Chalcones and Gastrointestinal Cancers: Experimental Evidence
Abstract
:1. Introduction
2. Molecular and Cellular Mechanisms of Action
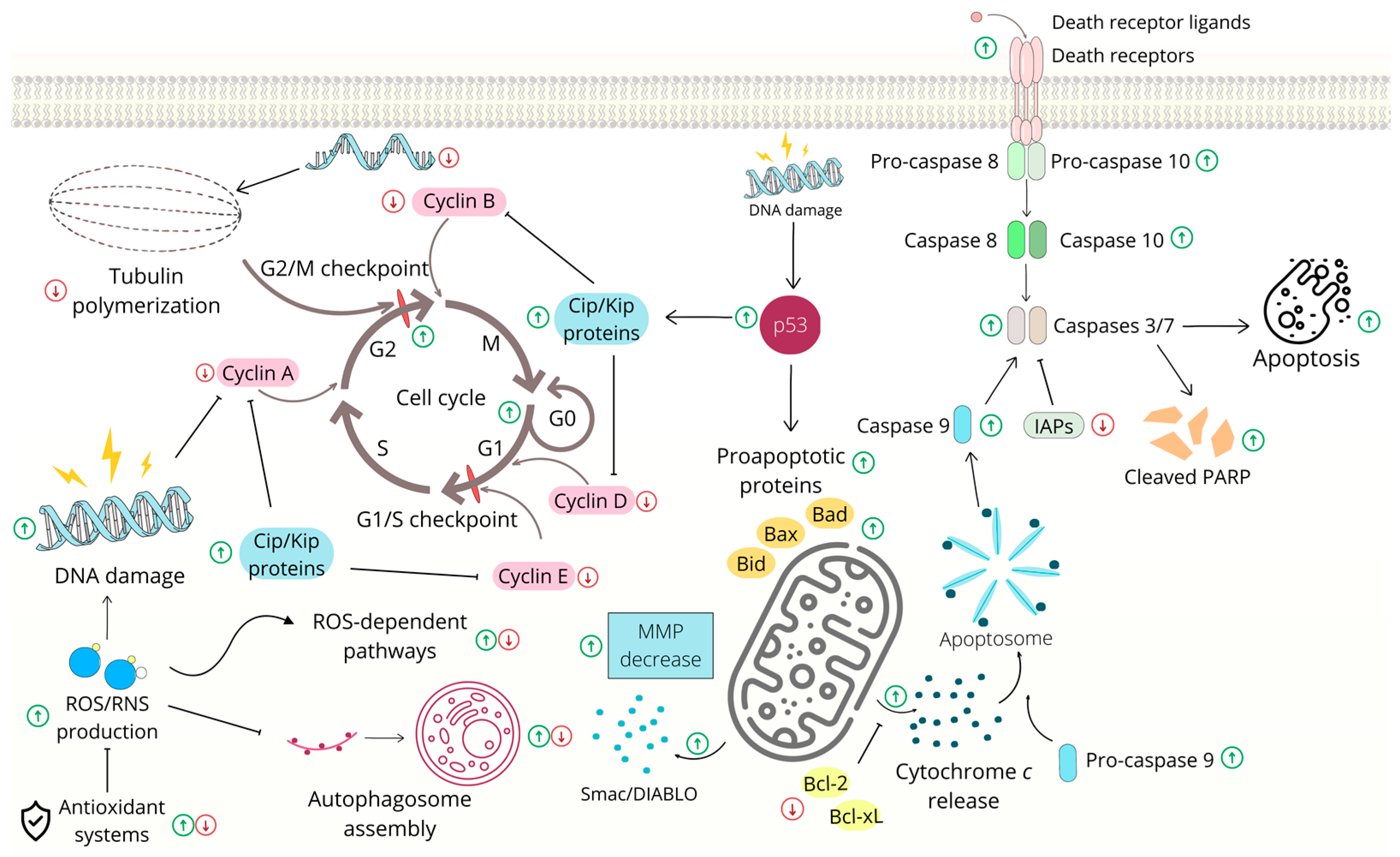
2.1. Effects on Cell Cycle and Apoptosis
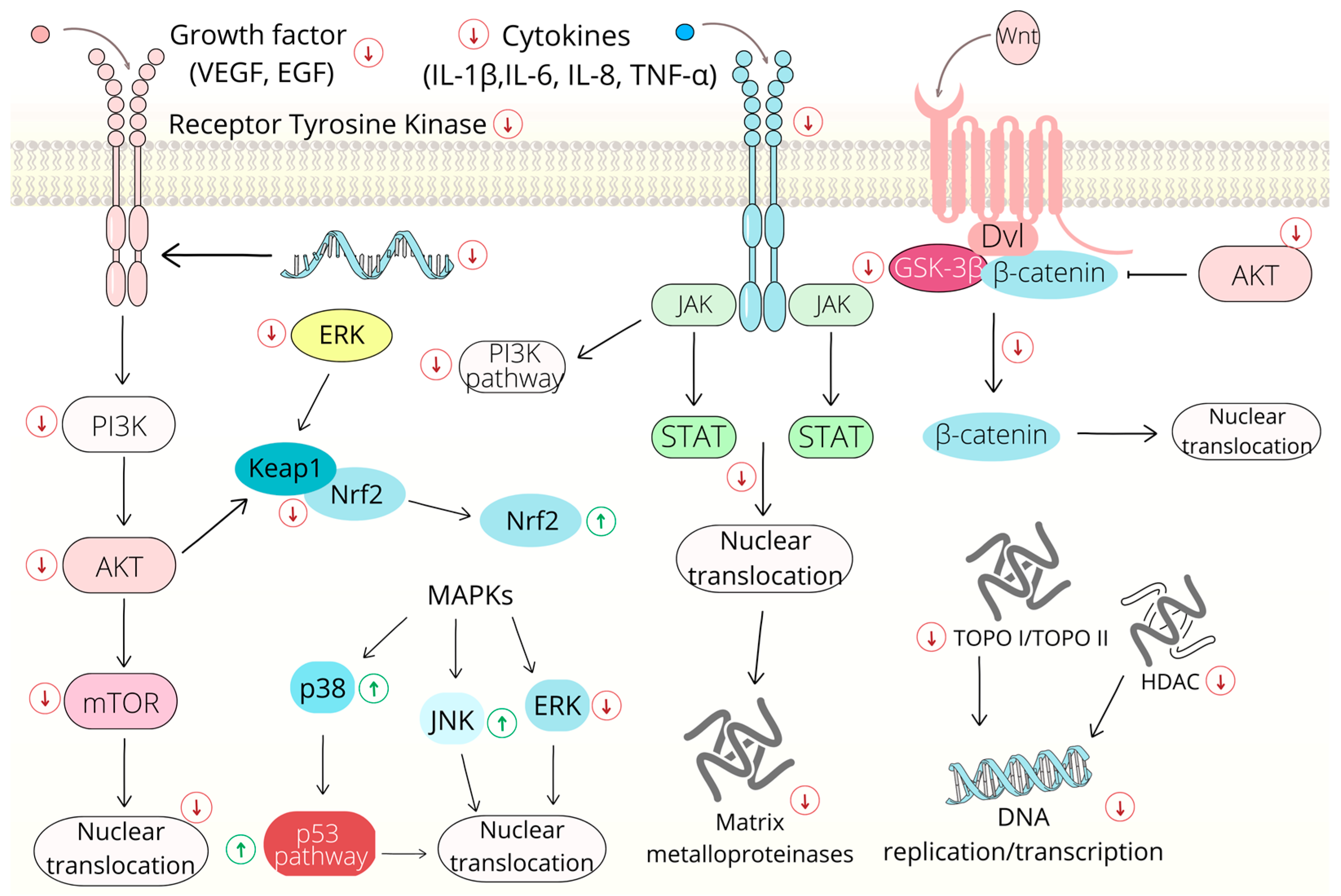
2.2. Modulation of Signaling Pathways
2.2.1. Wnt/β-Catenin Signaling Pathway
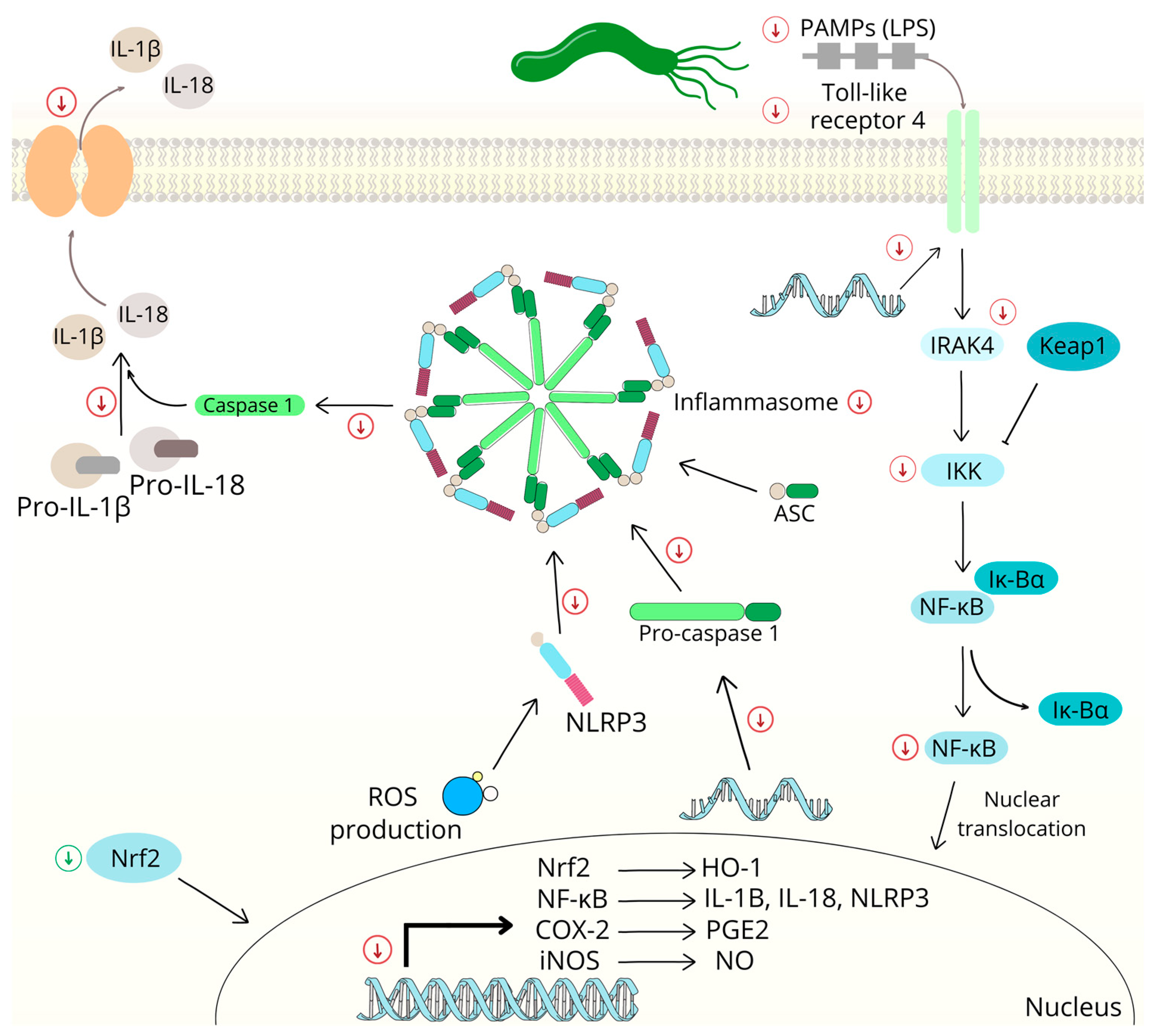
2.2.2. Nuclear Factor Kappa B Signaling Pathway
2.2.3. Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
2.3. Enzyme Inhibition
2.4. Antiangiogenic Effect of Chalcones
2.5. Chalcones and Inflammation
2.6. Chalcone-Induced Oxidative Stress
2.7. Chalcones and Multidrug Resistance
3. Animal Studies
3.1. Chemically Induced Colon Cancers
3.2. Xenograft Models of Colon Carcinogenesis
3.3. Chalcones and Gastric Cancer
3.4. Chalcones and Helicobacter pylori Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar] [CrossRef]
- Rohrl, K.; Guren, M.G.; Smastuen, M.C.; Rustoen, T. Symptoms during chemotherapy in colorectal cancer patients. Support. Care Cancer 2019, 27, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, A.B.; Lopes, R.M.; Schwartsmann, G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001, 1, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Teplova, V.V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural Polyphenols: Biological Activity, Pharmacological Potential, Means of Metabolic Engineering (Review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Kubczak, M.; Szustka, A.; Rogalinska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecien, M.; Nowakowski, L.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Galczynski, K. Antioxidative, Anti-Inflammatory, Anti-Obesogenic, and Antidiabetic Properties of Tea Polyphenols-The Positive Impact of Regular Tea Consumption as an Element of Prophylaxis and Pharmacotherapy Support in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.E.; Khalil, A. A comprehensive review on the antiviral activities of chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Lu, Q.; Xie, J.; Wu, J.; Chen, C. A novel chalcone derivative exerts anti-inflammatory and anti-oxidant effects after acute lung injury. Aging 2019, 11, 7805–7816. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives. Curr. Top. Med. Chem. 2017, 17, 3146–3169. [Google Scholar] [CrossRef]
- Rocha, S.; Ribeiro, D.; Fernandes, E.; Freitas, M. A Systematic Review on Anti-diabetic Properties of Chalcones. Curr. Med. Chem. 2020, 27, 2257–2321. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 1306. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Shukla, S.; Sood, A.K.; Goyal, K.; Singh, A.; Sharma, V.; Guliya, N.; Gulati, S.; Kumar, S. Chalcone Scaffolds as Anticancer Drugs: A Review on Molecular Insight in Action of Mechanisms and Anticancer Properties. Anti-Cancer Agents Med. Chem. 2021, 21, 1650–1670. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A. Activation of DNA damage response signaling in mammalian cells by ionizing radiation. Free. Radic. Res. 2021, 55, 581–594. [Google Scholar] [CrossRef]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. Apoptosis induction, PARP-1 inhibition, and cell cycle analysis of leukemia cancer cells treated with novel synthetic 1,2,3-triazole-chalcone conjugates. Bioorg. Chem. 2022, 123, 105762. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Chen, M.X.; Chen, W.B.; Hui, J.G.; Ji, J.S.; Hu, S.P.; Zhou, J.M.; Wang, Y.; Liang, G. Chemopreventive effect of chalcone derivative, L2H17, in colon cancer development. BMC Cancer 2015, 15, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Q.; Xu, J.P.; Zhu, Z.X.; Hu, Z.Q.; Cai, Q.P. Phloretin flavonoid exhibits selective antiproliferative activity in doxorubicin-resistant gastric cancer cells by inducing autophagy, inhibiting cell migration and invasion, cell cycle arrest and targeting ERK1/2 MAP pathway. J. BUON 2020, 25, 308–313. [Google Scholar] [PubMed]
- Drutovic, D.; Chripkova, M.; Pilatova, M.; Kruzliak, P.; Perjesi, P.; Sarissky, M.; Lupi, M.; Damia, G.; Broggini, M.; Mojzis, J. Benzylidenetetralones, cyclic chalcone analogues, induce cell cycle arrest and apoptosis in HCT116 colorectal cancer cells. Tumor Biol. 2014, 35, 9967–9975. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Drutovic, D.; Pilatova, M.B.; Tischlerova, V.; Perjesi, P.; Mojzis, J. Chalcone derivatives cause accumulation of colon cancer cells in the G2/M phase and induce apoptosis. Life Sci. 2016, 150, 32–38. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Pilatova, M.B.; Kudlickova, Z.; Vilkova, M.; Slepcikova, P.; Petik, P.; Mojzis, J. New chalcone derivative exhibits antiproliferative potential by inducing G2/M cell cycle arrest, mitochondrial-mediated apoptosis and modulation of MAPK signalling pathway. Chem.-Biol. Interact. 2018, 292, 37–49. [Google Scholar] [CrossRef]
- Edwards, M.L.; Stemerick, D.M.; Sunkara, P.S. Chalcones—A New Class of Antimitotic Agents. J. Med. Chem. 1990, 33, 1948–1954. [Google Scholar] [CrossRef]
- Dias, T.A.; Duarte, C.L.; Lima, C.F.; Proenca, M.F.; Pereira-Wilson, C. Superior anticancer activity of halogenated chalcones and flavonols over the natural flavonol quercetin. Eur. J. Med. Chem. 2013, 65, 500–510. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.S.; Koh, D.; Lee, Y.H.; Lim, Y.; Shin, S.Y. A new synthetic 2′-hydroxy-2,4,6-trimethoxy-5′,6′-naphthochalcone induces G2/M cell cycle arrest and apoptosis by disrupting the microtubular network of human colon cancer cells. Cancer Lett. 2014, 354, 348–354. [Google Scholar] [CrossRef]
- Liu, X.K.; An, L.J.; Li, Y.; Wang, Y.W.; Zhao, L.; Lv, X.; Guo, J.Y.; Song, A.L. Xanthohumol chalcone acts as a powerful inhibitor of carcinogenesis in drug-resistant human colon carcinoma and these effects are mediated via G2/M phase cell cycle arrest, activation of apoptotic pathways, caspase activation and targeting Ras/MEK/ERK pathway. J. BUON 2019, 24, 2442–2447. [Google Scholar] [PubMed]
- Moura, A.F.; de Castro, M.R.C.; Naves, R.F.; Araujo, A.J.; Dos Santos, M.C.L.; Marinho, J.D.B.; Noda-Perez, C.; Martins, F.T.; Pessoa, C.D.; Moraes, M.O. New Synthetic Sulfonamide Chalcone Induced Cell Cycle Arrest and Cell Death in Colorectal Adenocarcinoma Metastatic Cells (SW-620). Anti-Cancer Agents Med. Chem. 2022, 22, 2340–2351. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; He, M.; Li, Y.J.; Peng, Z.Y.; Wang, G.C. A review on synthetic chalcone derivatives as tubulin polymerisation inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, Y.J.; Mani, K.; Ashton, A.W.; Peng, C.F.; Krishnamurthy, B.; Hayakawa, Y.; Lee, P.; Korsmeyer, S.J.; Kitsis, R.N. Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol. Cell 2004, 15, 901–912. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Gazdova, M.; Kello, M.; Mojzis, J. Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones. Cancers 2021, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Uribe, B.; Garcia-Saez, A.J. Membranes in motion: Mitochondrial dynamics and their role in apoptosis. Biol. Chem. 2014, 395, 297–311. [Google Scholar] [CrossRef]
- Cosentino, K.; Garcia-Saez, A.J. Mitochondrial alterations in apoptosis. Chem. Phys. Lipids 2014, 181, 62–75. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Delta psi m) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Han, X.; Peng, B.; Xiao, B.B.; Cao, S.L.; Yang, C.R.; Wang, W.Z.; Wang, F.C.; Li, H.Y.; Yuan, X.L.; Shi, R.F.; et al. Synthesis and evaluation of chalcone analogues containing a 4-oxoquinazolin-2-yl group as potential anti-tumor agents. Eur. J. Med. Chem. 2019, 162, 586–601. [Google Scholar] [CrossRef]
- Lukovic, J.; Mitrovic, M.M.; Popovic, S.; Milosavljevic, Z.; Stanojevic-Pirkovic, M.; Andelkovic, M.; Zelen, I.; Sorak, M.; Muskinja, J.; Ratkovic, Z.; et al. Antitumor Effects of Vanillin Based Chalcone Analogs In Vitro. Acta Pol. Pharm. 2020, 77, 57–67. [Google Scholar] [CrossRef]
- Seo, H.W.; No, H.; Cheon, H.J.; Kim, J.K. Sappanchalcone, a flavonoid isolated from Caesalpinia sappan L., induces caspase-dependent and AIF-dependent apoptosis in human colon cancer cells. Chem.-Biol. Interact. 2020, 327, 109185. [Google Scholar] [CrossRef]
- Shin, S.Y.; Ahn, S.; Koh, D.; Lim, Y. p53-dependent and -independent mechanisms are involved in (E)-1-(2-hydroxyphenyl)-3-(2-methoxynaphthalen-1-yl)prop-2-en-1-one (HMP)-induced apoptosis in HCT116 colon cancer cells. Biochem. Biophys. Res. Commun. 2016, 479, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Brandao, P.; Loureiro, J.B.; Carvalho, S.; Hamadou, M.H.; Cravo, S.; Moreira, J.; Pereira, D.; Palmeira, A.; Pinto, M.; Saraiva, L.; et al. Targeting the MDM2-p53 protein-protein interaction with prenylchalcones: Synthesis of a small library and evaluation of potential antitumor activity. Eur. J. Med. Chem. 2018, 156, 711–721. [Google Scholar] [CrossRef]
- Iftikhar, S.; Khan, S.; Bilal, A.; Manzoor, S.; Abdullah, M.; Emwas, A.H.; Sioud, S.; Gao, X.; Chotana, G.A.; Faisal, A.; et al. Synthesis and evaluation of modified chalcone based p53 stabilizing agents. Bioorganic Med. Chem. Lett. 2017, 27, 4101–4106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leao, M.; Soares, J.; Gomes, S.; Raimundo, L.; Ramos, H.; Bessa, C.; Queiroz, G.; Domingos, S.; Pinto, M.; Inga, A.; et al. Enhanced cytotoxicity of prenylated chalcone against tumour cells via disruption of the p53-MDM2 interaction. Life Sci. 2015, 142, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Marins, M.; Chaichanasak, N.; Yoon, Y.; Fachin, A.L.; Pinhanelli, V.C.; Regasini, L.O.; Dos Santos, M.B.; Ayusso, G.M.; Marques, B.C.; et al. Trans-chalcone increases p53 activity via DNAJB1/HSP40 induction and CRM1 inhibition. PLoS ONE 2018, 13, e0202263. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.J.; Zhang, S.Y.; Song, J.; Liu, Y.C.; Zhang, L.; Zhao, R.H.; Zib, X.L.; Liu, H.M.; Zhang, Y.B. Design and antiproliferative activity of N-heterocycle-chalcone derivatives. J. Chem. Res. 2016, 40, 620–623. [Google Scholar] [CrossRef]
- Guan, Y.F.; Liu, X.J.; Yuan, X.Y.; Liu, W.B.; Li, Y.R.; Yu, G.X.; Tian, X.Y.; Zhang, Y.B.; Song, J.; Li, W.; et al. Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives. Molecules 2021, 26, 4899. [Google Scholar] [CrossRef]
- Lou, C.H.; Yang, G.M.; Cai, H.; Zou, M.C.; Xu, Z.S.; Li, Y.; Zhao, F.M.; Li, W.D.; Tong, L.; Wang, M.Y.; et al. 2′, 4′-Dihydroxychalcone-induced apoptosis of human gastric cancer MGC-803 cells via down-regulation of survivin mRNA. Toxicol. In Vitro 2010, 24, 1333–1337. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Hao, M.A.; Yang, X.Y.; Ba, Q.A.; Li, M.A.; Ni, S.J.; Wang, L.S.; Du, X.A. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011, 302, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C.; Wang, J.; Xu, Y.; Zhang, Y.F.; Xu, N.; Yin, L. Discovery of novel isoliquiritigenin analogue ISL-17 as a potential anti-gastric cancer agent. Biosci. Rep. 2020, 40, BSR20201199. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Hseu, Y.C.; Thiyagarajan, V.; Lin, K.Y.; Way, T.D.; Korivi, M.; Liao, J.W.; Yang, H.L. Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch. Toxicol. 2017, 91, 3341–3364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Lee, D.H.; Pan, C.H.; Park, S.J.; Oh, S.C.; Lee, S.Y. Role of phloretin as a sensitizer to TRAIL-induced apoptosis in colon cancer. Oncol. Lett. 2022, 24, 321. [Google Scholar] [CrossRef]
- Lu, C.F.; Wang, S.H.; Pang, X.J.; Zhu, T.; Li, H.L.; Li, Q.R.; Li, Q.Y.; Gu, Y.F.; Mu, Z.Y.; Jin, M.J.; et al. Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents. Molecules 2020, 25, 5530. [Google Scholar] [CrossRef]
- Pan, L.; Becker, H.; Gerhauser, C. Xanthohumol induces apoptosis in cultured 40-16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol. Nutr. Food Res. 2005, 49, 837–843. [Google Scholar] [CrossRef]
- Zhao, T.Q.; Zhao, Y.D.; Liu, X.Y.; Li, Z.H.; Wang, B.; Zhang, X.H.; Cao, Y.Q.; Ma, L.Y.; Liu, H.M. Novel 3-(2,6,9-trisubstituted-9H-purine)-8-chalcone derivatives as potent anti-gastric cancer agents: Design, synthesis and structural optimization. Eur. J. Med. Chem. 2019, 161, 493–505. [Google Scholar] [CrossRef]
- Ko, H.; Kim, Y.J.; Amor, E.C.; Lee, J.W.; Kim, H.C.; Kim, H.J.; Yang, H.O. Induction of Autophagy by Dimethyl Cardamonin Is Associated With Proliferative Arrest in Human Colorectal Carcinoma HCT116 and LOVO Cells. J. Cell. Biochem. 2011, 112, 2471–2479. [Google Scholar] [CrossRef]
- Wan, B.S.; Zhu, J.Q.; Chang, Q.; Zhou, H.H.; Shi, Z.; Min, L.; Cai, Y.J.; Guan, H.G. Alpha, 2′-dihydroxy-4,4′-dimethoxydihydrochalcone inhibits cell proliferation, invasion, and migration in gastric cancer in part via autophagy. Biomed Pharm. 2018, 98, 709–718. [Google Scholar] [CrossRef]
- Wei, F.; Jiang, X.; Gao, H.Y.; Gao, S.H. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int. J. Oncol. 2017, 51, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.Q.; Tang, S.; Ren, S.; Yang, H.; Liu, M.L.; Tao, Q.; Xu, H.B. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lv, J.; Sun, D.; Huang, Y.F. Therapeutic strategies targeting Wnt/β-catenin signaling for colorectal cancer. Int. J. Mol. Med. 2022, 49, 1. [Google Scholar] [CrossRef]
- Yu, S.W.; Han, R.Y.; Gan, R.L. The Wnt/β-catenin signalling pathway in Haematological Neoplasms. Biomark Res. 2022, 10, 74. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Shin, S.; Son, Y.; Liu, K.H.; Kang, W.; Oh, S. Cytotoxic activity of broussochalcone a against colon and liver cancer cells by promoting destruction complex-independent beta-catenin degradation. Food Chem. Toxicol. 2019, 131, 110550. [Google Scholar] [CrossRef]
- Park, S.; Gwak, J.; Han, S.J.; Oh, S. Cardamonin Suppresses the Proliferation of Colon Cancer Cells by Promoting beta-Catenin Degradation. Biol. Pharm. Bull. 2013, 36, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Predes, D.; Oliveira, L.F.S.; Ferreira, L.S.S.; Maia, L.A.; Delou, J.M.A.; Faletti, A.; Oliveira, I.; Amado, N.G.; Reis, A.H.; Fraga, C.A.M.; et al. The Chalcone Lonchocarpin Inhibits Wnt/β-Catenin Signaling and Suppresses Colorectal Cancer Proliferation. Cancers 2019, 11, 1968. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, B.F.; Predes, D.; Cerqueira, D.M.; Reis, A.H.; Amado, N.G.; Cayres, M.C.L.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Derricin and Derricidin Inhibit Wnt/β-Catenin Signaling and Suppress Colon Cancer Cell Growth In Vitro. PLoS ONE 2015, 10, e0120919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.X.; Qin, X.X.; Li, P.L.; Zhang, H.; Lin, T.; Miao, Z.W.; Ma, S.P. Isobavachalcone isolated from Psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3 beta/beta-catenin pathway in colorectal cancer cells. Drug Des. Dev. Ther. 2019, 13, 1449–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, G.C.; Yuan, X.; Li, Y.; Hou, G.Y.; Liu, X.L. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Investig. New Drug 2020, 38, 329–339. [Google Scholar] [CrossRef]
- Xia, L.Z.; Tan, S.M.; Zhou, Y.J.; Lin, J.G.; Wang, H.R.; Oyang, L.D.; Tian, Y.T.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NF kappa B-signaling pathway in cancer. Oncotargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.S.; Xing, Y.; Li, M.Y.; Zhang, Z.H.; Wang, J.Y.; Ri, M.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Jin, H.L.; et al. Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-KB and Ras/Raf/MEK pathways. J. Ethnopharmacol. 2021, 273, 113989. [Google Scholar] [CrossRef] [PubMed]
- Papierska, K.; Krajka-Kuzniak, V.; Kleszcz, R.; Stefanski, T.; Kurczab, R.; Kubicki, M. The synthesis of novel thioderivative chalcones and their influence on NF-kappa B, STAT3 and NRF2 signaling pathways in colorectal cancer cells. Sci. Rep. 2022, 12, 14915. [Google Scholar] [CrossRef]
- James, S.; Aparna, J.S.; Paul, A.M.; Lankadasari, M.B.; Mohammed, S.; Binu, V.S.; Santhoshkumar, T.R.; Reshmi, G.; Harikumar, K.B. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci. Rep. 2017, 7, 13945. [Google Scholar] [CrossRef]
- Qiao, W.L.; Shi, B.W.; Han, Y.D.; Tang, H.M.; Lin, J.; Hu, H.Y.; Lin, Q. Testes-specific protease 50 as an independent risk factor for poor prognosis in patients with non-small cell lung cancer. Oncol. Lett. 2018, 15, 8796–8804. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Lin, C.Z.; Cheng, X.B.; Hua, H.J.; Xiang, T.; Huang, Y.; Huang, X. Cardamonin reduces chemotherapy resistance of colon cancer cells via the TSP50/NF-kappa B pathway in vitro. Oncol. Lett. 2018, 15, 9641–9646. [Google Scholar] [CrossRef] [Green Version]
- Ocasio-Malave, C.; Donate, M.J.; Sanchez, M.M.; Sosa-Rivera, J.M.; Mooney, J.W.; Pereles-De Leon, T.A.; Carballeira, N.M.; Zayas, B.; Velez-Gerena, C.E.; Martinez-Ferrer, M.; et al. Synthesis of novel 4-Boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorganic Med. Chem. Lett. 2020, 30, 126760. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Shen, Y.C.; Yang, J.S.; Hwang, T.L.; Bastow, K.F.; Qian, K.D.; Lee, K.H.; Wu, T.S. New bichalcone analogs as NF-kappa B inhibitors and as cytotoxic agents inducing Fas/CD95-dependent apoptosis. Bioorganic Med. Chem. 2011, 19, 1895–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, N.; Meng, X.L.; Wang, S.H. Isobavachalcone: A comprehensive review of its plant sources, pharmacokinetics, toxicity, pharmacological activities and related molecular mechanisms. Phytother. Res. 2022, 36, 3120–3142. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Wang, Y.F.; Zhang, L.J.; Gao, Y.J. Hydroxysafflor Yellow A of Carthamus Tinctorius L., Represses the Malignant Development of Esophageal Cancer Cells via Regulating NF-kappa B Signaling Pathway. Cell Biochem. Biophys. 2020, 78, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, D.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Harsha, C.; Wang, H.; Kumar, A.P.; Arfuso, F.; Kunnumakkara, A.B. An Investigation on the Therapeutic Potential of Butein, A Tretrahydroxychalcone Against Human Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 3437–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Kang, K.S.; Choi, K.C.; Ko, H. Cardamonin induces autophagy and an antiproliferative effect through JNK activation in human colorectal carcinoma HCT116 cells. Bioorganic Med. Chem. Lett. 2015, 25, 2559–2564. [Google Scholar] [CrossRef]
- Jin, H.; Kim, H.S.; Yu, S.T.; Shin, S.R.; Lee, S.H.; Seo, G.S. Synergistic anticancer effect of docosahexaenoic acid and isoliquiritigenin on human colorectal cancer cells through ROS-mediated regulation of the JNK and cytochrome c release. Mol. Biol. Rep. 2021, 48, 1171–1180. [Google Scholar] [CrossRef]
- Phang, C.W.; Karsani, S.A.; Sethi, G.; Abd Malek, S.N. Flavokawain C Inhibits Cell Cycle and Promotes Apoptosis, Associated with Endoplasmic Reticulum Stress and Regulation of MAPKs and Akt Signaling Pathways in HCT 116 Human Colon Carcinoma Cells. PLoS ONE 2016, 11, e0148775. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Chen, H.Y.; Lee, M.H.; Li, H.T.; Ma, W.Y.; Peng, C.; Song, N.R.; Lee, K.W.; Bode, A.M.; Dong, Z.M.; et al. Licochalcone A, a Natural Inhibitor of c-Jun N-Terminal Kinase 1. Cancer Prev. Res. 2014, 7, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Huang, H.; Choi, B.Y.; Liu, X.J.; Zhang, M.; Zhou, S.L.; Song, M.Q.; Yin, F.X.; Chen, H.Y.; Shim, J.H.; et al. Cell growth inhibition by 3-deoxysappanchalcone is mediated by directly targeting the TOPK signaling pathway in colon cancer. Phytomedicine 2019, 61, 152813. [Google Scholar] [CrossRef]
- Jin, X.; Shi, Y.I. Isobavachalcone induces the apoptosis of gastric cancer cells via inhibition of the Akt and Erk pathways. Exp. Ther. Med. 2016, 11, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Shih, Y.W. Antitumor effects of the flavone chalcone: Inhibition of invasion and migration through the FAK/JNK signaling pathway in human gastric adenocarcinoma AGS cells. Mol. Cell. Biochem. 2014, 391, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.J.; Yuan, X.; Yu, L.N.; Gao, C.X.; Sun, X.L.; Wang, D.; Zheng, Q.S. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 10336. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Lv, C. Hydroxysafflor yellow A inhibits the proliferation, migration, and invasion of colorectal cancer cells through the PPARgamma/PTEN/Akt signaling pathway. Bioengineered 2021, 12, 11533–11543. [Google Scholar] [CrossRef] [PubMed]
- Wani, Z.A.; Guru, S.K.; Rao, A.V.S.; Sharma, S.; Mahajan, G.; Behl, A.; Kumar, A.; Sharma, P.R.; Kamal, A.; Bhushan, S.; et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem. Toxicol. 2016, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Q.; Xu, Z.; Li, W.S.; Chen, C.; Yao, X.Q.; Liu, F.K. Cyclooxygenase-2 knockdown using retinoic acid chalcone (RAC), a promising therapeutic strategy for colon cancer. Am. J. Cancer Res. 2015, 5, 2012–2021. [Google Scholar]
- Takahashi, T.; Takasuka, N.; Iigo, M.; Baba, M.; Nishino, H.; Tsuda, H.; Okuyama, T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004, 95, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.Y.; Yang, J.; Yu, W.K.; Li, H.B.; Cai, M.H.; Xu, J.L.; Xu, H.D.; Shi, Y.F.; Guan, X.Q.; Cheng, X.D.; et al. Discovery of benzochalcone derivative as a potential antigastric cancer agent targeting signal transducer and activator of transcription 3 (STAT3). J. Enzym. Inhib. Med. Chem. 2022, 37, 2004–2016. [Google Scholar] [CrossRef]
- Semaan, J.; Pinon, A.; Rioux, B.; Hassan, L.; Limami, Y.; Pouget, C.; Fagnere, C.; Sol, V.; Diab-Assaf, M.; Simon, A.; et al. Resistance to 3-HTMC-Induced Apoptosis Through Activation of PI3K/Akt, MEK/ERK, and p38/COX-2/PGE(2) Pathways in Human HT-29 and HCT116 Colorectal Cancer Cells. J. Cell. Biochem. 2016, 117, 2875–2885. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.L.; Wu, X.Q.; Yang, M.Y.; Wang, W.; Wang, L.H.; Tang, D.; Wang, D.R. Cardamonin exerts anti-gastric cancer activity via inhibiting LncRNA-PVT1-STAT3 axis. Biosci. Rep. 2019, 39, BSR20190357. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.L.; Hsieh, C.M.; Chan, N.L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef]
- Mohammed, H.H.H.; Abbas, S.H.; Hayallah, A.M.; Abuo-Rahma, G.E.A.; Mostafa, Y.A. Novel urea linked ciprofloxacin-chalcone hybrids having antiproliferative topoisomerases I/II inhibitory activities and caspases-mediated apoptosis. Bioorg. Chem. 2021, 106, 104422. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H.H.; Abd El-Hafeez, A.A.; Ebeid, K.; Mekkawy, A.I.; Abourehab, M.A.S.; Wafa, E.I.; Alhaj-Suliman, S.O.; Salem, A.K.; Ghosh, P.; Abuo-Rahma, G.E.A.; et al. New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization. J. Enzym. Inhib. Med. Chem. 2022, 37, 1346–1363. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, E.; Baek, K.H.; Kwon, H.B.; Woo, H.; Lee, E.S.; Kwon, Y.; Na, Y. Chalcones, inhibitors for topoisomerase I and cathepsin B and L, as potential anti-cancer agents. Bioorganic Med. Chem. Lett. 2013, 23, 3320–3324. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Lee, J.S.; Lee, I.S.; Kang, B.Y. Inhibition of topoisomerase I activity and efflux drug transporters’ expression by xanthohumol. from hops. Arch. Pharmacal. Res. 2007, 30, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Kassab, A.E.; Gedawy, E.M. Novel ciprofloxacin hybrids using biology oriented drug synthesis (BIODS) approach: Anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis, topoisomerase II inhibition, and antibacterial activity. Eur. J. Med. Chem. 2018, 150, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Nam, J.M. Synthesis and topoisomerase II inhibitory and cytotoxic activity of oxiranylmethoxy- and thiiranylmethoxy-chalcone derivatives. Bioorganic Med. Chem. Lett. 2011, 21, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Noh, J.H.; Lee, J.H.; Eun, J.W.; Ahn, Y.M.; Kim, S.Y.; Lee, S.H.; Park, W.S.; Yoo, N.J.; Lee, J.Y.; et al. Increased expression of historic deacetylase 2 is found in human gastric cancer. Apmis 2005, 113, 264–268. [Google Scholar] [CrossRef]
- Zhu, P.; Martin, E.; Mengwasser, J.; Schlag, P.; Janssen, K.P.; Gottlicher, M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell 2004, 5, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schioth, H.B. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [Google Scholar] [CrossRef] [PubMed]
- Jenke, R.; Ressing, N.; Hansen, F.K.; Aigner, A.; Buch, T. Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives. Cancers 2021, 13, 634. [Google Scholar] [CrossRef]
- Mohamed, M.F.A.; Shaykoon, M.S.A.; Abdelrahman, M.H.; Elsadek, B.E.M.; Aboraia, A.S.; Abuo-Rahma, G. Design, synthesis, docking studies and biological evaluation of novel chalcone derivatives as potential histone deacetylase inhibitors. Bioorg. Chem. 2017, 72, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Schnekenburger, M.; Zloh, M.; Golais, F.; Diederich, M.; Tasdemir, D. Natural chalcones as dual inhibitors of HDACs and NF-kappaB. Oncol. Rep. 2012, 28, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Chen, X.; Gao, J.; Su, L.; Zhang, L.; Xu, H.; Luan, Y. Anti-tumor activity evaluation of novel tubulin and HDAC dual-targeting inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yoon, H.; Ahn, S.; Kim, D.W.; Kim, S.H.; Koh, D.; Lee, Y.H.; Lim, Y. Chromenylchalcones showing cytotoxicity on human colon cancer cell lines and in silico docking with aurora kinases. Bioorg. Med. Chem. 2013, 21, 4250–4258. [Google Scholar] [CrossRef]
- Shin, S.Y.; Yoon, H.; Hwang, D.; Ahn, S.; Kim, D.W.; Koh, D.; Lee, Y.H.; Lim, Y. Benzochalcones bearing pyrazoline moieties show anti-colorectal cancer activities and selective inhibitory effects on aurora kinases. Bioorg. Med. Chem. 2013, 21, 7018–7024. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.H.H.; Mohamed, M.F.A.; Allam, R.M.; Nafady, A.; Mohamed, S.K.; Gouda, A.E.; Beshr, E.A.M. Design, synthesis, and molecular docking of novel pyrazole-chalcone analogs of lonazolac as 5-LOX, iNOS and tubulin polymerization inhibitors with potential anticancer and anti-inflammatory activities. Bioorg. Chem. 2022, 129, 106171. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Li, W.; Liu, H.D.; Yu, X.F. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 2019, 15, 2497–2508. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Kim, H.S.; Seo, G.S.; Lee, S.H. A new chalcone derivative, 3-phenyl-1-(2,4,6-tris(methoxymethoxy)phenyl) prop-2-yn-1-one), inhibits phorbol ester-induced metastatic activity of colorectal cancer cells through upregulation of heme oxygenase-1. Eur. J. Pharmacol. 2018, 841, 1–9. [Google Scholar] [CrossRef]
- Lu, T.; Zheng, C.J.; Fan, Z.M. Cardamonin suppressed the migration, invasion, epithelial mesenchymal transition (EMT) and lung metastasis of colorectal cancer cells by down-regulating ADRB2 expression. Pharm. Biol. 2022, 60, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Alswah, M.; Bayoumi, A.H.; Elgamal, K.; Elmorsy, A.; Ihmaid, S.; Ahmed, H.E.A. Design, Synthesis and Cytotoxic Evaluation of Novel Chalcone Derivatives Bearing Triazolo [4,3-a]-quinoxaline Moieties as Potent Anticancer Agents with Dual EGFR Kinase and Tubulin Polymerization Inhibitory Effects. Molecules 2017, 23, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, M.; Koike, J.; Yoshikawa, N.; Kondo, M.; Hemmi, H. Licochalcone A Inhibits BDNF and TrkB Gene Expression and Hypoxic Growth of Human Tumor Cell Lines. Int. J. Mol. Sci. 2020, 21, 506. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Kim, Y.; Jeong, D.; Kim, J.H.; Kim, S.; Son, Y.J.; Yoo, B.C.; Jeong, E.J.; Kim, T.W.; Lee, I.H.; et al. Pyrrole-Derivative of Chalcone, (E)-3-Phenyl-1-(2-Pyrrolyl)-2-Propenone, Inhibits Inflammatory Responses via Inhibition of Src, Syk, and TAK1 Kinase Activities. Biomol. Ther. 2016, 24, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Hoff, P.M.; Machado, K.K. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat. Rev. 2012, 38, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhong, X.; Lin, L.; Xie, J.; Lian, Y.; Xu, L. Comparative efficacy and adverse reactions of apatinib-chemotherapy combinations versus chemotherapy alone for treatment of advanced colorectal cancer: A meta-analysis of randomized controlled trials. Am. J. Transl. Res. 2022, 14, 6703–6711. [Google Scholar]
- Hironaka, S. Anti-angiogenic therapies for gastric cancer. Asia-Pac. J. Clin. Oncol. 2019, 15, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Salati, M.; Caputo, F.; Bocconi, A.; Cerri, S.; Baldessari, C.; Piacentini, F.; Dominici, M.; Gelsomino, F. Successes and failures of angiogenesis blockade in gastric and gastro-esophageal junction adenocarcinoma. Front. Oncol. 2022, 12, 993573. [Google Scholar] [CrossRef]
- Khater, M.; Watson, K.A.; Boateng, S.Y.; Greco, F.; Osborn, H.M.I. Halogenated Flavonoid Derivatives Display Antiangiogenic Activity. Molecules 2022, 27, 4757. [Google Scholar] [CrossRef]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef]
- Varinska, L.; van Wijhe, M.; Belleri, M.; Mitola, S.; Perjesi, P.; Presta, M.; Koolwijk, P.; Ivanova, L.; Mojzis, J. Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur. J. Pharmacol. 2012, 691, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirossay, L.; Varinska, L.; Mojzis, J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int. J. Mol. Sci. 2017, 19, 27. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.F.; Santali, E.Y.; El-Haggar, R. Novel piperazine-chalcone hybrids and related pyrazoline analogues targeting VEGFR-2 kinase; design, synthesis, molecular docking studies, and anticancer evaluation. J. Enzym. Inhib. Med. Chem. 2021, 36, 307–318. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, R.; Samanta, P.; Sarkar, R.; Biswas, S.; Saha, P.; Hajra, S.; Bhowmik, A. Targeting HIF-1alpha by Natural and Synthetic Compounds: A Promising Approach for Anti-Cancer Therapeutics Development. Molecules 2022, 27, 5192. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Ji, J.; Haam, K.; Han, T.H.; Lim, S.; Kang, M.J.; Lim, S.S.; Ban, H.S. Licochalcone A inhibits hypoxia-inducible factor-1alpha accumulation by suppressing mitochondrial respiration in hypoxic cancer cells. Biomed Pharm. 2021, 133, 111082. [Google Scholar] [CrossRef]
- Guruswamy, D.K.M.; Balaji, K.D.S.; Dharmappa, K.K.; Jayarama, S. Novel 3-(3, 5-difluoro-4-hydroxyphenyl)-1-(naphthalen-2-yl) prop-2-en-1-one as a potent inhibitor of MAP-kinase in HeLa cell lines and anti-angiogenic activity is mediated by HIF-1alpha in EAC animal model. Oncotarget 2020, 11, 4661–4676. [Google Scholar] [CrossRef]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R.; et al. Cardamonin inhibits breast cancer growth by repressing HIF-1alpha-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. CR 2019, 38, 377. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Ma, J.Y. Isoliquiritin Apioside Suppresses in vitro Invasiveness and Angiogenesis of Cancer Cells and Endothelial Cells. Front. Pharm. 2018, 9, 1455. [Google Scholar] [CrossRef] [Green Version]
- Lemes, S.R.; Junior, L.A.; da Silva Manoel, D.; de Sousa, M.A.M.; Fonseca, R.D.; Lima, R.S.; Noda-Perez, C.; de Melo Reis, P.R.; Cardoso, C.G.; de Paula Silveira-Lacerda, E.; et al. Optical properties and antiangiogenic activity of a chalcone derivate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 685–695. [Google Scholar] [CrossRef]
- Gonzalez, J.J.; Ortega, E.; Rothemund, M.; Gold, M.; Vicente, C.; de Haro, C.; Bautista, D.; Schobert, R.; Ruiz, J. Luminescent Gold(I) Complexes of 1-Pyridyl-3-anthracenylchalcone Inducing Apoptosis in Colon Carcinoma Cells and Antivascular Effects. Inorg. Chem. 2019, 58, 12954–12963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, L.L.; Fu, D.Y.; Qin, T.T.; Ran, Y.G.; Xu, F.; Du, X.R.; Gao, H.Y.; Sun, S.J.; Yang, T.J.; et al. Discovery of chalcone-modified estradiol analogs as antitumour agents that Inhibit tumour angiogenesis and epithelial to mesenchymal transition. Eur. J. Med. Chem. 2019, 176, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.R.; Wang, Y.Y.; Yuan, M.H.; Zhao, Q.; Zhang, Y.X.; Yao, Y.F.; Duan, Y.T. Angiogenesis, Anti-Tumor, and Anti-Metastatic Activity of Novel alpha-Substituted Hetero-Aromatic Chalcone Hybrids as Inhibitors of Microtubule Polymerization. Front. Chem. 2021, 9, 766201. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.R.; Yuan, M.H.; Kang, Y.Y.; Qin, J.L.; Zhang, Y.X.; Duan, Y.T.; Wang, L.F.; Yao, Y.F. Identification of novel non-toxic and anti-angiogenic alpha-fluorinated chalcones as potent colchicine binding site inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 339–354. [Google Scholar] [CrossRef]
- Wang, C.L.; Chen, Y.X.; Wang, Y.; Liu, X.X.; Liu, Y.Z.; Li, Y.; Chen, H.L.; Fan, C.P.; Wu, D.F.; Yang, J. Inhibition of COX-2, mPGES-1 and CYP4A by isoliquiritigenin blocks the angiogenic Akt signaling in glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J. Exp. Clin. Cancer Res. 2019, 38, 371. [Google Scholar] [CrossRef] [Green Version]
- Pollard, J. Bacteria, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 528. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Im, J.P.; Ye, B.D.; Kim, J.M.; Jung, H.C.; Song, I.S.; Kim, J.S. Rectal Administration of Lipopolysaccharide and Ovalbumin Ameliorates Acute Murine Colitis. Dig. Dis. Sci. 2011, 56, 2292–2298. [Google Scholar] [CrossRef]
- Chen, L.; Guo, S.J.; Ranzer, M.J.; DiPietro, L.A. Toll-Like Receptor 4 Has an Essential Role in Early Skin Wound Healing. J. Investig. Dermatol. 2013, 133, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-kappa B-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.X.; Liu, G.Y.; Yang, Y.F.; Wu, X.W.; Xu, W.; Yang, X.W. Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 1627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cao, L.; Sun, Y.; Qing, D.G.; Xu, X.Q.; Wang, J.C.; Si, J.Y.; Li, N. The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis. Molecules 2021, 26, 4149. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.N.; Wu, Y.Q.; Deng, B.G.; Li, J.S.; Cao, H.Y.; Qu, Y.; Qian, X.L.; Zhong, G.S. Isoliquiritigenin decreases the incidence of colitis-associated colorectal cancer by modulating the intestinal microbiota. Oncotarget 2016, 7, 85318–85331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Baba, K. Xanthoangelol Isolated from Angelica keiskei Roots Prevents Dextran Sulfate Sodium-Treated Colitis in Mice. Nat. Prod. J. 2020, 10, 655–663. [Google Scholar] [CrossRef]
- Folmer, F.; Blasius, R.; Morceau, F.; Tabudravu, J.; Dicato, M.; Jaspars, M.; Diederich, M. Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem. Pharmacol. 2006, 71, 1206–1218. [Google Scholar] [CrossRef]
- Gerhauser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Israf, D.A.; Khaizurin, T.A.; Syahida, A.; Lajis, N.H.; Khozirah, S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-kappaB nuclear translocation and Ikappa-B phosphorylation in RAW 264.7 macrophage cells. Mol. Immunol. 2007, 44, 673–679. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.J.; Yun, K.J.; Cho, Y.W.; Park, H.J.; Lee, K.T. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur. J. Pharmacol. 2008, 584, 175–184. [Google Scholar] [CrossRef]
- Kwon, H.S.; Park, J.H.; Kim, D.H.; Kim, Y.H.; Park, J.H.Y.; Shin, H.K.; Kim, J.K. Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J. Mol. Med. 2008, 86, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Yakubu, M.T.; Oladiji, A.T. Electrophilic and reactive oxygen species detoxification potentials of chalcone dimers is mediated by redox transcription factor Nrf-2. J. Biochem. Mol. Toxicol. 2014, 28, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Nozawa, H.; Daikonnya, A.; Kondo, K.; Kitanaka, S. Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biol. Pharm. Bull. 2003, 26, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem.-Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef]
- Bister, V.; Salmela, M.T.; Heikkila, P.; Anttila, A.; Rintala, R.; Isaka, K.; Andersson, S.; Saarialho-Kere, U. Matrilysins-1 and -2 (MMP-7 and -26) and metalloelastase (MMP-12), unlike MMP-19, are up-regulated in necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 60–66. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Kuroda, M.; Katada, K.; Ichikawa, H.; Kokura, S.; Yoshida, N.; Okanoue, T.; Yoshikawa, T. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm. Res. 2004, 53, 462–468. [Google Scholar] [CrossRef]
- Lee, S.H.; Sohn, D.H.; Jin, X.Y.; Kim, S.W.; Choi, S.C.; Seo, G.S. 2′,4′,6′-tris(methoxymethoxy) chalcone protects against trinitrobenzene sulfonic acid-induced colitis and blocks tumor necrosis factor-alpha-induced intestinal epithelial inflammation via heme oxygenase 1-dependent and independent pathways. Biochem. Pharmacol. 2007, 74, 870–880. [Google Scholar] [CrossRef]
- Huang, Z.H.; Yin, L.Q.; Guan, L.P.; Li, Z.H.; Tan, C. Screening of chalcone analogs with anti-depressant, anti-inflammatory, analgesic, and COX-2-inhibiting effects. Bioorganic Med. Chem. Lett. 2020, 30, 127173. [Google Scholar] [CrossRef]
- Mahmoud, A.; Elkhalifa, D.; Alali, F.; Al Moustafa, A.E.; Khalil, A. Novel Polymethoxylated Chalcones as Potential Compounds Against KRAS-Mutant Colorectal Cancers. Curr. Pharm. Des. 2020, 26, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Hatem, E.; El Banna, N.; Huang, M.E. Multifaceted Roles of Glutathione and Glutathione-Based Systems in Carcinogenesis and Anticancer Drug Resistance. Antioxid. Redox Signal. 2017, 27, 1217–1234. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef]
- Hodnick, W.F.; Milosavljevic, E.B.; Nelson, J.H.; Pardini, R.S. Electrochemistry of flavonoids. Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 1988, 37, 2607–2611. [Google Scholar] [CrossRef]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, X.; Xue, W.; Zhao, S.; Zhang, X.; Pei, J. Curcumin induced human gastric cancer BGC-823 cells apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway. Int. J. Mol. Sci. 2014, 15, 15754–15765. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.J.; Li, J.H.; Li, P.; Cui, Z.W.; Zhang, S.Y.; Li, J.F. Antiproliferative Evaluation In Vitro of a New Chalcone Inducing Apoptosis by ROS Generation Against MGC-803 Cells. Pharm. Chem. J. 2019, 53, 539–543. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, T.Y.; Zhang, Y.B.; Xu, H.D.; Li, Y.C.; Zi, X.L.; Yu, H.Y.; Li, J.F.; Jin, C.Y.; Liu, H.M. A new brominated chalcone derivative suppresses the growth of gastric cancer cells in vitro and in vivo involving ROS mediated up-regulation of DR5 and 4 expression and apoptosis. Toxicol. Appl. Pharm. 2016, 309, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.Y.; Li, T.Y.; Zhang, L.; Wang, X.Y.; Dong, H.Q.; Li, L.L.; Fu, D.J.; Li, Y.C.; Zi, X.L.; Liu, H.M.; et al. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells. Sci. Rep. 2017, 7, 9873. [Google Scholar] [CrossRef]
- Kuo, Y.F.; Su, Y.Z.; Tseng, Y.H.; Wang, S.Y.; Wang, H.M.; Chueh, P.J. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radical. Biol. Med. 2010, 49, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Lin, R.W.; Shen, Y.C.; Lin, K.Y.; Liao, J.W.; Thiyagarajan, V.; Yang, H.L. Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways. Cancers 2020, 12, 2475. [Google Scholar] [CrossRef] [PubMed]
- Phang, C.W.; Karsani, S.A.; Abd Malek, S.N. Induction of Apoptosis and Cell Cycle Arrest by Flavokawain C on HT-29 Human Colon Adenocarcinoma via Enhancement of Reactive Oxygen Species Generation, Upregulation of p21, p27, and GADD153, and Inactivation of Inhibitor of Apoptosis Proteins. Pharmacogn. Mag. 2017, 13, S321–S328. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.S.; Sun, T.L.; Du, J.; Zhang, B.K.; Xiang, D.X.; Li, W.Q. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 2018, 40, 3213–3222. [Google Scholar] [CrossRef]
- De Spirt, S.; Eckers, A.; Wehrend, C.; Micoogullari, M.; Sies, H.; Stahl, W.; Steinbrenner, H. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic. Biol. Med. 2016, 91, 164–171. [Google Scholar] [CrossRef]
- Wu, P.; Yu, T.; Wu, J.; Chen, J. Licochalcone a Induces ROS-Mediated Apoptosis through TrxR1 Inactivation in Colorectal Cancer Cells. BioMed Res. Int. 2020, 2020, 5875074. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Vilkova, M.; Vaskova, J.; Michalkova, R.; Mojzisova, G.; Mojzis, J. Antiproliferative Effect of Acridine Chalcone Is Mediated by Induction of Oxidative Stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Jung, E.; Lim, Y.; Lee, H.-J.; Rhee, J.H.; Yoo, M.; Ahn, S.; Koh, D. Synthesis, Crystal Structure, Hirshfeld Surface Analysis and Docking Studies of a Novel Flavone–Chalcone Hybrid Compound Demonstrating Anticancer Effects by Generating ROS through Glutathione Depletion. Crystals 2022, 12, 108. [Google Scholar]
- Liu, C.; Song, J.; Cui, X.-X.; Liu, W.-B.; Li, Y.-R.; Yu, G.-X.; Tian, X.-Y.; Wang, Y.-F.; Liu, Y.; Zhang, S.-Y. Discovery of novel 1,2,4-triazine-chalcone hybrids as anti-gastric cancer agents via an axis of ROS-ERK-DR5 in vitro and in vivo. Arab. J. Chem. 2022, 15, 103644. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Cai, Y.; Pan, Y.; Ye, F.; Zhang, Y.; Zhao, Y.; Yang, S.; Li, X.; Liang, G. Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J. Med. Chem. 2011, 54, 8110–8123. [Google Scholar] [CrossRef]
- Shi, W.J.; Gao, J.B. Molecular mechanisms of chemoresistance in gastric cancer. World J. Gastrointest. Oncol. 2016, 8, 673–681. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Shen, X.F.; Chen, G.; Du, J.F. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers 2022, 14, 2928. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zheng, Y.C.; Ma, L.L.; Tian, L.L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.R.; Jeddi, F.; Soozangar, N.; Somi, M.H.; Shirmohamadi, M.; Khaze, V.; Samadi, N. Nrf2/P-glycoprotein axis is associated with clinicopathological characteristics in colorectal cancer. BioMed Pharm. 2018, 104, 458–464. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.E.; Mo, Z.Z.; Shi, X.C.; Qu, C.X. Clinical significance and correlation of miR-200c and P-gp expression in gastric cancer and the effects on multidrug resistance. J. Gastrointest. Oncol. 2022, 13, 581–592. [Google Scholar] [CrossRef]
- Geng, M.; Wang, L.; Chen, X.; Cao, R.X.; Li, P.F. The association between chemosensitivity and Pgp, GST-pi and Topo II expression in gastric cancer. Diagn. Pathol. 2013, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.W.; Xu, L.; Hao, J.H.; Qin, C.Y.; Liu, H. Expression of P-glycoprotein and Multidrug Resistance-associated Protein is Associated with Multidrug Resistance in Gastric Cancer. J. Int. Med. Res. 2010, 38, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ding, Z.; Peng, Y.; Pan, F.; Li, J.; Zou, L.; Zhang, Y.; Liang, H. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS ONE 2014, 9, e98882. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; To, K.K.; Wang, L.; Zhang, L.; Lu, L.; Shen, J.; Chan, R.L.; Li, M.; Yeung, J.H.; Cho, C.H. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine 2014, 21, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Liu, Y. Reversing multidrug resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS ONE 2014, 9, e90180. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Z.; Zhu, Y.; Pan, Q.; Liu, Y.; Qi, X.; Jin, L.; Jin, J.; Ma, X.; Hua, D. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J. Biol. Chem. 2015, 290, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Lin, C.; Zhang, Y.; Zhang, X.; Zhang, C.; Zhang, P.; Xie, X.; Ren, Z. miR-506 enhances the sensitivity of human colorectal cancer cells to oxaliplatin by suppressing MDR1/P-gp expression. Cell Prolif. 2017, 50, e12341. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Rosello, S.; Arnold, D.; Normanno, N.; Taieb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 10–32. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.B.; Batovska, D.I.; Todorova, I.T.; Stamboliyska, B.A.; Serly, J.; Molnar, J. Comparative Study on the MDR Reversal Effects of Selected Chalcones. Int. J. Med. Chem. 2011, 2011, 530780. [Google Scholar] [CrossRef] [Green Version]
- Parveen, Z.; Brunhofer, G.; Jabeen, I.; Erker, T.; Chiba, P.; Ecker, G.F. Synthesis, biological evaluation and 3D-QSAR studies of new chalcone derivatives as inhibitors of human P-glycoprotein. Bioorganic Med. Chem. 2014, 22, 2311–2319. [Google Scholar] [CrossRef]
- Silbermann, K.; Shah, C.P.; Sahu, N.U.; Juvale, K.; Stefan, S.M.; Kharkar, P.S.; Wiese, M. Novel chalcone and flavone derivatives as selective and dual inhibitors of the transport proteins ABCB1 and ABCG2. Eur. J. Med. Chem. 2019, 164, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dong, J.J.; Yuan, X.; Wen, H.T.; Wu, L.G.J.; Liu, J.N.; Sui, H.; Deng, W.L. A New Chalcone Derivative C49 Reverses Doxorubicin Resistance in MCF-7/DOX Cells by Inhibiting P-Glycoprotein Expression. Front. Pharmacol. 2021, 12, 653306. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.H.; Dong, J.J.; Cai, Y.C.; Shi, X.M.; Wang, H.; Liu, G.X.; Tang, Y.; Liu, J.W.; Ma, L. Design, synthesis and biological evaluation of chalcones as reversers of P-glycoprotein-mediated multidrug resistance. Eur. J. Med. Chem. 2019, 180, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Cizmarikova, M.; Takac, P.; Spengler, G.; Kincses, A.; Nove, M.; Vilkova, M.; Mojzis, J. New Chalcone Derivative Inhibits ABCB1 in Multidrug Resistant T-cell Lymphoma and Colon Adenocarcinoma Cells. Anticancer Res. 2019, 39, 6499–6505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palko-Labuz, A.; Kostrzewa-Suslow, E.; Janeczko, T.; Sroda-Pomianek, K.; Pola, A.; Uryga, A.; Michalak, K. Cyclization of flavokawain B reduces its activity against human colon cancer cells. Hum. Exp. Toxicol. 2020, 39, 262–275. [Google Scholar] [CrossRef]
- Krawczenko, A.; Bielawska-Pohl, A.; Wojtowicz, K.; Jura, R.; Paprocka, M.; Wojdat, E.; Kozlowska, U.; Klimczak, A.; Grillon, C.; Kieda, C.; et al. Expression and activity of multidrug resistance proteins in mature endothelial cells and their precursors: A challenging correlation. PLoS ONE 2017, 12, e0172371. [Google Scholar] [CrossRef] [Green Version]
- Palko-Labuz, A.; Blaszczyk, M.; Sroda-Pomianek, K.; Wesolowska, O. Isobavachalcone as an Active Membrane Perturbing Agent and Inhibitor of ABCB1 Multidrug Transporter. Molecules 2021, 26, 4637. [Google Scholar] [CrossRef]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Rolando, B.; Kopecka, J.; Campia, I.; Chegaev, K.; Lazzarato, L.; Federico, A.; Fruttero, R.; Ghigo, D. Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to overcome drug resistance. Mol. Pharm. 2013, 10, 161–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.Y.; Zhang, W.; Wang, J.Q.; Lei, Z.N.; Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Tan, C.P.; Wang, B.; Chen, Z.S. Biological evaluation of non-basic chalcone CYB-2 as a dual ABCG2/ABCB1 inhibitor. Biochem. Pharmacol. 2020, 175, 113848. [Google Scholar] [CrossRef]
- Tuy, H.D.; Shiomi, H.; Mukaisho, K.I.; Naka, S.; Shimizu, T.; Sonoda, H.; Mekata, E.; Endo, Y.; Kurumi, Y.; Sugihara, H.; et al. ABCG2 expression in colorectal adenocarcinomas may predict resistance to irinotecan. Oncol. Lett. 2016, 12, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.P.; Lusvarghi, S.; Hsiao, S.H.; Liu, T.C.; Li, Y.Q.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V. Licochalcone A Selectively Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs. J. Nat. Prod. 2020, 83, 1461–1472. [Google Scholar] [CrossRef]
- Zoldakova, M.; Kornyei, Z.; Brown, A.; Biersack, B.; Madarasz, E.; Schobert, R. Effects of a combretastatin A4 analogous chalcone and its Pt-complex on cancer cells: A comparative study of uptake, cell cycle and damage to cellular compartments. Biochem. Pharmacol. 2010, 80, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Sugisawa, N.; Ohnuma, S.; Ueda, H.; Murakami, M.; Sugiyama, K.; Ohsawa, K.; Kano, K.; Tokuyama, H.; Doi, T.; Aoki, J.; et al. Novel Potent ABCB1 Modulator, Phenethylisoquinoline Alkaloid, Reverses Multidrug Resistance in Cancer Cell. Mol. Pharm. 2018, 15, 4021–4030. [Google Scholar] [CrossRef]
- Andersen, V.; Vogel, U.; Godiksen, S.; Frenzel, F.B.; Saebo, M.; Hamfjord, J.; Kure, E.; Vogel, L.K. Low ABCB1 gene expression is an early event in colorectal carcinogenesis. PLoS ONE 2013, 8, e72119. [Google Scholar] [CrossRef]
- Mealey, K.L.; Barhoumi, R.; Burghardt, R.C.; Safe, S.; Kochevar, D.T. Doxycycline induces expression of P glycoprotein in MCF-7 breast carcinoma cells. Antimicrob. Agents Chemother. 2002, 46, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed Pharm. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Wesolowska, O.; Kostrzewa-Suslow, E.; Uryga, A.; Michalak, K. MDR reversal and pro-apoptotic effects of statins and statins combined with flavonoids in colon cancer cells. Biomed Pharm. 2019, 109, 1511–1522. [Google Scholar] [CrossRef]
- Correa, S.; Binato, R.; Du Rocher, B.; Castelo-Branco, M.T.; Pizzatti, L.; Abdelhay, E. Wnt/β-catenin pathway regulates ABCB1 transcription in chronic myeloid leukemia. BMC Cancer 2012, 12, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.Y.; Zhang, W.; Zeng, X.; Liu, C.Q. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013, 104, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, E.K.; Park, J.H.; Kim, Y.H.; Park, J.H. Antitumor and antimetastatic effects of licochalcone A in mouse models. J. Mol. Med. 2010, 88, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Li, G.; Gao, Z. Xanthohumol protects against Azoxymethane-induced colorectal cancer in Sprague-Dawley rats. Environ. Toxicol. 2020, 35, 136–144. [Google Scholar] [CrossRef]
- Baba, M.; Asano, R.; Takigami, I.; Takahashi, T.; Ohmura, M.; Okada, Y.; Sugimoto, H.; Arika, T.; Nishino, H.; Okuyama, T. Studies on cancer chemoprevention by traditional folk medicines XXV. Inhibitory effect of isoliquiritigenin on azoxymethane-induced murine colon aberrant crypt focus formation and carcinogenesis. Biol. Pharm. Bull. 2002, 25, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.X.; Zhang, X.H.; Chen, X.W.; Li, Y.; Ke, Z.Q.; Tang, T.; Chai, H.Y.; Guo, A.M.; Chen, H.L.; Yang, J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE(2) and IL-6. Toxicol. Appl. Pharm. 2014, 279, 311–321. [Google Scholar] [CrossRef]
- James, S.; Aparna, J.S.; Babu, A.; Paul, A.M.; Lankadasari, M.B.; Athira, S.R.; Kumar, S.S.; Vijayan, Y.; Namitha, N.N.; Mohammed, S.; et al. Cardamonin Attenuates Experimental Colitis and Associated Colorectal Cancer. Biomolecules 2021, 11, 661. [Google Scholar] [CrossRef]
- Pande, A.N.; Biswas, S.; Reddy, N.D.; Jayashree, B.S.; Kumar, N.; Rao, C.M. In vitro and in vivo anticancer studies of 2’-hydroxy chalcone derivatives exhibit apoptosis in colon cancer cells by HDAC inhibition and cell cycle arrest. EXCLI J. 2017, 16, 448–463. [Google Scholar] [CrossRef]
- Jin, G.; Zhao, Z.; Chakraborty, T.; Mandal, A.; Roy, A.; Roy, S.; Guo, Z. Decrypting the Molecular Mechanistic Pathways Delineating the Chemotherapeutic Potential of Ruthenium-Phloretin Complex in Colon Carcinoma Correlated with the Oxidative Status and Increased Apoptotic Events. Oxidative Med. Cell. Longev. 2020, 2020, 7690845. [Google Scholar] [CrossRef]
- Hayashi, A.; Gillen, A.C.; Lott, J.R. Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern. Med. Rev. 2000, 5, 546–552. [Google Scholar] [PubMed]
- Jin, H.; Seo, G.S.; Lee, S.H. Isoliquiritigenin-mediated p62/SQSTM1 induction regulates apoptotic potential through attenuation of caspase-8 activation in colorectal cancer cells. Eur. J. Pharmacol. 2018, 841, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Son, S.H.; Park, K.K.; Park, J.H.; Lim, S.S.; Kim, S.H.; Chung, W.Y. Licochalcone A inhibits the growth of colon carcinoma and attenuates cisplatin-induced toxicity without a loss of chemotherapeutic efficacy in mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Son, S.H.; Park, K.K.; Park, J.H.; Lim, S.S.; Chung, W.Y. Isoliquiritigenin inhibits tumor growth and protects the kidney and liver against chemotherapy-induced toxicity in a mouse xenograft model of colon carcinoma. J. Pharmacol. Sci. 2008, 106, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Shin, E.K.; Kim, D.H.; Lee, H.H.; Park, J.H.; Kim, J.K. Antiangiogenic effect of licochalcone A. Biochem. Pharmacol. 2010, 80, 1152–1159. [Google Scholar] [CrossRef]
- Yin, F.; Zhao, R.; Gorja, D.R.; Fu, X.; Lu, N.; Huang, H.; Xu, B.; Chen, H.; Shim, J.H.; Liu, K.; et al. Novel dual inhibitor for targeting PIM1 and FGFR1 kinases inhibits colorectal cancer growth in vitro and patient-derived xenografts in vivo. Acta Pharm. Sin. B 2022, 12, 4122–4137. [Google Scholar] [CrossRef]
- Huang, Y.T.; Lin, C.I.; Chien, P.H.; Tang, T.T.; Lin, J.; Chao, J.I. The depletion of securin enhances butein-induced apoptosis and tumor inhibition in human colorectal cancer. Chem. Biol. Interact. 2014, 220, 41–50. [Google Scholar] [CrossRef]
- Park, J.M.; Park, S.H.; Hong, K.S.; Han, Y.M.; Jang, S.H.; Kim, E.H.; Hahm, K.B. Special licorice extracts containing lowered glycyrrhizin and enhanced licochalcone A prevented Helicobacter pylori-initiated, salt diet-promoted gastric tumorigenesis. Helicobacter 2014, 19, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Wang, F.B.; Tan, F.; Long, X.; Pan, Y.; Zhao, X. Intervention effects of lotus leaf flavonoids on gastric mucosal lesions in mice infected with Helicobacter pylori. RSC Adv. 2020, 10, 23510–23521. [Google Scholar] [CrossRef]
- Pincock, S. Nobel Prize winners Robin Warren and Barry Marshall. Lancet 2005, 366, 1429. [Google Scholar] [CrossRef]
- Khoder, G.; Muhammad, J.S.; Mahmoud, I.; Soliman, S.S.M.; Burucoa, C. Prevalence of Helicobacter pylori and Its Associated Factors among Healthy Asymptomatic Residents in the United Arab Emirates. Pathogens 2019, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Mentis, A.F.A.; Boziki, M.; Grigoriadis, N.; Papavassiliou, A.G. Helicobacter pylori infection and gastric cancer biology: Tempering a double-edged sword. Cell. Mol. Life Sci. 2019, 76, 2477–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzahrani, S.; Lina, T.T.; Gonzalez, J.; Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Effect of Helicobacter pylori on gastric epithelial cells. World J. Gastroenterol. 2014, 20, 12767–12780. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar Mahapatra, D.; Asati, V.; Bharti, S.K. An updated patent review of therapeutic applications of chalcone derivatives (2014-present). Expert Opin. Ther. Pat. 2019, 29, 385–406. [Google Scholar] [CrossRef]
- Bodet, C.; Burucoa, C.; Rouillon, S.; Bellin, N.; Taddeo, V.A.; Fiorito, S.; Genovese, S.; Epifano, F. Antibacterial activities of oxyprenylated chalcones and napthtoquinone against Helicobacter pylori. Nat. Prod. Commun. 2014, 9, 1299–1301. [Google Scholar] [CrossRef] [Green Version]
- Yoshiyama, H.; Nakamura, H.; Okamoto, T.; Okita, K.; Nakazawa, T. A novel in vitro effect of the mucosal protective agent sofalcone--inhibition of chemotactic motility in Helicobacter pylori. Aliment. Pharmacol. Ther. 2000, 14 (Suppl. S1), 230–236. [Google Scholar] [CrossRef]
- Higuchi, K.; Watanabe, T.; Tanigawa, T.; Tominaga, K.; Fujiwara, Y.; Arakawa, T. Sofalcone, a gastroprotective drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori: A randomized controlled comparative trial with cimetidine, an H2-receptor antagonist. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. S1), S155–S160. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Kim, S.; Joo, S.; Jeong, S.; Yoo, J.W.; Jung, Y. Sofalcone, a gastroprotective drug, covalently binds to KEAP1 to activate Nrf2 resulting in anti-colitic activity. Eur. J. Pharmacol. 2019, 865, 172722. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.H.; Rao, Y.K.; Fang, S.H.; Sing, Y.T.; Tzeng, Y.M. Identification of 3′, 4′, 5′-trimethoxychalcone analogues as potent inhibitors of Helicobacter pylori-induced inflammation in human gastric epithelial cells. Bioorganic Med. Chem. Lett. 2010, 20, 5462–5465. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Santagostini, L.; Forino, M.; Piazza, S.; Colombo, E.; Taglialatela-Scafati, O.; Fico, G.; Dell’Agli, M. A bio-guided assessment of the anti-inflammatory activity of hop extracts (Humulus lupulus L. cv. Cascade) in human gastric epithelial cells. J. Funct. Foods 2019, 57, 95–102. [Google Scholar] [CrossRef]
- Pachathundikandi, S.K.; Blaser, N.; Backert, S. Mechanisms of Inflammasome Signaling, microRNA Induction and Resolution of Inflammation by Helicobacter pylori. Curr. Top. Microbiol. 2019, 421, 267–302. [Google Scholar] [CrossRef]
- Choi, H.R.; Lim, H.; Lee, J.H.; Park, H.; Kim, H.P. Interruption of Helicobacter pylori-Induced NLRP3 Inflammasome Activation by Chalcone Derivatives. Biomol. Ther. 2021, 29, 410–418. [Google Scholar] [CrossRef]
- De Ventura, T.; Perrone, M.; Missiroli, S.; Pinton, P.; Marchetti, P.; Strazzabosco, G.; Turrin, G.; Illuminati, D.; Cristofori, V.; Fantinati, A.; et al. Synthesis and NLRP3-Inflammasome Inhibitory Activity of the Naturally Occurring Velutone F and of Its Non-Natural Regioisomeric Chalconoids. Int. J. Mol. Sci. 2022, 23, 8957. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative Stress Resulting From Helicobacter pylori Infection Contributes to Gastric Carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.R.; Lee, H.J.; Ryu, J.H. Chalcones from Angelica keiskei attenuate the inflammatory responses by suppressing nuclear translocation of NF-kappaB. J. Med. Food 2014, 17, 1306–1313. [Google Scholar] [CrossRef]
- Jang, S.H.; Cho, S.; Lee, E.S.; Kim, J.M.; Kim, H. The phenyl-thiophenyl propenone RK-I-123 reduces the levels of reactive oxygen species and suppresses the activation of NF-kappaB and AP-1 and IL-8 expression in Helicobacter pylori-infected gastric epithelial AGS cells. Inflamm. Res. 2013, 62, 689–696. [Google Scholar] [CrossRef]

| Chalcone | IC50 or Used Concentration | Cell Line | Mechanism of Action | Reference |
|---|---|---|---|---|
| (E)-3,4-dihydroxy-2′-methoxychalcone | 10 µmol/L | CT26.WT | Inhibition of proliferation, colony formation, migration and invasion, induction of apoptosis ↓ Bcl-2, phospho-Akt, IkB-α, nuclear p65 ↑ Bax, cleaved caspases—3, cleaved PARP, E-cadherin | [23] |
| (E)-2-(2′,4′-dimethoxybenzylidene)-1-tetralone | 3.44 µmol/L | HCT116 | Induction of apoptosis, G2/M phase cell cycle arrest, DNA damage and changes in microtubule structure ↑ activity of caspases—3/-7, cleaved PARP, | [25] |
| (E)-2-(4′-methoxybenzylidene)-1- Benzosuberone, (E)-2-(2′,4′- dimethoxybenzylidene)-1-tetralone | 0.5 and 5 µmol/L | Caco-2 | Cell cycle arrest in G2/M phase cell cycle arrest, induction of apoptosis, DNA fragmentation ↓ MMP, expression of Bcl-2, Bcl-xL, α, α1 and β5 tubulins ↑ activity of caspase -3, production of ROS, expression of Bax, | [26] |
| (2 E)-3-(acridin-9-yl)-1-(2,6-dimethoxyphenyl)prop-2-en-1-one | 10 µmol/L | HCT116 | Inhibition of proliferation, induction of apoptosis, G2/M cell cycle arrest, tubulin dysregulation ↓ MMP, phospho-survivin ↑ cytochrome c, Smac/Diablo, activity of caspases -3, -7 and -9, cleaved PARP, phospho-ATM, phospho-SMC, phospho-H2A.X, phospho-p53, p53, phospho-Bad, Bad, p21, phospho-p38 MAPK, phospho-Erk1/2, phospho-JNK, phospho-Akt | [27] |
| (2E)-3-(3-bromophenyl)-1-(2-hydroxyphenyl)prop-2-en-1- one, 2-(4-bromophenyl)-3-hydroxy-4H-chromen-4-one | 10–15 µmol/L | HCT116 | Inhibition of proliferation, induction of apoptosis, S and G2/M cell cycle arrest, nuclear condensation ↑ p53, p27, cleaved PARP | [29] |
| 2′-hydroxy-2,4,6-trimethoxy-5′,6′-naphthochalcone | 10 µmol/L | SW620 | Inhibition of proliferation, colony formation, induction of apoptosis, G2/M cell cycle arrest, disruption of the microtubular network, DNA fragmentation ↓ cyclins D1, A, B1, ↑ phospho-Aurora A/B/C, activation of caspases -2, -3, -7, -9, cleaved PARP, phospho-ATM, phospho-ATR, phospho-γ-H2A.X, phospho-p53, p53, Bax, phospho-Chk2, | [30] |
| Xanthohumol | 5–20 µmol/L | HT-29 | Induction of apoptosis, G2/M cell cycle arrest ↓ Bcl-2, cyclin B1, Ras, phospho-MEK, phospho-Erk ↑ Bax, activation caspases -3, -9 | [31] |
| (E)-2-(3-(2,5-dimethoxyphenyl)-3-oxoprop-1-enyl)quinazolin-4(3H)-one | 3.56 µmol/L | HCT116 | Induction of apoptosis, G2/M cell cycle arrest ↓ MMP ↑ cleaved caspases -3, -7, -9, cleaved PARP | [40] |
| Sappanchalcone | 10–50 µmol/L | HCT116 | Induction of apoptosis ↓ MMP, Bcl-2 ↑ Bax, cleaved caspases -3, -7, -8, -9, cleaved PARP, ROS production, AIF, phospho-p53, p-53 | [42] |
| 2′,4′-dihydroxychalcone | 5–20 µmol/L | MGC-803 | Inhibition of proliferation, nuclear condensation and ear fragmentation, G2/M cell cycle arrest ↓ expression of survivin ↑ activity of caspases -3 | [50] |
| (E)-1-(4-((2-methylquinolin-4-yl)amino)phenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one | 300–1500 nmol/L | MGC-803 | Inhibition of proliferation and colony formation, G2/M cell cycle arrest ↑ cleaved caspases -3, -9, cleaved PARP, ROS production | [49] |
| Halogenated derivatives of chalcones | 2.5–20 µmol/L | RAW 264.7 and mouse peritoneal macrophages | Activity against LPS-induced release of cytokines ↓ TNF-α, IL-1β, IL-6, IL-12 and COX-2 expression, phospho-JNK, IkB, phospho-p38, phospho-Erk, p65 | [194] |
| Phloretin | 9–36 µmol/L | SNU-1 | Induction of autophagy, G0/G1 cell cycle arrest, inhibition of invasiveness and migration, ↓ cyclins D1 and D2, phospho-p38, phospho-Erk1/2 ↑ Beclin-1, LC3B | [24] |
| (E)-3-(3-fluoro-4-hydroxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one | 40 μmol/L | SGC-7901, BGC-823 | Inhibition of cell proliferation and induction of apoptosis, G2/M cell cycle arrest ↓ cdc2, cyclin B1, Bcl-2, p62, phospho-Akt, phospho-mTOR ↑ cleaved PARP, Bax, ROS generation, Beclin-1, LC3A/B | [52] |
| Flavokawain B | 2.5–10 μg/mL | AGS cells | Inhibition of cell proliferation and colony formation, induction of autophagy, G2/M cell cycle arrest ↓ phospho-mTOR, ATG4B, cyclins A, B1, Cdk1, cdc25C, phospho-HER2, HER2, phospho-PI3K, PI3K, phospho-Akt, Akt, Bax ↑ LC3A/B, p62, cleaved PARP, Beclin-1/Bcl-2 ratio, ROS production, phospho-JNK1/2, phospho-Erk1/2 | [53] |
| (E)-4-chloro-N-(4-(3-oxo-3-(3,4,5-trimethoxyphenyl) prop-1-en-1-yl) phenyl) butanamide | 1.5–6 μmol/L | MGC-803 | Inhibition of proliferation and colony formation, induction of apoptosis ↓ cyclin B1, Cdk1, Bid, Bcl-2, XIAP, c-IAP1 ↑ DR5, cleaved caspases -3, -7, -8, -9, cleaved PARP, Noxa | [55] |
| 2′-dihydroxy-4,4′-dimethoxydihydrochalcone | 8-32 μmol/L | MKN45 | Inhibition of cell proliferation, induction of apoptosis and autophagy, inhibition of invasiveness and migration ↓ MMP2, MMP9 ↑ ROS generation, Beclin-1, Atg6, Atg7, LC3-II, phospho-MEK, phospho-Erk | [59] |
| Liquiritin | 80 μmol/L | SGC7901/DDP | Inhibition of proliferation and colony formation, invasiveness and migration, induction of apoptosis and autophagy, DNA damage ↓ cyclin D1, A, Cdk4, MMP, p62 ↑ p21, p53, cleaved caspases -3, -8, -9, cleaved PARP, Beclin-1, LC3I/II | [60] |
| Derricin and derricidin | 30, 50 μmol/L | HCT116, DLD-1 | Inhibition of proliferation, S and G2/M cell cycle arrest ↓ β-catenin, Wnt reporter activity | [71] |
| Isobavachalcone | 50, 100 μmol/L | HCT116, SW480 | Inhibition of proliferation and colony formation, induction of apoptosis ↓ Bcl-2, XIAP, survivin, β-catenin, phospho-GSK-3β, phospho-Akt ↑ cleaved PARP, cleaved caspase -3, Bax, phospho-β-catenin | [72] |
| Licochalcone A | 10–50 μmol/L | HCT116, HeLa, A549, Hep3B | Inhibition of proliferation and colony formation, induction of apoptosis ↓ PD-L1, Ras, phospho-Raf, phospho-MEK, phospho-p65, phospho-IkBα, phospho-IKKα/β, TRAF2, RIP1 ↑ cleaved PARP, cleaved caspase-8 | [75] |
| Thioderivatives of chalcones | 1–5 μmol/L | HCT116, DLD-1 | Inhibition of proliferation, invasiveness and migration, induction of apoptosis, S phase cell cycle arrest ↓ p50 in nuclear fraction, p65 nuclear fraction, COX-2, EGFR, phospho-Akt, STAT3, Bcl-xL, c-Myc, ↑ nuclear phospho-Nrf2, SOD, GSTP | [76] |
| Cardamonin | 20–80 μmol/L | HCT116 | Inhibition of proliferation, induction of apoptosis ↓ c-Myc, Oct4, Cyclin E, TSP50, NF-kB ↑ activity of caspases -3, -9, Bax, | [79] |
| Hydroxysafflor Yellow A | 20 μmol/L | KYSE-30 | Inhibition of proliferation, invasiveness and migration, induction of apoptosis ↓ ICAM1, VCAM1, MMP9, TNF-α, phospho-p65, phospho-IkBα | [83] |
| Butein | 1–50 μmol/L | SAS, KB | Inhibition of proliferation, invasiveness and migration, induction of apoptosis ↓ phospho-p65, p65, COX-2, MMP-9, survivin | [84] |
| Flavokawain C | 60 μmol/L | HCT116 | Inhibition of proliferation and colony formation, invasiveness and migration, induction of apoptosis, S and G2/M cell cycle arrest, DNA damage ↓ MMP, c-IAP1, XIAP, c-FLIPL, survivin, Cdk2, Cdk4, phospho-Rb, Rb, phospho-Akt ↑ cleaved caspases -3, -8, -9, cleaved PARP, Smac/DIABLO, AIF and cytochrome c release, Bak, p21, p27, GADD153, phospho-Erk | [88] |
| Cardamonin | 10 μmol/L | BGC-823/5- FU, BGC-823 | Induction of apoptosis, inhibition of proliferation, G2/M phase cell cycle arrest ↓ function of P-gp, P-gp, TCF4, β-catenin | [73] |
| 3-deoxysappanchalcone | 5–20 μmol/L | HCT15, HCT116 | Inhibition of proliferation and colony formation, G2/M cell cycle arrest ↓ cyclin B1, phospho-TOPK, TOPK, phospho-Erk, phospho-RSK, phospho-c-Jun ↑ p53, p21, cleaved PARP, cleaved caspases -3, -7 | [90] |
| Isobavachalcone | 20, 40 μmol/L | MGC803 | Inhibition of proliferation, invasiveness and migration, induction of apoptosis ↓ phospho-Akt, phospho-Erk, Bcl-2, ↑ Bax, active caspase -3 | [91] |
| Chalcone | 0.5–2 μg/mL | AGS | Inhibition of cell viability, adhesion, migration and invasion ↓ MMP9, MMP2, phospho-FAK, phospho-JNK1/2, NF-kB, phospho-IkBα ↑ IkBα | [92] |
| Licochalcone A | 100 μmol/L | BGC-823 | Inhibition of proliferation, induction of apoptosis ↓ GSH/GSSG ratio, phospho-PI3K, phospho-Akt, ↑ ROS production, cleaved PARP, phospho-Erk, phospho-JNK, phospho-p38 | [93] |
| Hydroxysafflor yellow A | 25–100 μmol/L | HCT116 | Inhibition of proliferation and colony formation, invasion and migration, induction od apoptosis ↓ PCNA, Bcl-2, N-cadherin, vimentin, phospho-Akt ↑ Bax, cleaved caspase -3, E-cadherin, PPARγ, PTEN | [94] |
| 3-(3-((E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoyl)phenyl)-2-methyl-3,4- dihydro-4-quinazolinone | 20–40 μmol/L | HCT116 | Induction of apoptosis, S and G2/M cell cycle arrest ↓ Bcl-2, cytosolic Bax, mitochondrial cytochrome c, phospho-100α, phospho-100γ, phospho-Akt, Akt, ↑ mitochondrial Bax, cytosolic cytochrome c, cleaved caspases -3, -9, cleaved PARP, p21, p27, Skp-1, Skp-2, Ub, phospho-mTOR, mTOR, phospho-p70S6K, phospho-STAT3, STAT3 | [95] |
| (E)-1–(1-Hydroxy-4,5,8-trimethoxynaphthalen-2-yl)-3-(quinolin-6-yl)prop-2-en-1-one | 1, 2 μmol/L | MKN1 | Induction of apoptosis, inhibition of colony formation, migration and invasion | [98] |
| 3-hydroxy-3′,4,4′,5′-tetra-methoxy-chalcone | 0.5, 35, 55 μmol/L | HT-29, HCT116 | Inhibition of proliferation, G2/M cell cycle arrest, induction of apoptosis, DNA damage ↓ cdc2, ↑ p21, phospho-p53, DR5, cleaved caspases -3, -8, cleaved PARP, phospho-Akt, phospho-Erk, phospho-p38, COX-2, PGE2 | [99] |
| Cardamonin | 10–30 μmol/L | AGS | Inhibition of proliferation, colony formation and migration, induction of apoptosis ↓ Bcl-2, Cdk1, cyclin B1, cdc25C, α-SMA, vimentin, Snail, phospho-STAT3, EMT ↑ Bax, caspase -3, p21, E-cadherin, | [100] |
| 1,2,3-triazole linked ciprofloxacin-chalcone | 0.1–100 μmol/L | HCT116 | Inhibition of proliferation, G2/M cell cycle arrest, inhibition of topoisomerase I and II and tubulin polymerization ↓ α-tubulin ↑ γH2AX, phospho-ATR, phospho-Chk1, phospho-cdc25C | [103] |
| Xanthohumol | 2–8 μmol/L | HCT116, SW6320 | Inhibition of colony formation and proliferation ↓ HK1, HK2, phospho-histone H3, cytosolic Bax, phospho-EGFR, phospho-Akt, phospho-Erk1/2 ↑ cleaved caspase -3, cytosolic cytochrome c, mitochondrial Bax, mitochondrial VDAC1 | [119] |
| 3-phenyl-1-(2,4,6- tris(methoxymethoxy)phenyl)prop-2-yn-1-one) | 4 μmol/L | HCT116, SW6320 | Inhibition of migration, invasion and proliferation ↓ nuclear NF-kB, phospho-IkBα, MMP-7, ↑ IkBα, HO-1, p21, cleaved PARP, cleaved caspases -3, -8, -9 | [120] |
| Cardamonin | 10 μmol/L | HT-29, HCT116 | Inhibition of migrtion, invasion and proliferation ↓ MMP-2, MMP-9, N-cadherin, ADRB2, EMT ↑ E-cadherin | [121] |
| (E)-3-Phenyl-1-(2-Pyrrolyl)-2-Propenone | 10–100 μmol/L | HEK293, RAW264.7 | ↓ iNOS, TNF-α, p50, c-Jun, c-Fos, phospho-IkBα, IkBα, phospho-Syk, phospho-Src, phospho-JNK, phospho-Erk, phospho-IRAK1, phospho-c-Raf, phospho-TAK1, phospho-MEK1, phospho-MKK4 | [124] |
| (Z)-3–(6-bromo-1H-indol-3-yl)-2-fluoro-1–(3,4,5-trimethoxyphenyl)prop-2-en-1-one (4c) | 18–72 nmol/L | MGC-803, HUVEC | Inhibition of tubulin polymerization, G2/M phase cell cycle arrest, induction of apoptosis, inhibition of migration and invasion ↓ cyclin B1, phospho-cdc2, MMP ↑ p21, cleaved caspases -3, -7, -9, cleaved PARP, ROS generation | [144] |
| Isoliquiritigenin | 0.4–1.6 μmol/L | RAW264.7 | ↓ iNOS, COX-2, IL-6, TNF-α, phospho-IkBα, nuclear p65, nuclear p50, phospho-IKK, phospho-Erk, phospho-p38 | [161] |
| Licochalcone A | 2.5–20 μmol/L | RAW264.7 | ↓ iNOS, COX-2, PGE2, IL-1β, IL-6, phospho-IkBα, phospho-p38, nuclear NF-kB, ↑ IL-10, IkBα, AP-1 DNA-binding activity | [162] |
| Bromo chalcone derivative H72 | 1–6 μmol/L | MGC803, HGC27 | Inhibition of proliferation, induction of apoptosis, ROS production ↓ Bid, Bcl-xL, XIAP, survivin, MMP ↑ Bim, DR4, DR5, cytochrome c, cleaved PARP, cleaved caspases -3, -9 | [183] |
| Flavokawain A derivate S17 | 10 μmol/L | MGC803 | Inhibition of proliferation, induction of apoptosis, ROS production ↓ p18, XIAP, Nrf2 ↑ cleaved caspases -3, -8, -9, cleaved PARP, p43/p41, Bim, Bax, Bid, Bad, DR5, phospho-Nrf2, HO-1, NQO1 | [184] |
| Flavokawain C | 40–80 μmol/L | HCT116, HT-29 | Induction of apoptosis, inhibition of proliferation, G2/M phase cell cycle arrest, ROS production ↓ MMP, c-IAP1, c-IAP2, XIAP ↑ activity of caspases -3, -8, -9, cleaved PARP, p21, p27, GADD153 | [187] |
| Chalcone | Dose | Exposure Duration | Tumor Model | Antitumor Effect | Reference |
|---|---|---|---|---|---|
| Chalcone derivative L2H17 | 25–50 mg/kg | 60 days | CT26.WT tumor induced in mouse model | Extended survival | [23] |
| (E)-2-Chloro-4′-methoxychalcone, | 15 mg/kg | 7 days | ICR and C57BL/6 (B6) mice | Inhibition of TNF-α, IL-1β, IL-6 and IL-12 expression and prolonged survival in LPS-induced acute inflammatory model | [194] |
| Flavokawain B | Intraperitoneal (1.5 mg/kg) and oral (7.5 mg/kg) | 51 days | AGS-xenografted nude mice | Inhibition of tumor growth, reduction of tumor volume and weight, prolonged the survival rate and induced autophagy ↑ LCI/II, Beclin-1, ATG7 | [53] |
| 2′-dihydroxy-4,4′-dimethoxydihydrochalcone | 12-48 mg/kg | 32 days | MKN45 xenograftedBALB/C-nu mice | Significant decrease in tumor volumes, inhibition of Ki67 expression | [59] |
| Liquiritin | 15 mg/kg | 21 days | SGC7901/DDP xenografted BALB/C-nu mice | Reduced the size, volume and weight of tumor, inhibition of Ki67 expression, induction of apoptosis and autophagy ↓ cyclin D1, Cdk4, p62 ↑ p21, p53, cleaved caspases -3, -8, -9, cleaved PARP, LC3I/II, Beclin-1 | [60] |
| Cardamonin | 25 mg/kg | 30 days | BGC-823/5-FU xenograft mouse model | Reduced tumor volumes and tumor weights | [73] |
| Licochalcone A | 15, 50 mg/kg | 30 days | HCT116 cells in a xenograft model (BALB/c nude mice) | Inhibition of tumor growth, reduced levels of PD-L1, p65, Ras and VEGF in tumor tissues | [75] |
| Licochalcone A | 200, 400 µmol/L | 30 days | BGC-823 gastric carcinoma in KM mice | Tumor growth inhibition | [93] |
| 3-(3-((E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoyl)phenyl)-2-methyl-3,4- dihydro-4-quinazolinone | 30, 40 mpk | 13 days | Ehrlich Ascites Carcinoma cells in Swiss albino mice/Sarcoma-180 in BALB/c mice | Tumor growth inhibition and reduced tumor volume/weight | [95] |
| (E)-1–(1-Hydroxy-4,5,8-trimethoxynaphthalen-2-yl)-3-(quinolin-6-yl)prop-2-en-1-one | 5 mg/kg | 28 days | MKN1 xenografted model (BALA/c nude mice) | Tumor growth inhibition and reduced tumor and metastasis mass | [98] |
| Xanthohumol | 10 mg/kg | 16 days | HT29 and HCT116 xenograft mouse model | Reduced tumor volume and weight, decreased phosphorylation of Akt, expression of Ki67 and HK2 | [119] |
| Licochalcone A | 1–10 mg/kg | 5 days | LPS-treated BALB/c mice | Extended survival, reduced plasmatic concentrations of NO, IL-6, TNF-α | [162] |
| Bromo chalcone derivative H72 | 40 mg/kg/day | 21 days | MGC803 xenograft model | Reduced tumor weight | [183] |
| Flavokawain A derivate S17 | 40 mg/kg/day | 24 days | MGC803 xenograft model | Lowered tumor growth rate, increased expression od cleaved caspases -3 and -8, ROS production | [184] |
| Licorice Extracts Containing Enhanced Licochalcone A | 25–100 mg/kg | 24 weeks | H. pylori-infected animal model (mice) | Reduced inflammation, mucosal erosion/ulcer, dysplasia, adenoma formation ↓ bFGF, FcrRIIB, ICAM-1, lungkine, thymus-CK1, TRANCE, TROY, COX-2, iNOS, TNF-α, IL-1β, IL-6, phospho-STAT3, phospho-JAK2 | [248] |
| Lotus leaf flavonoids extract | 25–100 mg/kg | 2 weeks | H. pylori-infected BALB/c mice | Reduction of gastric lesions, decreased levels of endothelin-1, substance P, increased levels of somatostatin, and vasoactive intestinal peptide, IL-6, IL-12, TNF-α, INF-γ, MPO, KRT16, KRT6b, TGM3, NLRP3, IL-1β, TLR4 | [249] |
| Xanthohumol | 5 mg/kg | 8 weeks | Sprague-Dawley rats | Inhibition of formation of aberrant crypt foci, reduced MDA level, reduced expression of iNOS and COX-2, cyclin D1, c-Myc and Bcl-2, suppression of Wnt/β-catenin signaling and Ki76 positive cells, increased Bax and caspase -3, SOD, CAT, GPx | [235] |
| Licochalcone A | 5–30 mg/kg | 8 weeks | Licochalcone A | Reduction of tumor formation and tumor volume, decreased cytokine levels in colon (IL-1β, IL-6, TNF-α, KC, MCP-1), inhibition of IκBα and expression of iNOS, COX-2, β-catenin and MMP-9 | [234] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalkova, R.; Kello, M.; Cizmarikova, M.; Bardelcikova, A.; Mirossay, L.; Mojzis, J. Chalcones and Gastrointestinal Cancers: Experimental Evidence. Int. J. Mol. Sci. 2023, 24, 5964. https://doi.org/10.3390/ijms24065964
Michalkova R, Kello M, Cizmarikova M, Bardelcikova A, Mirossay L, Mojzis J. Chalcones and Gastrointestinal Cancers: Experimental Evidence. International Journal of Molecular Sciences. 2023; 24(6):5964. https://doi.org/10.3390/ijms24065964
Chicago/Turabian StyleMichalkova, Radka, Martin Kello, Martina Cizmarikova, Annamaria Bardelcikova, Ladislav Mirossay, and Jan Mojzis. 2023. "Chalcones and Gastrointestinal Cancers: Experimental Evidence" International Journal of Molecular Sciences 24, no. 6: 5964. https://doi.org/10.3390/ijms24065964
APA StyleMichalkova, R., Kello, M., Cizmarikova, M., Bardelcikova, A., Mirossay, L., & Mojzis, J. (2023). Chalcones and Gastrointestinal Cancers: Experimental Evidence. International Journal of Molecular Sciences, 24(6), 5964. https://doi.org/10.3390/ijms24065964









