Ovarian Stimulation in Mice Resulted in Abnormal Placentation through Its Effects on Proliferation and Cytokine Production of Uterine NK Cells
Abstract
:1. Introduction
2. Results
2.1. Ovarian Stimulation Resulted in Lower Fetal Weight on GD 18.5
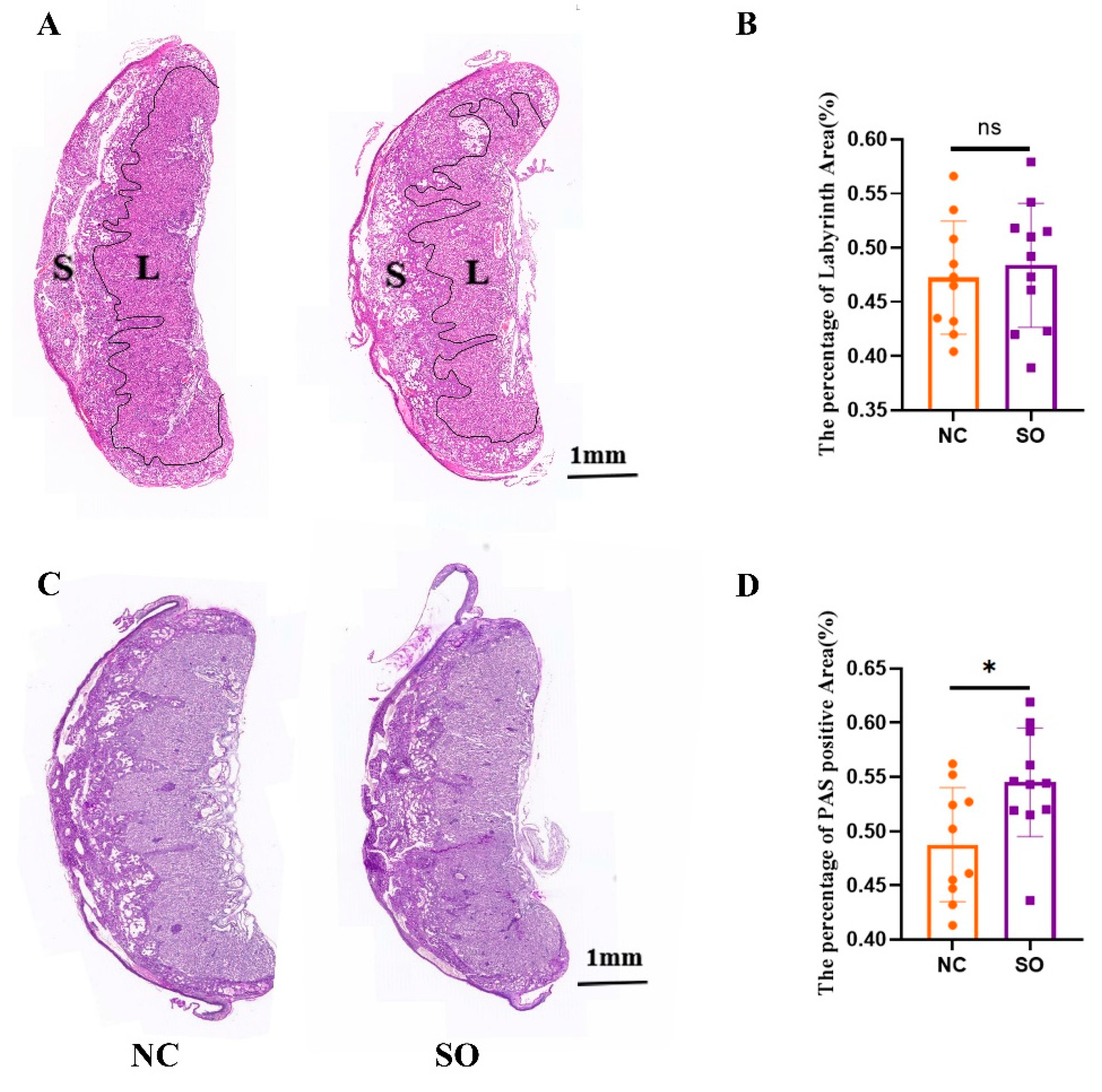
2.2. The Placental Morphology Was Perturbed by Ovarian Stimulation
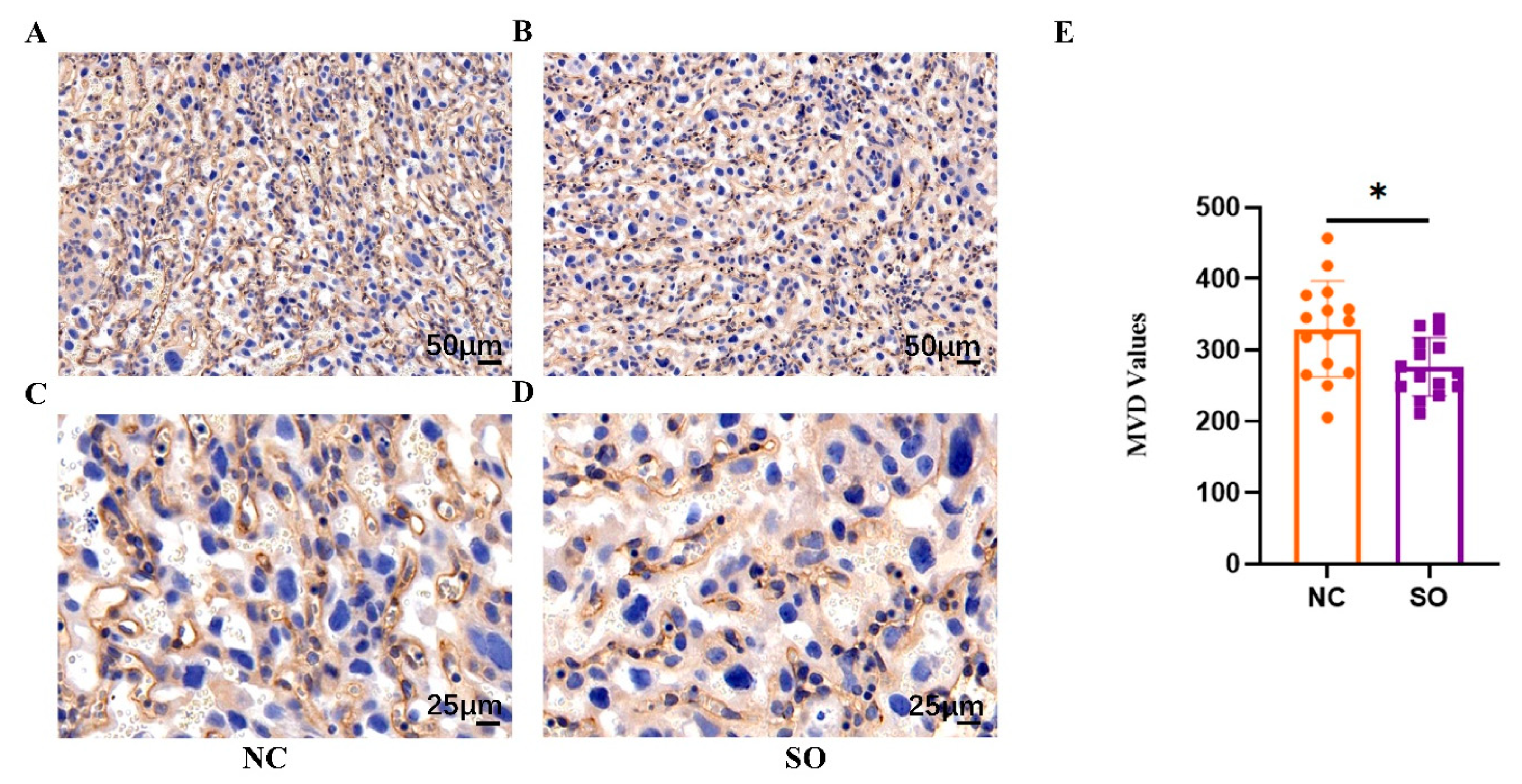
2.3. Ovarian Stimulation Resulted in Lower Placental Angiogenesis on GD 14.5
2.4. Effects of Ovarian Stimulation on the Uterine Natural Killer Cells in the Decidual Basalis at Different Gestational Ages
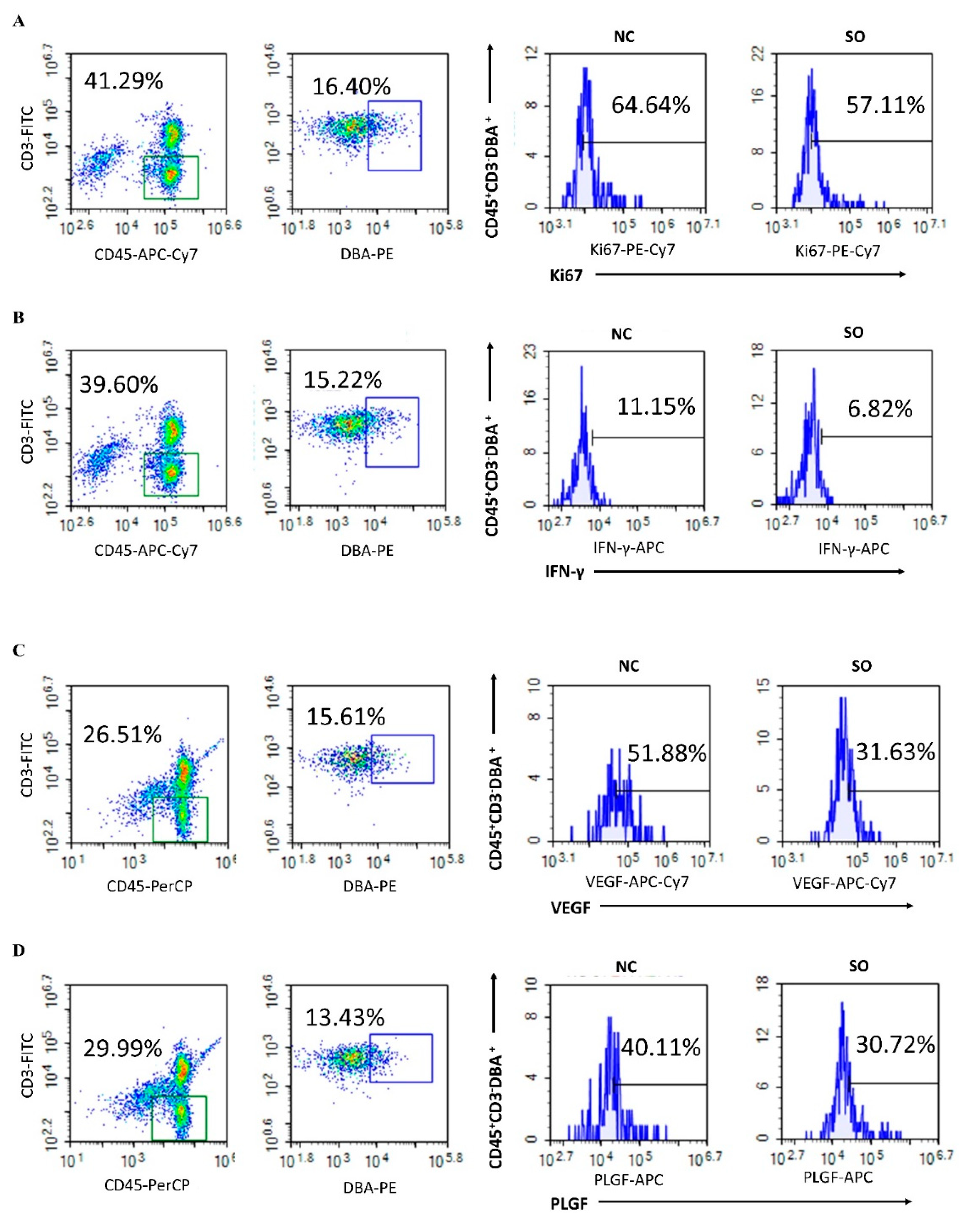
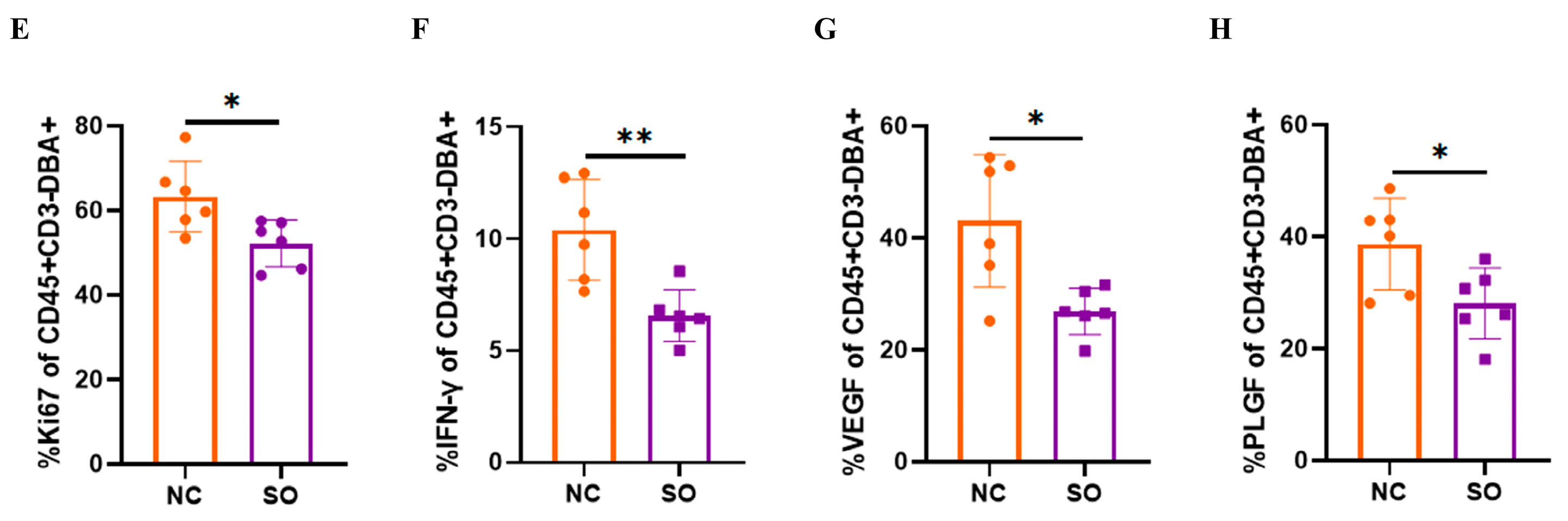
2.5. Effects of Ovarian Stimulation on the Function of Uterine Natural Killer Cells in the Decidual Basalis
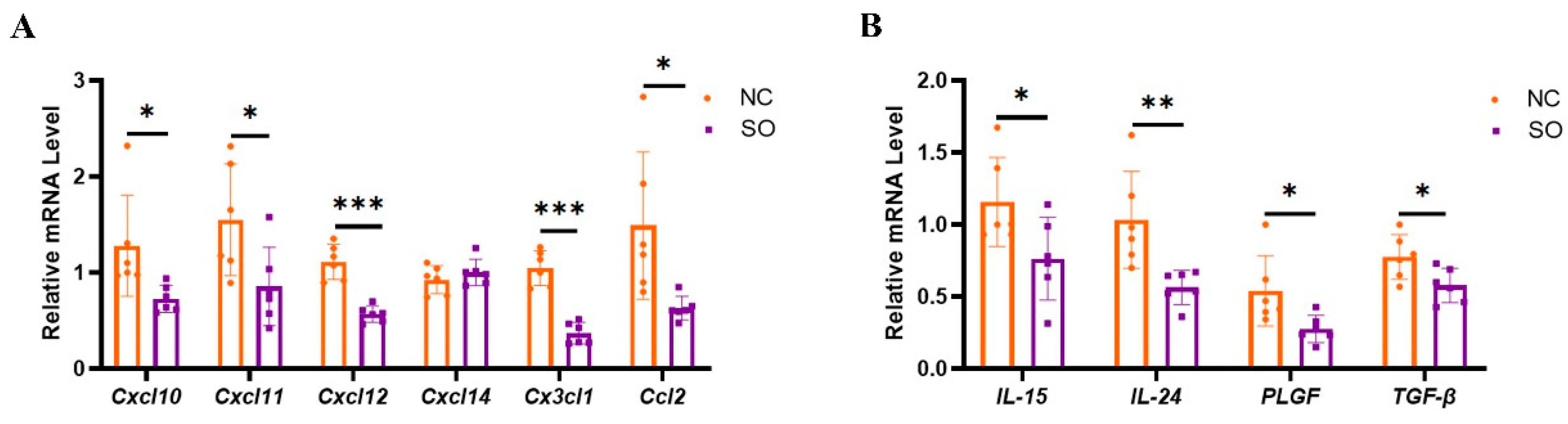
2.6. Key Chemokines and Cytokines That Regulated the Recruitment, Proliferation, and Function of uNK Cells in GD 9.5 Decidual Tissue Were Downregulated by Ovarian Stimulation
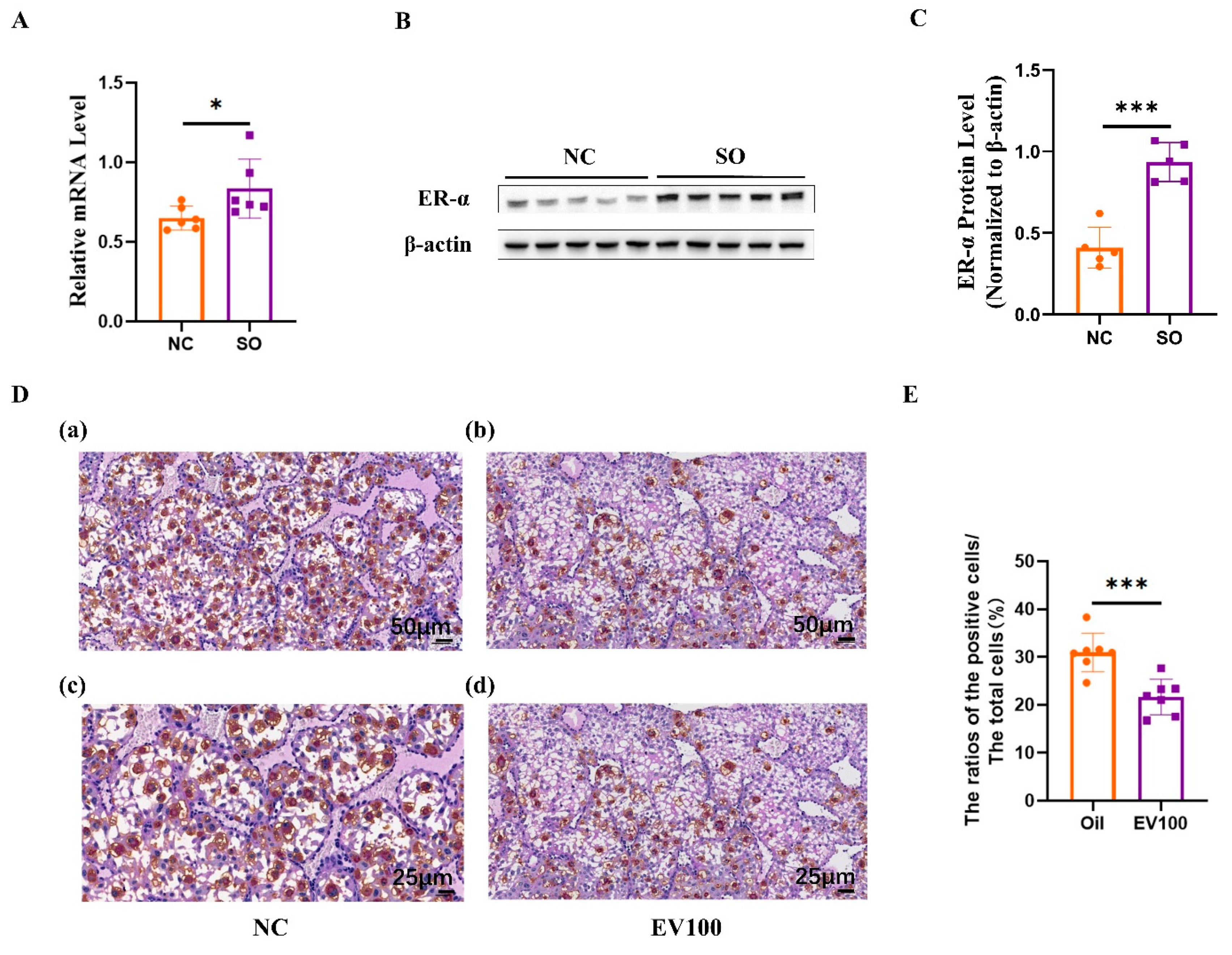
2.7. Aberrant Estrogen Signaling May Contribute to the Disorder of uNK Cells Related to Ovarian Stimulation
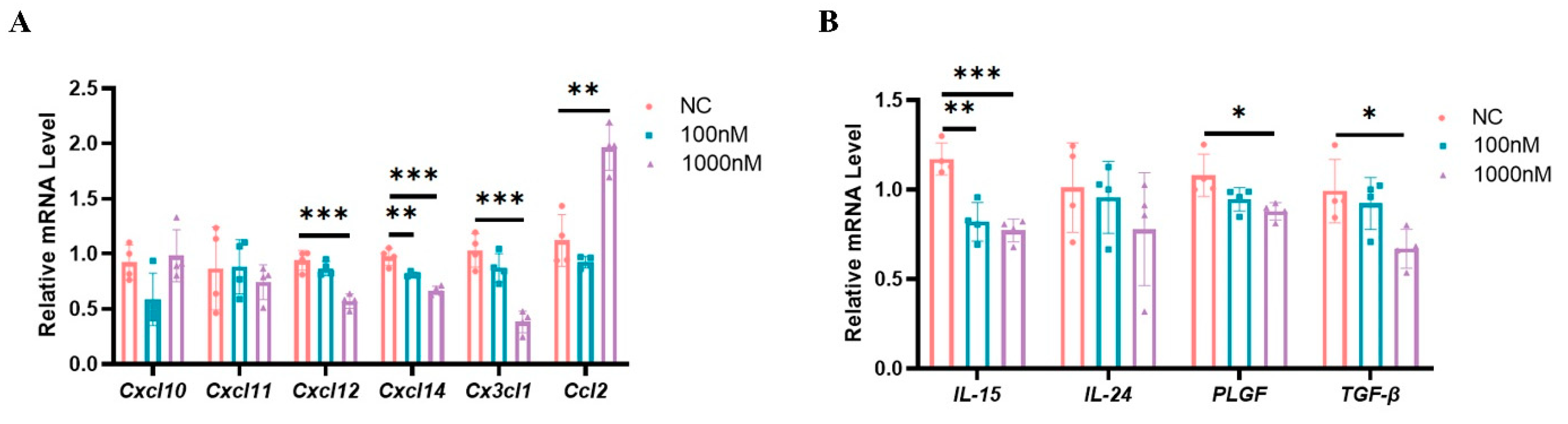
2.8. High Estrogen Levels Suppressed the Expression of Chemokines and Cytokines That Regulate the Functions of uNK Cells in Mouse Decidual Stromal Cells
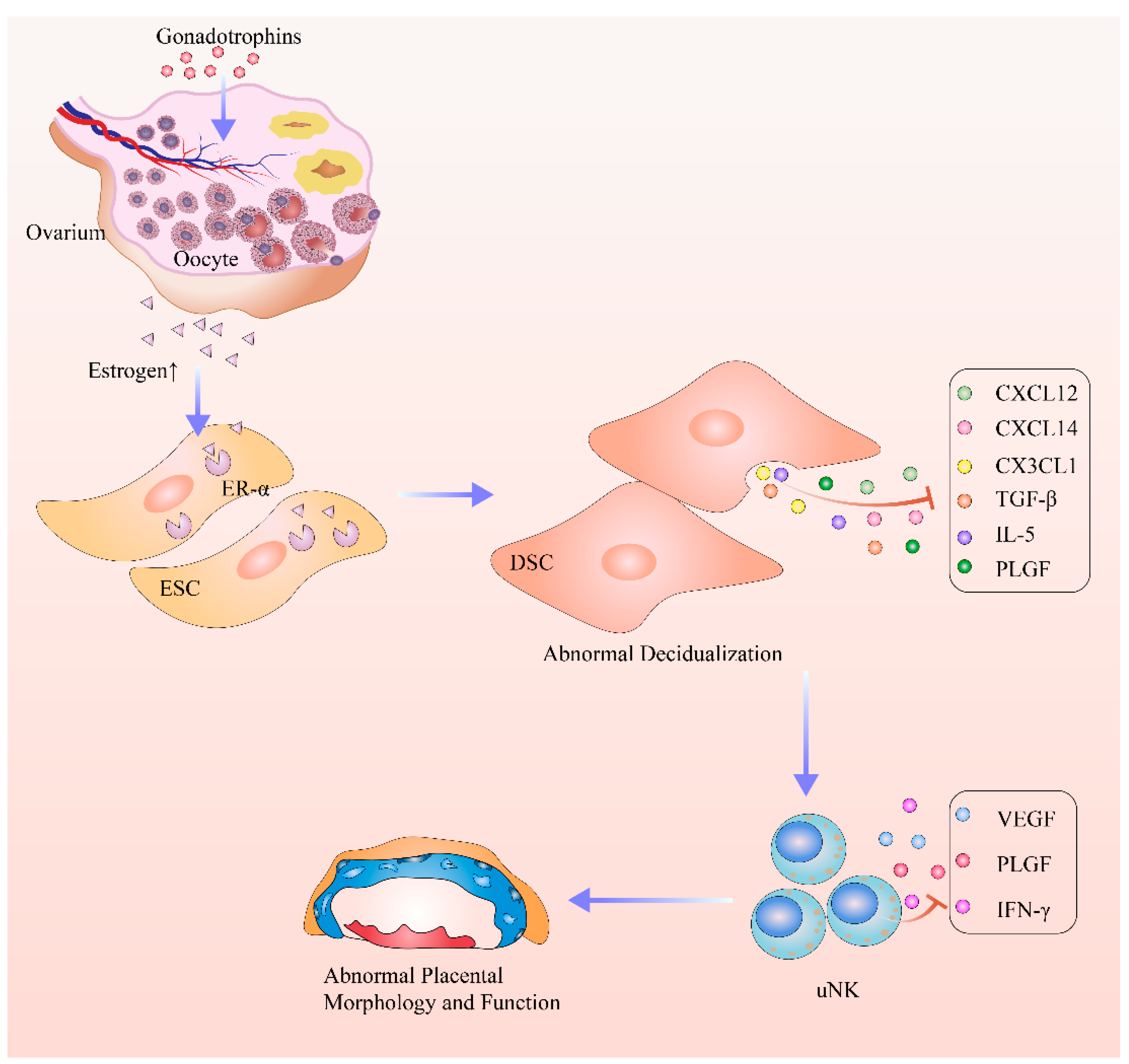
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Embryo Transfer
4.3. Placenta Dissection
4.4. Placental HE and PAS Staining
4.5. Immunohistochemistry for Determination of Placental Microvascular Density
4.6. DBA Lectin and PAS Dual Staining
4.7. Isolation and In Vitro Decidualization of Mouse Endometrial Stromal Cells
4.8. RNA Extraction, cDNA Preparation, and Real-Time PCR Analysis
4.9. Western Blot
4.10. Flow Cytometry (FCM)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Peng, Y.; Ma, X.; Kong, S.; Tan, S.; Wei, Y.; Zhao, Y.; Zhang, W.; Wang, Y.; Yan, L.; et al. Integrated multi-omics reveal epigenomic disturbance of assisted reproductive technologies in human offspring. EBioMedicine 2020, 61, 103076. [Google Scholar] [CrossRef]
- Wennerholm, U.B.; Bergh, C. Perinatal outcome in children born after assisted reproductive technologies. Ups. J. Med. Sci. 2020, 125, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Berntsen, S.; Soderstrom-Anttila, V.; Wennerholm, U.B.; Laivuori, H.; Loft, A.; Oldereid, N.B.; Romundstad, L.B.; Bergh, C.; Pinborg, A. The health of children conceived by ART: ‘the chicken or the egg?’. Hum. Reprod. Update 2019, 25, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.S.; Kirubakaran, R.; Mascarenhas, M.; Sunkara, S.K. Perinatal outcomes after stimulated versus natural cycle IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2018, 36, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Wessel, J.A.; Mol, F.; Danhof, N.A.; Bensdorp, A.J.; Tjon-Kon Fat, R.I.; Broekmans, F.J.M.; Hoek, A.; Mol, B.W.J.; Mochtar, M.H.; van Wely, M.; et al. Birthweight and other perinatal outcomes of singletons conceived after assisted reproduction compared to natural conceived singletons in couples with unexplained subfertility: Follow-up of two randomized clinical trials. Hum. Reprod. 2021, 36, 817–825. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Jung, E.J.; Cho, H.J.; Byun, J.M.; Jeong, D.H.; Lee, K.B.; Sung, M.S.; Kim, K.T.; Kim, Y.N. Placental pathologic changes and perinatal outcomes in placenta previa. Placenta 2018, 63, 15–20. [Google Scholar] [CrossRef]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Aplin, J.D.; Myers, J.E.; Timms, K.; Westwood, M. Tracking placental development in health and disease. Nat. Rev. Endocrinol. 2020, 16, 479–494. [Google Scholar] [CrossRef]
- Senapati, S.; Wang, F.; Ord, T.; Coutifaris, C.; Feng, R.; Mainigi, M. Superovulation alters the expression of endometrial genes critical to tissue remodeling and placentation. J. Assist. Reprod. Genet. 2018, 35, 1799–1808. [Google Scholar] [CrossRef]
- Farhi, J.; Ben-Haroush, A.; Andrawus, N.; Pinkas, H.; Sapir, O.; Fisch, B.; Ashkenazi, J. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod. Biomed. Online 2010, 21, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Weinerman, R.; Ord, T.; Bartolomei, M.S.; Coutifaris, C.; Mainigi, M. The superovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model. Biol. Reprod. 2017, 97, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Mainigi, M.A.; Olalere, D.; Burd, I.; Sapienza, C.; Bartolomei, M.; Coutifaris, C. Peri-implantation hormonal milieu: Elucidating mechanisms of abnormal placentation and fetal growth. Biol. Reprod. 2014, 90, 26. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knofler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef] [Green Version]
- Jabrane-Ferrat, N.; Siewiera, J. The up side of decidual natural killer cells: New developments in immunology of pregnancy. Immunology 2014, 141, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dunk, C.E.; Shynlova, O.; Caniggia, I.; Lye, S.J. TGFb1 suppresses the activation of distinct dNK subpopulations in preeclampsia. EBioMedicine 2019, 39, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Zhou, X.; Jin, L. Natural killer cells: Functional differences in recurrent spontaneous abortiondagger. Biol. Reprod. 2020, 102, 524–531. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, N.; Ma, Y.; Lu, J.; Wang, J.; Chen, S.; Wang, X. Ovarian stimulation reduces fetal growth by dysregulating uterine natural killer cells in mice. Mol. Reprod. Dev. 2021, 88, 618–627. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [Green Version]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Nadeau-Vallee, M.; Obari, D.; Palacios, J.; Brien, M.E.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Winuthayanon, W.; Lierz, S.L.; Delarosa, K.C.; Sampels, S.R.; Donoghue, L.J.; Hewitt, S.C.; Korach, K.S. Juxtacrine Activity of Estrogen Receptor alpha in Uterine Stromal Cells is Necessary for Estrogen-Induced Epithelial Cell Proliferation. Sci. Rep. 2017, 7, 8377. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.D.; Sison, C.A.M.; Yildiz, S.; Miyazaki, K.; Coon, V.J.; Yin, P.; Bulun, S.E. Genome-wide estrogen receptor-alpha binding and action in human endometrial stromal cells. F&S Sci. 2020, 1, 59–66. [Google Scholar]
- Chai, J.; Lee, K.F.; Ng, E.H.; Yeung, W.S.; Ho, P.C. Ovarian stimulation modulates steroid receptor expression and spheroid attachment in peri-implantation endometria: Studies on natural and stimulated cycles. Fertil. Steril. 2011, 96, 764–768. [Google Scholar] [CrossRef]
- Pawar, S.; Laws, M.J.; Bagchi, I.C.; Bagchi, M.K. Uterine Epithelial Estrogen Receptor-alpha Controls Decidualization via a Paracrine Mechanism. Mol. Endocrinol. 2015, 29, 1362–1374. [Google Scholar] [CrossRef] [Green Version]
- Ezoe, K.; Daikoku, T.; Yabuuchi, A.; Murata, N.; Kawano, H.; Abe, T.; Okuno, T.; Kobayashi, T.; Kato, K. Ovarian stimulation using human chorionic gonadotrophin impairs blastocyst implantation and decidualization by altering ovarian hormone levels and downstream signaling in mice. Mol. Hum. Reprod. 2014, 20, 1101–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.H.; Yamada, A.T.; Croy, B.A. DBA-lectin reactivity defines natural killer cells that have homed to mouse decidua. Placenta 2009, 30, 968–973. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Hatta, K.; Lima, P.D.; Yadi, H.; Colucci, F.; Yamada, A.T.; Croy, B.A. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol. Reprod. 2012, 87, 81. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef] [Green Version]
- Paffaro, V.A., Jr.; Bizinotto, M.C.; Joazeiro, P.P.; Yamada, A.T. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003, 24, 479–488. [Google Scholar] [CrossRef]
- Yadi, H.; Burke, S.; Madeja, Z.; Hemberger, M.; Moffett, A.; Colucci, F. Unique receptor repertoire in mouse uterine NK cells. J. Immunol. 2008, 181, 6140–6147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croy, B.A.; Zhang, J.H.; Tayade, C.; Colucci, F.; Yadi, H.; Yamada, A.T. Analysis of Uterine Natural Killer Cells in Mice. In Natural Killer Cell Protocols: Cellular and Molecular Methods; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 612, pp. 465–503. [Google Scholar]
- Imudia, A.N.; Awonuga, A.O.; Doyle, J.O.; Kaimal, A.J.; Wright, D.L.; Toth, T.L.; Styer, A.K. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil. Steril. 2012, 97, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Styer, A.K.; Wright, D.L.; Wolkovich, A.M.; Veiga, C.; Toth, T.L. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil. Steril. 2008, 89, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Fortier, A.L.; Lopes, F.L.; Darricarrere, N.; Martel, J.; Trasler, J.M. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum. Mol. Genet. 2008, 17, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Ertzeid, G.; Storeng, R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum. Reprod. 2001, 16, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Mor, G.; Liao, A. Epigenetic modifications working in the decidualization and endometrial receptivity. Cell Mol. Life Sci. 2020, 77, 2091–2101. [Google Scholar] [CrossRef]
- Sentman, C.L.; Meadows, S.K.; Wira, C.R.; Eriksson, M. Recruitment of uterine NK cells: Induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J. Immunol. 2004, 173, 6760–6766. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Chen, H.; Deng, Z.; Yue, J.; Chen, Q.; Cao, Y.; Ning, L.; Lei, X.; Duan, E. Genetic deletion of Cxcl14 in mice alters uterine NK cells. Biochem. Biophys. Res. Commun. 2013, 435, 664–670. [Google Scholar] [CrossRef]
- Gong, H.; Chen, Y.; Xu, J.; Xie, X.; Yu, D.; Yang, B.; Kuang, H. The regulation of ovary and conceptus on the uterine natural killer cells during early pregnancy. Reprod. Biol. Endocrinol. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Strunz, B.; Bister, J.; Jonsson, H.; Filipovic, I.; Crona-Guterstam, Y.; Kvedaraite, E.; Sleiers, N.; Dumitrescu, B.; Brannstrom, M.; Lentini, A.; et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci. Immunol. 2021, 6, eabb7800. [Google Scholar] [CrossRef] [PubMed]
- Tayade, C.; Hilchie, D.; He, H.; Fang, Y.; Moons, L.; Carmeliet, P.; Foster, R.A.; Croy, B.A. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J. Immunol. 2007, 178, 4267–4275. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Mantena, S.R.; Kannan, A.; Evans, D.B.; Bagchi, M.K.; Bagchi, I.C. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12542–12547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, H.; Tanaka, S.; Tsuzuki-Nakao, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; et al. The transcription factor HAND2 up-regulates transcription of the IL15 gene in human endometrial stromal cells. J. Biol. Chem. 2020, 295, 9596–9605. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, K.; Hennes, A.; Vriens, J. Isolation of Mouse Endometrial Epithelial and Stromal Cells for In Vitro Decidualization. J. Vis. Exp. 2017, 121, e55168. [Google Scholar]

| Gestational Age | Group | Litters | Litter Size | Placental Weight (mg) | Fetal Weight (mg) | Placental Efficiency (%) |
|---|---|---|---|---|---|---|
| GD 18.5 | NC | 10 | 10.40 ± 0.80 | 107.62 ± 3.33 | 1523.74 ± 52.23 | 14.17 ± 0.54 |
| SO | 10 | 10.00 ± 2.24 | 101.63 ± 8.71 | 1207.84 ± 136.15 | 12.03 ± 2.12 | |
| p | 0.620 | 0.070 | <0.001 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Jin, N.; Lei, H.; Dong, J.; Xiong, Y.; Qian, C.; Chen, S.; Wang, X. Ovarian Stimulation in Mice Resulted in Abnormal Placentation through Its Effects on Proliferation and Cytokine Production of Uterine NK Cells. Int. J. Mol. Sci. 2023, 24, 5907. https://doi.org/10.3390/ijms24065907
Ma R, Jin N, Lei H, Dong J, Xiong Y, Qian C, Chen S, Wang X. Ovarian Stimulation in Mice Resulted in Abnormal Placentation through Its Effects on Proliferation and Cytokine Production of Uterine NK Cells. International Journal of Molecular Sciences. 2023; 24(6):5907. https://doi.org/10.3390/ijms24065907
Chicago/Turabian StyleMa, Rong, Ni Jin, Hui Lei, Jie Dong, Yujing Xiong, Chenxi Qian, Shuqiang Chen, and Xiaohong Wang. 2023. "Ovarian Stimulation in Mice Resulted in Abnormal Placentation through Its Effects on Proliferation and Cytokine Production of Uterine NK Cells" International Journal of Molecular Sciences 24, no. 6: 5907. https://doi.org/10.3390/ijms24065907
APA StyleMa, R., Jin, N., Lei, H., Dong, J., Xiong, Y., Qian, C., Chen, S., & Wang, X. (2023). Ovarian Stimulation in Mice Resulted in Abnormal Placentation through Its Effects on Proliferation and Cytokine Production of Uterine NK Cells. International Journal of Molecular Sciences, 24(6), 5907. https://doi.org/10.3390/ijms24065907






