An Uncommon Case of Moyamoya Syndrome Is Accompanied by an Arteriovenous Malformation with the Involvement of Dural Arteries
Abstract
:1. Introduction
2. Case Presentation
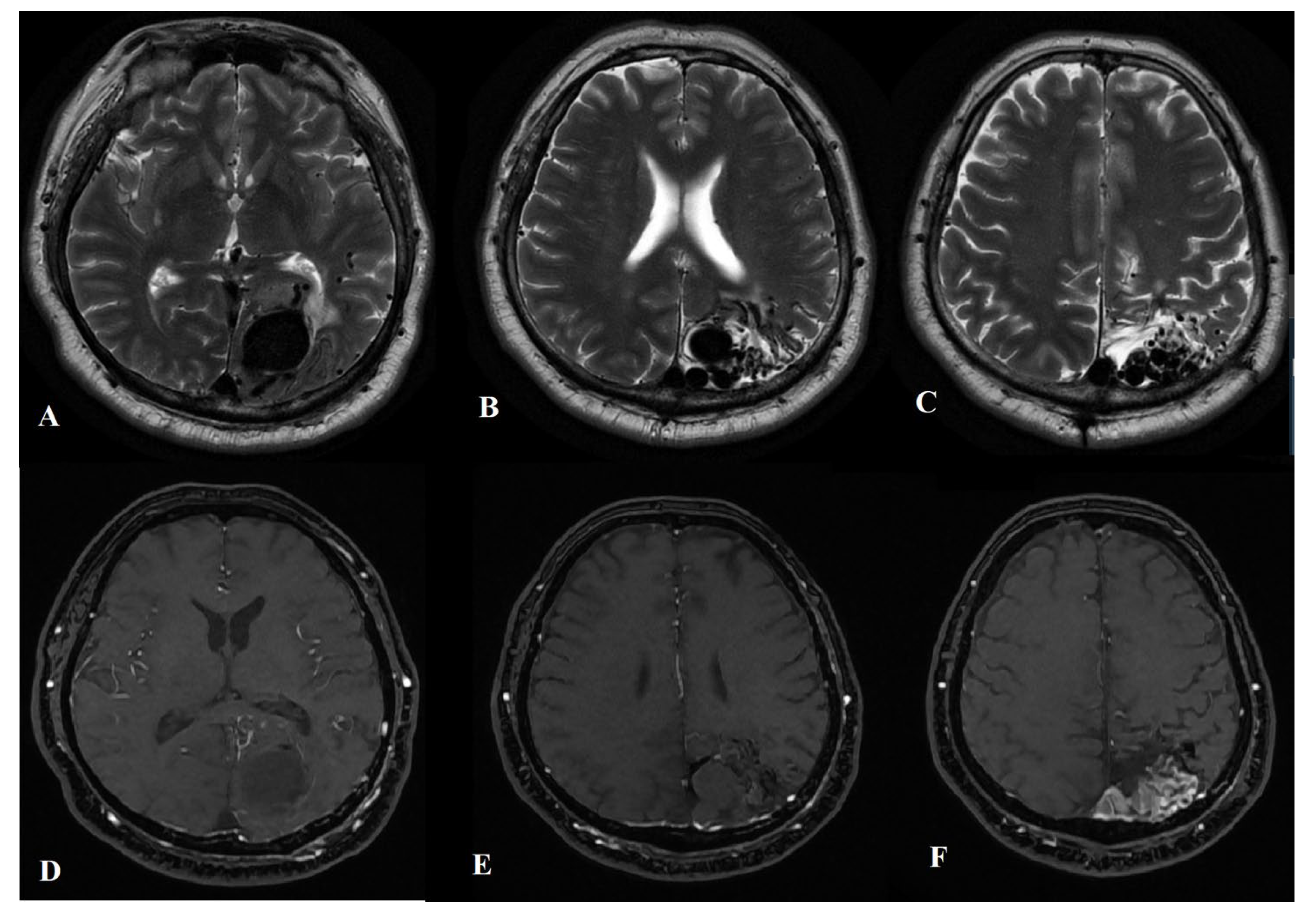
2.1. Diagnostic Procedures
2.2. Treatment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, M.T.; Zafar, A.; Choudhari, K.A.; Smith, A.; Coley, S.; Jankowski, S.; Randall, M.; Patel, U.J. Management of Concomitant Moyamoya Disease, Arterial Venous Malformation, and Intracranial Aneurysm: Case Illustration, Literature Review, and Management Algorithm. World Neurosurg. 2018, 119, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Bervini, D.; Morgan, M.K.; Stoodley, M.A.; Heller, G.Z. Transdural Arterial Recruitment to Brain Arteriovenous Malformation: Clinical and Management Implications in a Prospective Cohort Series. J. Neurosurg. 2017, 127, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.-W.; Jo, K.-I.; Yeon, J.-Y.; Kim, K.H.; Jeon, P.; Kim, J.-S.; Hong, S.-C.; Shin, H.J.; Lee, J.-I. Clinical Features of Superficially Located Brain Arteriovenous Malformations with Transdural Arterial Communication. Cerebrovasc. Dis. 2016, 41, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.R. Moyamoya Biomarkers. J. Korean Neurosurg. Soc. 2015, 57, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phi, J.H.; Wang, K.C.; Lee, J.Y.; Kim, S.K. Moyamoya Syndrome: A Window of Moyamoya Disease. J. Korean Neurosurg. Soc. 2015, 57, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, K.; Zhang, Y.; Wang, X.; Yu, J. Treatment Strategies for Aneurysms Associated with Moyamoya Disease. Int. J. Med. Sci. 2015, 12, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leacy, R.; Ansari, S.A.; Schirmer, C.M.; Cooke, D.L.; Prestigiacomo, C.J.; Bulsara, K.R.; Hetts, S.W.; SNIS Standards and Guidelines Committee; SNIS Board of Directors. Endovascular treatment in the multimodality management of brain arteriovenous malformations: Report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J. Neurointerv. Surg. 2022, 14, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Feria, A.; Brzezicki, G. Ruptured Temporal Horn Aneurysm Associated with Moyamoya Collateral Vessel Coexisting with Contralateral Arteriovenous Malformation—Technical Note with Clinical Vignette. Neurol. I Neurochir. Pol. 2021, 55, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, E.J.; Cheon, J.-E.; Kim, J.E.; Kang, H.-S.; Kim, S.-K. Clinical Features and Treatment Outcomes of Cerebral Arteriovenous Malformation Associated With Moyamoya Disease. World Neurosurg. 2021, 154, e633–e640. [Google Scholar] [CrossRef] [PubMed]
- Killory, B.D.; Gonzalez, L.F.; Wait, S.D.; Ponce, F.A.; Albuquerque, F.C.; Spetzler, R.F. Simultaneous Unilateral Moyamoya Disease and Ipsilateral Dural Arteriovenous Fistula: Case Report. Neurosurgery 2008, 62, E1375–E1376. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zhao, Y.; Chen, X.; Xu, K.; Yu, J. Moyamoya Disease Concurrent with Dural Arteriovenous Fistula: A Case Report and Literature Review. Exp. Ther. Med. 2020, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Torazawa, S.; Miyawaki, S.; Shinya, Y.; Kawashima, M.; Hasegawa, H.; Dofuku, S.; Uchikawa, H.; Kin, T.; Shin, M.; Nakatomi, H.; et al. De Novo Development of Moyamoya Disease after Stereotactic Radiosurgery for Brain Arteriovenous Malformation in a Patient With RNF213 p.Arg4810Lys (Rs112735431). World Neurosurg. 2020, 140, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, D.; Mattle, H.P.; Sturzenegger, M.; Schroth, G. Pulsatile tinnitus—A review of 84 patients. J. Neurol. 1998, 245, 137–142. [Google Scholar] [CrossRef] [PubMed]
- An, Y.-H.; Han, S.; Lee, M.; Rhee, J.; Kwon, O.-K.; Hwang, G.; Jung, C.; Bae, Y.J.; An, G.S.; Lee, K.; et al. Dural arteriovenous fistula masquerading as pulsatile tinnitus: Radiologic Assessment and Clinical Implications. Sci. Rep. 2016, 6, 36601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, J.A.; Lavine, S.D. Role of embolization for cerebral arteriovenous malformations. Methodist DeBakey Cardiovasc. J. 2014, 10, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-C.; Guo, W.-Y.; Wu, H.-M.; Chang, F.-C.; Shiau, C.-Y.; Chung, W.-Y. The Rare Association of Moyamoya Disease and Cerebral Arteriovenous Malformations: A Case Report. Korean J. Radiol. 2008, 9, S65–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Presti, A.; Weil, A.G.; Fallah, A.; Peterson, E.C.; Niazi, T.N.; Bhatia, S. Treatment of a Cerebral Pial Arteriovenous Fistula in a Patient with Sickle Cell Disease-Related Moyamoya Syndrome: Case Report. J. Neurosurg. Pediatr. 2015, 16, 207–211. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurimanov, C.; Mammadinova, I.; Makhambetov, Y.; Akshulakov, S. An Uncommon Case of Moyamoya Syndrome Is Accompanied by an Arteriovenous Malformation with the Involvement of Dural Arteries. Int. J. Mol. Sci. 2023, 24, 5911. https://doi.org/10.3390/ijms24065911
Nurimanov C, Mammadinova I, Makhambetov Y, Akshulakov S. An Uncommon Case of Moyamoya Syndrome Is Accompanied by an Arteriovenous Malformation with the Involvement of Dural Arteries. International Journal of Molecular Sciences. 2023; 24(6):5911. https://doi.org/10.3390/ijms24065911
Chicago/Turabian StyleNurimanov, Chingiz, Iroda Mammadinova, Yerbol Makhambetov, and Serik Akshulakov. 2023. "An Uncommon Case of Moyamoya Syndrome Is Accompanied by an Arteriovenous Malformation with the Involvement of Dural Arteries" International Journal of Molecular Sciences 24, no. 6: 5911. https://doi.org/10.3390/ijms24065911
APA StyleNurimanov, C., Mammadinova, I., Makhambetov, Y., & Akshulakov, S. (2023). An Uncommon Case of Moyamoya Syndrome Is Accompanied by an Arteriovenous Malformation with the Involvement of Dural Arteries. International Journal of Molecular Sciences, 24(6), 5911. https://doi.org/10.3390/ijms24065911








