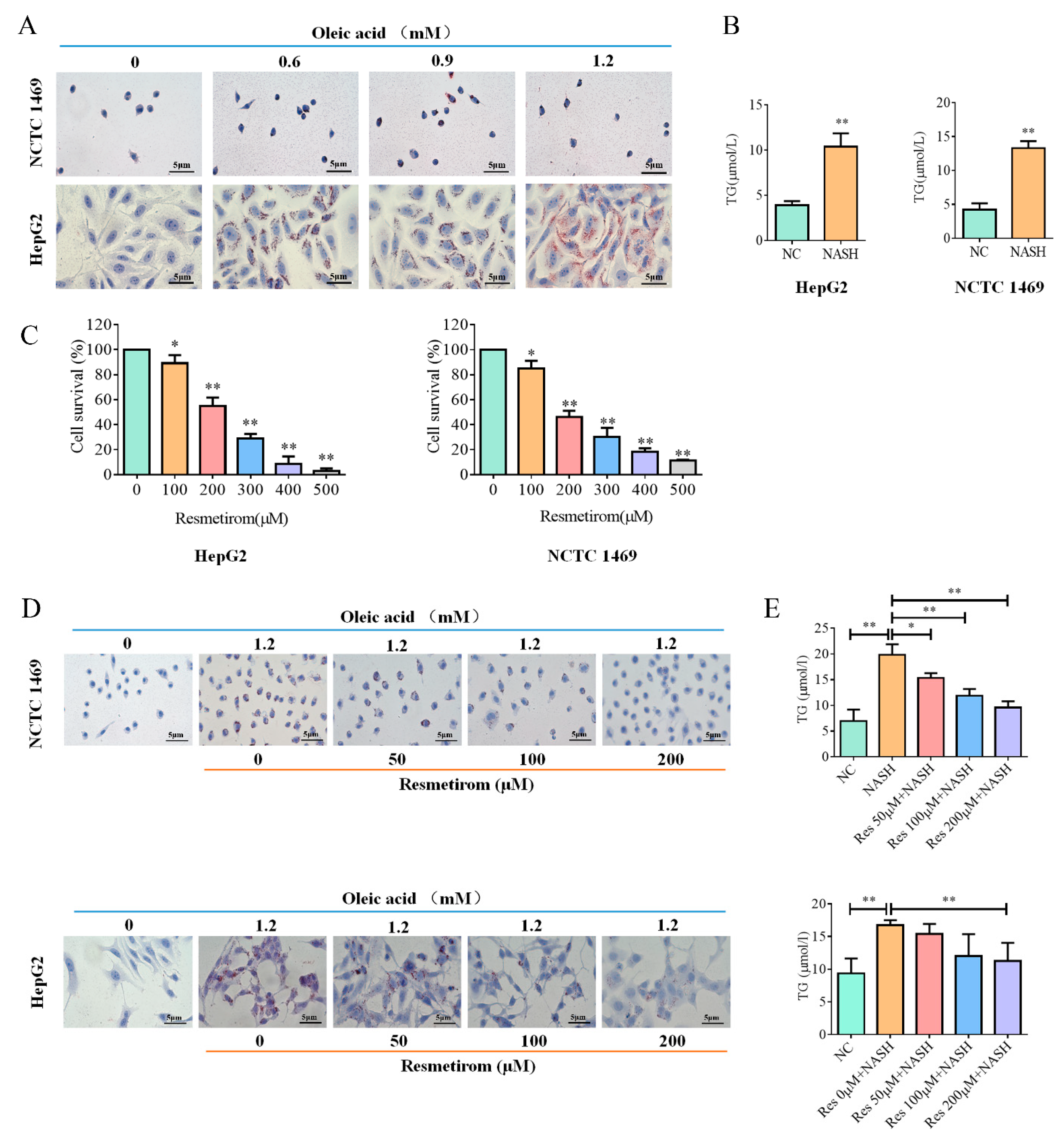
2.1. Resmetirom Effectively Impaired Lipid Accumulation in Liver Cells Induced by Oleic Acid
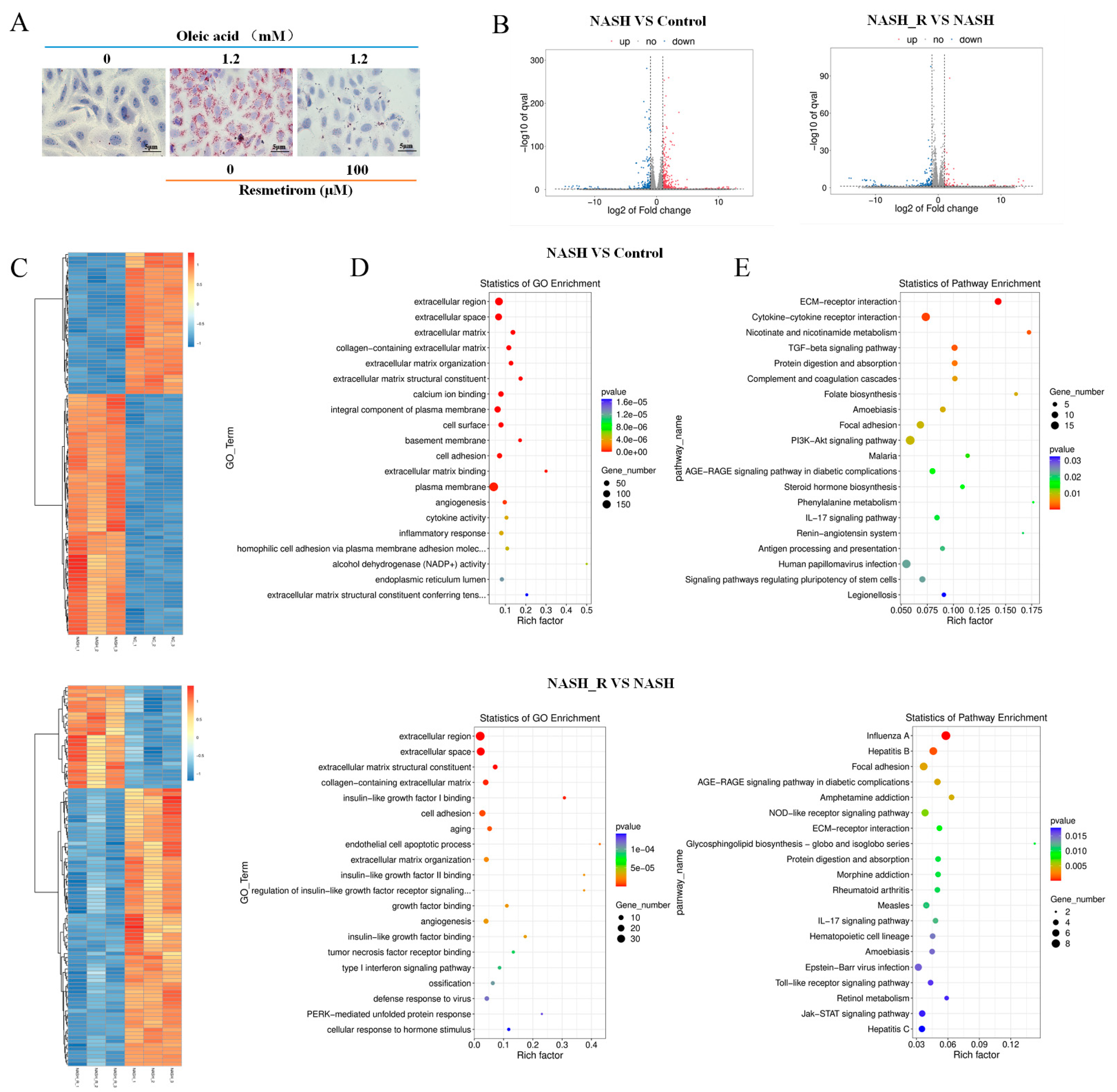
2.2. mRNA Sequencing Indicated That Resmetirom Might Improve NAFLD/NASH by Influencing the Activation of Toll-like Receptor and Jak-STAT3 Signaling Pathways
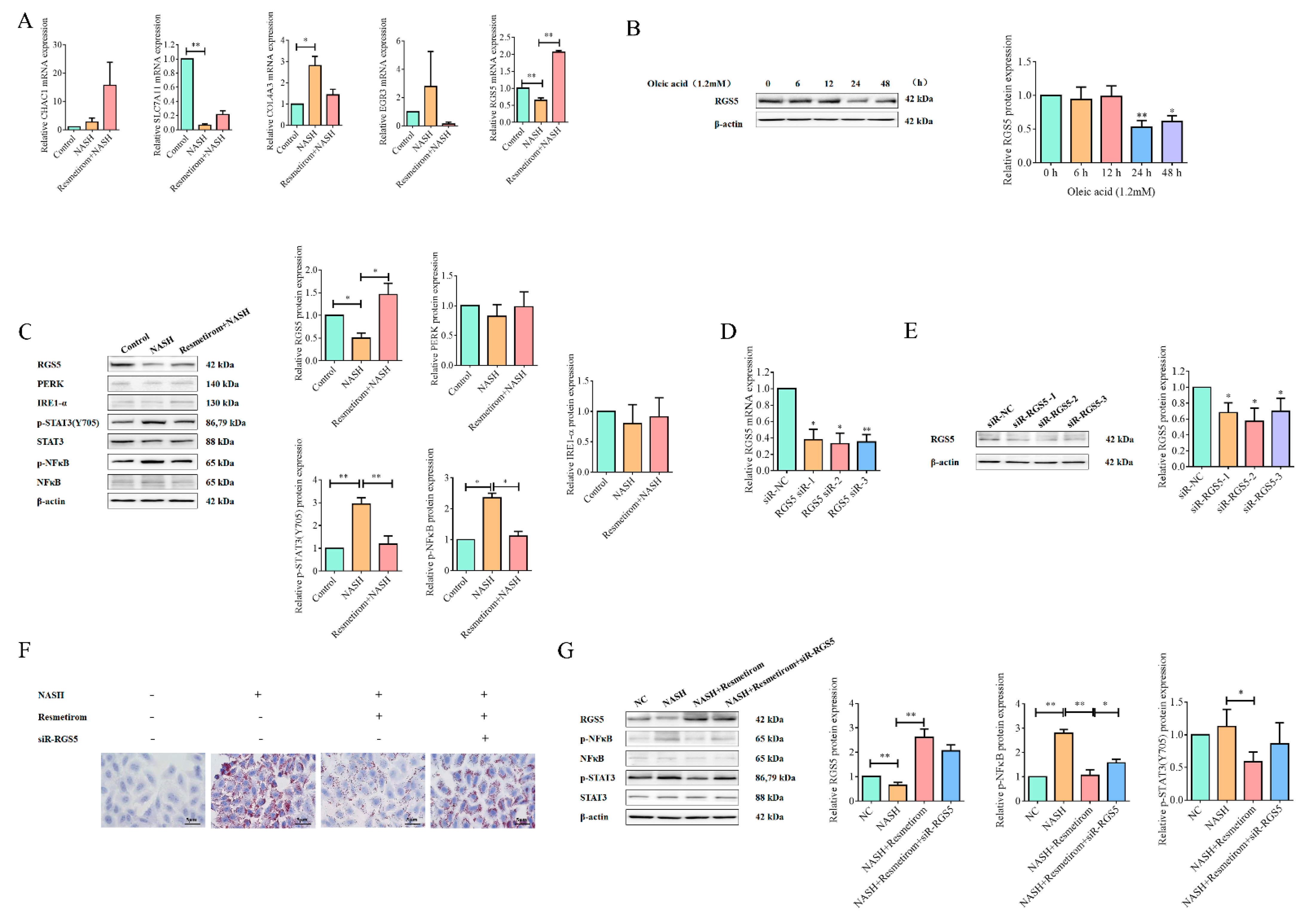
2.3. Resmetirom Might Ameliorate NAFLD/NASH in an RGS5-Dependent Manner
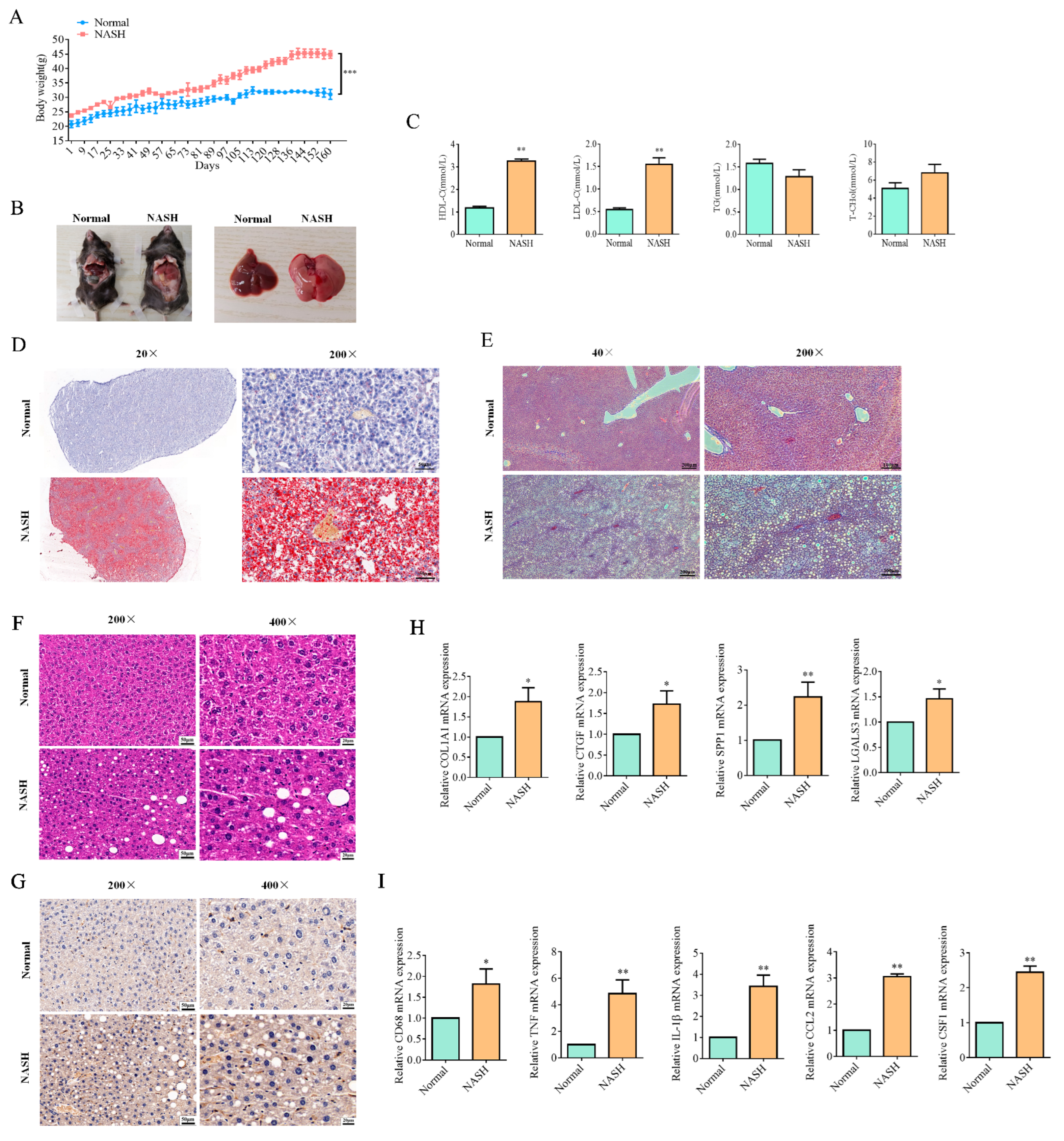
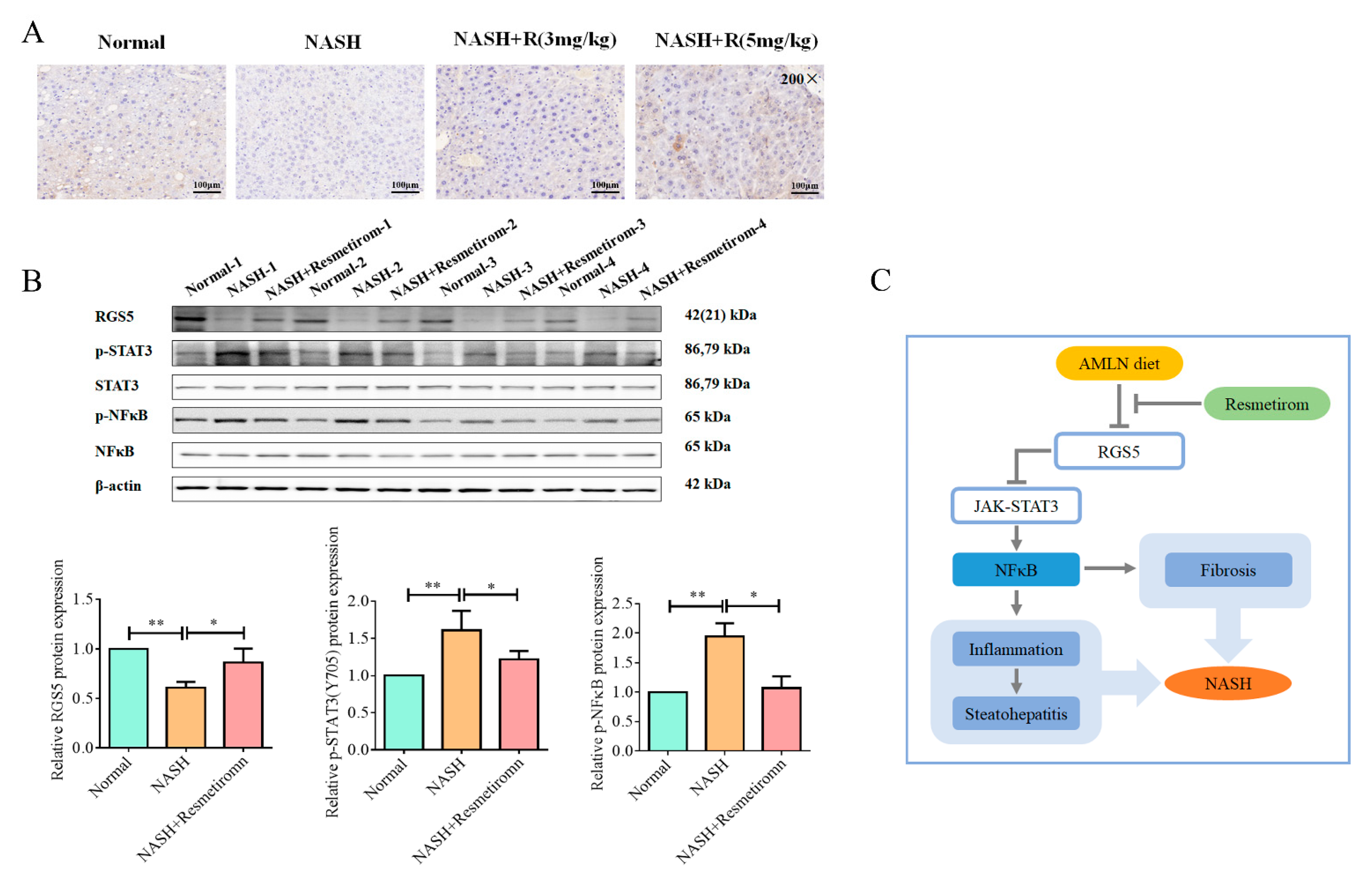
2.4. Resmetirom Significantly Prevented NASH in Mouse Model
2.5. Resmetirom Effectively Improved NASH In Vivo by Recovering RGS5 Expression and Inactivating STAT3 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. In Vitro Cell Culture Model for NAFLD/NASH Disease
4.3. MTT Assay for Resmetirom Cytotoxicity Detection
4.4. mRNA Sequencing and Bioinformatic Analysis
4.5. qRT-PCR Assay
4.6. Western Blot Assay
4.7. siRNA and Transfection
4.8. Animal Model for NASH Disease and Resmetirom Treatment
4.9. ELISA
4.10. Pathological Experiment
4.10.1. Oil Red O Staining
4.10.2. Masson Staining
4.10.3. HE Staining
4.10.4. TUNEL Assay
4.10.5. IHC Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Wang, X.; Tanaka, N.; Hu, X.; Kimura, T.; Lu, Y.; Jia, F.; Sato, Y.; Nakayama, J.; Moriya, K.; Koike, K.; et al. A high-cholesterol diet promotes steatohepatitis and liver tumorigenesis in HCV core gene transgenic mice. Arch. Toxicol. 2019, 93, 1713–1725. [Google Scholar] [CrossRef]
- Tanaka, N.; Kimura, T.; Fujimori, N.; Nagaya, T.; Komatsu, M.; Tanaka, E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 2019, 25, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Abdelmalek, M.F. Nonalcoholic fatty liver disease: Another leap forward. Nat. Reviews. Gastroenterol. Hepatol. 2021, 18, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, R.; Shen, Z.; Cai, G. Combination of luteolin and lycopene effectively protect against the "two-hit" in NAFLD through Sirt1/AMPK signal pathway. Life Sci. 2020, 256, 117990. [Google Scholar] [CrossRef]
- Kimura, T.; Tanaka, N.; Fujimori, N.; Sugiura, A.; Yamazaki, T.; Joshita, S.; Komatsu, M.; Umemura, T.; Matsumoto, A.; Tanaka, E. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fibrosis. World J. Gastroenterol. 2018, 24, 1440–1450. [Google Scholar] [CrossRef]
- Jia, F.; Hu, X.; Kimura, T.; Tanaka, N. Impact of Dietary Fat on the Progression of Liver Fibrosis: Lessons from Animal and Cell Studies. Int. J. Mol. Sci. 2021, 22, 10303. [Google Scholar] [CrossRef]
- Balenga, N.A.; Jester, W.; Jiang, M.; Panettieri, R.A., Jr.; Druey, K.M. Loss of regulator of G protein signaling 5 promotes airway hyperresponsiveness in the absence of allergic inflammation. J. Allergy Clin. Immunol. 2014, 134, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, J.; Nie, H.; Zhang, X.J.; Zhang, P.; She, Z.G.; Li, H.; Ji, Y.X.; Cai, J. Hepatic Regulator of G Protein Signaling 5 Ameliorates Nonalcoholic Fatty Liver Disease by Suppressing Transforming Growth Factor Beta-Activated Kinase 1-c-Jun-N-Terminal Kinase/p38 Signaling. Hepatology 2021, 73, 104–125. [Google Scholar] [CrossRef]
- Park, J.; Zhao, Y.; Zhang, F.; Zhang, S.; Kwong, A.C.; Zhang, Y.; Hoffmann, H.H.; Bushweller, L.; Wu, X.; Ashbrook, A.W.; et al. IL-6/STAT3 axis dictates the PNPLA3-mediated susceptibility to non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, 45–56. [Google Scholar] [CrossRef]
- Jiao, J.; Sanchez, J.I.; Saldarriaga, O.A.; Solis, L.M.; Tweardy, D.J.; Maru, D.M.; Stevenson, H.L.; Beretta, L. Spatial molecular and cellular determinants of STAT3 activation in liver fibrosis progression in non-alcoholic fatty liver disease. JHEP Rep. 2023, 5, 100628. [Google Scholar] [CrossRef]
- Huh, J.Y.; Saltiel, A.R. Roles of IkappaB kinases and TANK-binding kinase 1 in hepatic lipid metabolism and nonalcoholic fatty liver disease. Exp. Mol. Med. 2021, 53, 1697–1705. [Google Scholar] [CrossRef]
- Ye, D.; Li, F.Y.; Lam, K.S.; Li, H.; Jia, W.; Wang, Y.; Man, K.; Lo, C.M.; Li, X.; Xu, A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut 2012, 61, 1058–1067. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [Green Version]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid. Off. J. Am. Thyroid. Assoc. 2019, 29, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Taub, R.A.; Barbone, J.M.; Harrison, S.A. Hepatic Fat Reduction Due to Resmetirom in Patients With Nonalcoholic Steatohepatitis Is Associated With Improvement of Quality of Life. Clin. Gastroenterol. Hepatol. 2022, 20, 1354–1361.e1357. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, P.; Yang, B.; Wang, M.; Wang, T.; Sun, W.; Wang, X.; Zheng, W.; Song, X.; Li, J. A network pharmacology approach: Inhibition of the NF-κB signaling pathway contributes to the NASH preventative effect of an Oroxylum indicum seed extract in oleic acid-stimulated HepG2 cells and high-fat diet-fed rats. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 88, 153498. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Haczeyni, F.; Chitturi, S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. Adv. Exp. Med. Biol. 2018, 1061, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, B.; Meng, M.; Zhao, W.; Wang, D.; Yuan, Y.; Zheng, Y.; Qiu, J.; Li, Y.; Li, G.; et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 150–162. [Google Scholar] [CrossRef]
- Fouda, S.; Khan, A.; Chan, S.M.H.; Mahzari, A.; Zhou, X.; Qin, C.X.; Vlahos, R. Exposure to cigarette smoke precipitates simple hepatosteatosis to NASH in high-fat diet fed mice by inducing oxidative stress. Clin. Sci. 2021, 135, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Kowalik, M.A.; Columbano, A.; Perra, A. Thyroid Hormones, Thyromimetics and Their Metabolites in the Treatment of Liver Disease. Front. Endocrinol. 2018, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Guaraldi, G.; Maurice, J.B.; Marzolini, C.; Monteith, K.; Milic, J.; Tsochatzis, E. New Drugs for NASH and HIV Infection: Great Expectations for a Great Need. Hepatology 2020, 71, 1831–1844. [Google Scholar] [CrossRef]
- Alkhouri, N. Thyromimetics as emerging therapeutic agents for nonalcoholic steatohepatitis: Rationale for the development of resmetirom (MGL-3196). Expert Opin. Investig. Drugs 2020, 29, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, Q.; Chen, Y. Effect of silibinin on CFLAR-JNK pathway in oleic acid-treated HepG2 cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 108, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Gao, J.; Zheng, R.; Yu, M.; Ren, Y.; Yan, T.; Huang, Y.; Li, Y. Antagonizing circRNA_002581-miR-122-CPEB1 axis alleviates NASH through restoring PTEN-AMPK-mTOR pathway regulated autophagy. Cell Death Dis. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Cheng, S.Y. New insights into regulation of lipid metabolism by thyroid hormone. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jorgensen, S.M.D.; Thomsen, K.L.; Moller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Gronbaek, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Barreby, E.; Chen, P.; Aouadi, M. Macrophage functional diversity in NAFLD-more than inflammation. Nat. Rev. Endocrinol. 2022, 18, 461–472. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Jung, K.H.; Yoo, W.; Stevenson, H.L.; Deshpande, D.; Shen, H.; Gagea, M.; Yoo, S.Y.; Wang, J.; Eckols, T.K.; Bharadwaj, U.; et al. Multifunctional Effects of a Small-Molecule STAT3 Inhibitor on NASH and Hepatocellular Carcinoma in Mice. Clin. Cancer Res. 2017, 23, 5537–5546. [Google Scholar] [CrossRef] [Green Version]
- Seki, N.; Sugano, S.; Suzuki, Y.; Nakagawara, A.; Ohira, M.; Muramatsu, M.; Saito, T.; Hori, T. Isolation, tissue expression, and chromosomal assignment of human RGS5, a novel G-protein signaling regulator gene. J. Hum. Genet. 1998, 43, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yan, H.; Guo, J.; Han, Y.; Zhang, C.; Liu, X.; Du, J.; Tian, X.L. Down-regulated RGS5 by genetic variants impairs endothelial cell function and contributes to coronary artery disease. Cardiovasc. Res. 2021, 117, 240–255. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Moallem, S.A.; Johnston, T.P.; Sahebkar, A. Liver Protective Effect of Fenofibrate in NASH/NAFLD Animal Models. PPAR Res. 2022, 2022, 5805398. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.L.; Oro, D.; Tolbol, K.S.; Thrane, S.T.; Nielsen, J.C.; Cohen, T.S.; Tabor, D.E.; Fernandes, F.; Tovchigrechko, A.; Veidal, S.S.; et al. Towards a standard diet-induced and biopsy-confirmed mouse model of non-alcoholic steatohepatitis: Impact of dietary fat source. World J. Gastroenterol. 2019, 25, 4904–4920. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-Actin | AGCAGTTGTAGCTACCCGCCCA | GGCGGGCACGTTGAAGGTCT |
| Col1a1 | AAGAACCCTGCCCGCACAT | AGCCCTCGCTTCCGTACTCG |
| Ctgf | ATCTCCACCCGAGTTACCA | CGCAGAACTTAGCCCTGTATG |
| Spp1 | TGATGAGACCGTCACTGCT | GCTGCCCTTTCCGTTGTTG |
| Lgals3 | GGGAAAGGAAGAAAGACAGT | TTAGATCATGGCGTGGTTAG |
| Cd68 | GATCTTGCTAGGACCGCTTAT | TGGTGGCAGGGTTATGAGT |
| Tnf | GGCGGTGCCTATGTCTCA | CCTCCACTTGGTGGTTTG |
| Il-1b | AGCATCCAGCTTCAAATC | ATCTCGGAGCCTGTAGTG |
| Ccl2 | GCCTGCTGTTCACAGTTGC | TGGACCCATTCCTTCTTGG |
| Csf1 | CACTGGGCACTAACTGGGTC | GCTCCTGGTGGTCTTCACG |
| CHAC1 | CCTCCAGAGTTTACTGCCATGAC | GTAGGATCTCCGCCACTGATTC |
| SLC7A11 | GCAGTTGCTGGGCTGATTTA | TGTTCTGGTTATTTTCTCCGAC |
| COL4A3 | TCCTCACGGCTGGATTTCTC | GCACACCTGACAGCGACTT |
| RGS5 | GAAACCAGCCAAGACCCAGAA | AGAGACCAACCTCTTTAGGAGCC |
| EGR3 | TGCCTGACAATCTGTACCCC | TCCCAAGTAGGTCACGGTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, L.; Geng, L.; Tanaka, N.; Ye, B. Resmetirom Ameliorates NASH-Model Mice by Suppressing STAT3 and NF-κB Signaling Pathways in an RGS5-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 5843. https://doi.org/10.3390/ijms24065843
Wang X, Wang L, Geng L, Tanaka N, Ye B. Resmetirom Ameliorates NASH-Model Mice by Suppressing STAT3 and NF-κB Signaling Pathways in an RGS5-Dependent Manner. International Journal of Molecular Sciences. 2023; 24(6):5843. https://doi.org/10.3390/ijms24065843
Chicago/Turabian StyleWang, Xiaojing, Liangjing Wang, Lin Geng, Naoki Tanaka, and Bin Ye. 2023. "Resmetirom Ameliorates NASH-Model Mice by Suppressing STAT3 and NF-κB Signaling Pathways in an RGS5-Dependent Manner" International Journal of Molecular Sciences 24, no. 6: 5843. https://doi.org/10.3390/ijms24065843





