A Therapeutic Vaccine Targeting Rat BORIS (CTCFL) for the Treatment of Rat Breast Cancer Tumors
Abstract
:1. Introduction
2. Results
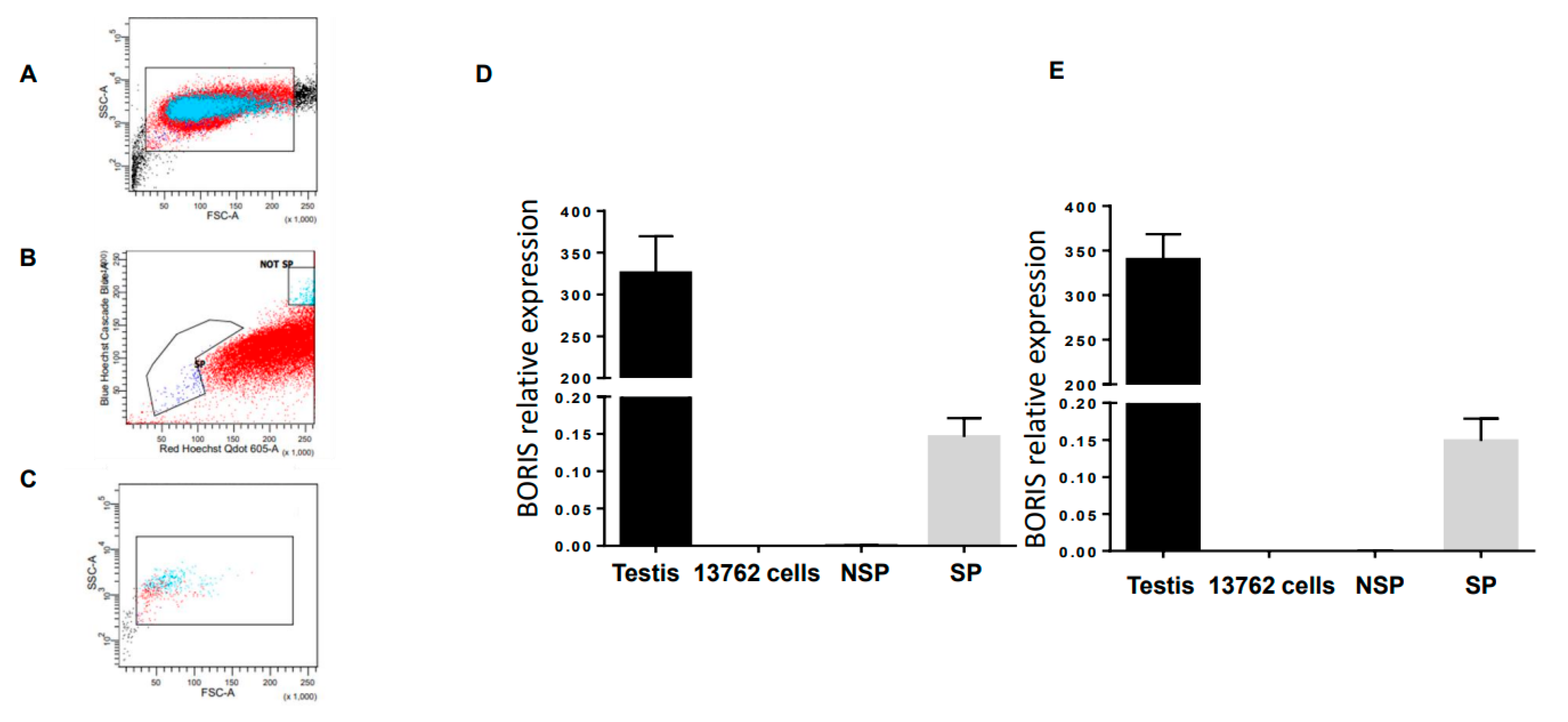
2.1. BORIS Expression in the 13762 MAT B III Carcinoma Cell Line
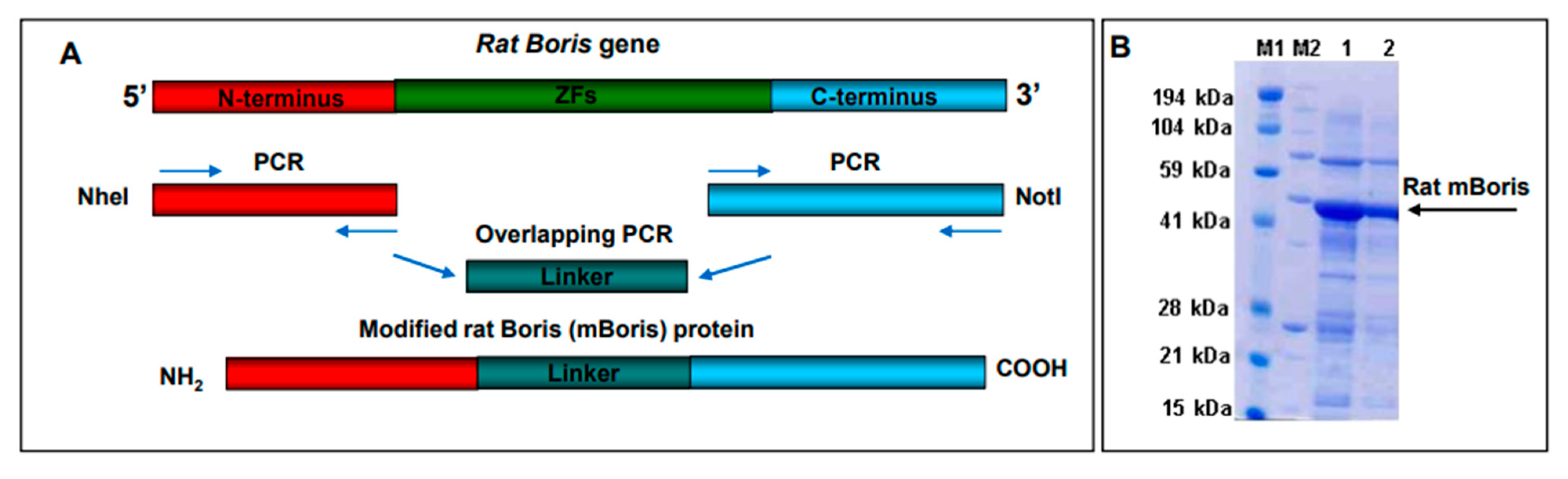
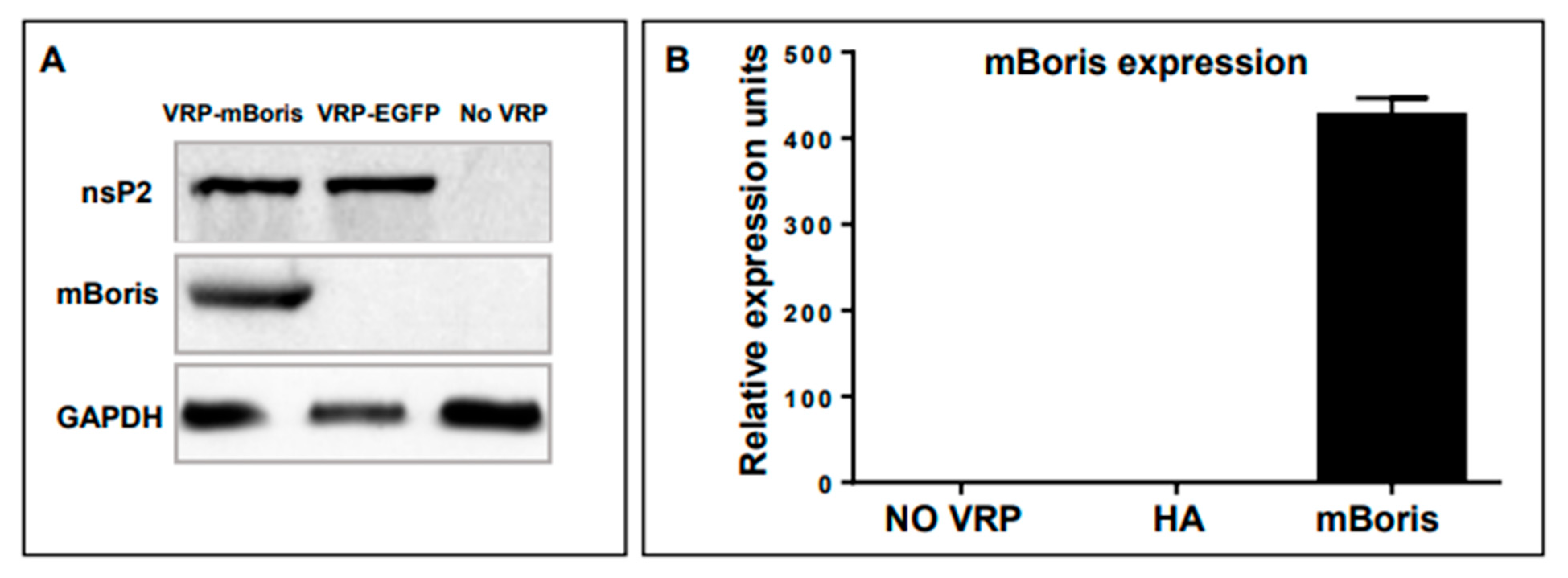
2.2. Generation of a VRP-BORIS Vaccine
2.3. Evaluation of Therapeutic Potency of VRP-mBORIS Vaccine
3. Discussion
4. Materials and Methods
4.1. Animals and Cell Lines
4.2. Side Population Flow Cytometry and Sorting
4.3. Generation of VRP and Recombinant Protein Reagents
4.4. Detection of VRP-Encoded mBORIS by Western Blotting
4.5. Detection of VRP-Encoded mBORIS mRNA by Real-Time qPCR
4.6. Real-Time PCR
4.7. Tumor Inoculation and VRP Immunotherapy Administration
4.8. Proliferation Assay
4.9. ELISpot Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27 (Suppl. 2), S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.-E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias-Escudero, M.; Arias-González, N.; Martínez-Cáceres, E. Regulatory cells and the effect of cancer immunotherapy. Mol. Cancer 2023, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front. Immunol. 2023, 14, 1127071. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J. Transforming growth factor-β signalling in tumour resistance to the anti-PD-(L)1 therapy: Updated. J. Cell Mol. Med. 2023, 27, 311–321. [Google Scholar] [CrossRef]
- Houghton, A.N. Cancer antigens: Immune recognition of self and altered self. J. Exp. Med. 1994, 180, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.; Goubier, A.; Kohrt, H.E. A quantitative analysis of therapeutic cancer vaccines in phase 2 or phase 3 trial. J. Immunother. Cancer 2015, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Antigen-specific vaccines for cancer treatment. Hum. Vaccines Immunother. 2014, 10, 3332–3346. [Google Scholar] [CrossRef]
- Ogi, C.; Aruga, A. Clinical evaluation of therapeutic cancer vaccines. Hum. Vaccines Immunother. 2013, 9, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Melief, C.J.; van Hall, T.; Arens, R.; Ossendorp, F.; Van Der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Q.; Zhang, R.; Xie, L.; Shu, Y.; Gao, S.; Wang, P.; Su, X.; Qin, Y.; Wang, Y.; et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct. Target. Ther. 2021, 6, 26. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221, Erratum in Nature 2018, 555, 402. [Google Scholar] [CrossRef] [Green Version]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Bastin, D.J.; Montroy, J.; Kennedy, M.A.; Martel, A.B.; Shorr, R.; Ghiasi, M.; Boucher, D.M.; Wong, B.; Gresham, L.; Diallo, J.-S.; et al. Safety and efficacy of autologous cell vaccines in solid tumors: A systematic review and meta-analysis of randomized control trials. Sci. Rep. 2023, 13, 3347. [Google Scholar] [CrossRef]
- Al-Khadairi, G.; Decock, J. Cancer Testis Antigens and Immunotherapy: Where Do We Stand in the Targeting of PRAME? Cancers 2019, 11, 984. [Google Scholar] [CrossRef] [Green Version]
- Manjili, M.H. A Theoretical Basis for the Efficacy of Cancer Immunotherapy and Immunogenic Tumor Dormancy: The Adaptation Model of Immunity. Adv. Cancer Res. 2018, 137, 17–36. [Google Scholar] [CrossRef]
- Pugacheva, E.M.; Rivero-Hinojosa, S.; Espinoza, C.A.; Méndez-Catalá, C.F.; Kang, S.; Suzuki, T.; Kosaka-Suzuki, N.; Robinson, S.; Nagarajan, V.; Ye, Z.; et al. Comparative analyses of CTCF and BORIS occupancies uncover two distinct classes of CTCF binding genomic regions. Genome Biol. 2015, 16, 161. [Google Scholar] [CrossRef] [Green Version]
- Loukinov, D. Targeting CTCFL/BORIS for the immunotherapy of cancer. Cancer Immunol. Immunother. 2018, 67, 1955–1965. [Google Scholar] [CrossRef]
- Pugacheva, E.M.; Teplyakov, E.; Wu, Q.; Li, J.; Chen, C.; Meng, C.; Liu, J.; Robinson, S.; Loukinov, D.; Boukaba, A.; et al. The cancer-associated CTCFL/BORIS protein targets multiple classes of genomic repeats, with a distinct binding and functional preference for humanoid-specific SVA transposable elements. Epigenet. Chromatin 2016, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Alberti, L.; Renaud, S.; Losi, L.; Leyvraz, S.; Benhattar, J. High expression of hTERT and stemness genes in BORIS/CTCFL positive cells isolated from embryonic cancer cells. PLoS ONE 2014, 9, e109921. [Google Scholar] [CrossRef]
- Alberti, L.; Losi, L.; Leyvraz, S.; Benhattar, J. Different Effects of BORIS/CTCFL on Stemness Gene Expression, Sphere Formation and Cell Survival in Epithelial Cancer Stem Cells. PLoS ONE 2015, 10, e0132977. [Google Scholar] [CrossRef] [PubMed]
- Loukinov, D.; Ghochikyan, A.; Mkrtichyan, M.; Ichim, T.E.; Lobanenkov, V.V.; Cribbs, D.H.; Agadjanyan, M.G. Antitumor efficacy of DNA vaccination to the epigenetically acting tumor promoting transcription factor BORIS and CD80 molecular adjuvant. J. Cell Biochem. 2006, 98, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Ghochikyan, A.; Mkrtichyan, M.; Loukinov, D.; Mamikonyan, G.; Pack, S.D.; Movsesyan, N. Epigenetically acting tumor promoting transcription factor BORIS is widely expressed TAA inducing anti-tumor specific T cell responses. J. Immunol. 2007, 178, 556–573. [Google Scholar]
- Mkrtichyan, M.; Ghochikyan, A.; Davtyan, H.; Movsesyan, N.; Loukinov, D.; Lobanenkov, V.; Cribbs, D.H.; Laust, A.K.; Nelson, E.L.; Agadjanyan, M.G. Cancer-testis antigen, BORIS based vaccine delivered by dendritic cells is extremely effective against a very aggressive and highly metastatic mouse mammary carcinoma. Cell Immunol. 2011, 270, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Smith, I.M.; Glazer, C.A.; Mithani, S.K.; Ochs, M.F.; Sun, W.; Bhan, S.; Vostrov, A.; Abdullaev, Z.; Lobanenkov, V.; Gray, A.; et al. Coordinated activation of candidate proto-oncogenes and cancer testes antigens via promoter demethylation in head and neck cancer and lung cancer. PLoS ONE 2009, 4, e4961. [Google Scholar] [CrossRef]
- Nishimoto, K.P.; Laust, A.K.; Nelson, E.L. A human dendritic cell subset receptive to the Venezuelan equine encephalitis virus-derived replicon particle constitu-tively expresses IL-32. J. Immunol. 2008, 181, 4010–4018. [Google Scholar] [CrossRef] [Green Version]
- Laust, A.K.; Sur, B.W.; Wang, K.; Hubby, B.; Smith, J.F.; Nelson, E.L. VRP immunotherapy targeting neu: Treatment efficacy and evidence for immunoediting in a stringent rat mammary tumor model. Breast Cancer Res. Treat. 2007, 106, 371–382. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Moran, T.P.; Collier, M.; McKinnon, K.P.; Davis, N.L.; Johnston, R.E.; Serody, J.S. A novel viral system for generating antigen-specific T cells. J. Immunol. 2005, 175, 3431–3438. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, G.H.; Johnston, R.E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 2000, 74, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.L.; West, A.; Reap, E.; Macdonald, G.; Collier, M.; Dryga, S.; Maughan, M.; Connell, M.; Walker, C.; McGrath, K.; et al. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life 2002, 53, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E.; Olmsted, R.; Houghton, A.N.; Wolchok, J.D. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS ONE 2010, 5, e12670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, D.; Devi, G.R.; Burnett, B.K.; Clay, T.M.; et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Investig. 2010, 120, 3234–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgents, J.E.; Moran, T.P.; West, M.L.; Davis, N.L.; Johnston, R.E.; Serody, J.S. The immunosuppressive tumor environment is the major impediment to successful therapeutic vaccination in Neu transgenic mice. J. Immunother. 2010, 33, 482–491. [Google Scholar] [CrossRef]
- Kanodia, S.; Da Silva, D.M.; Karamanukyan, T.; Bogaert, L.; Fu, Y.-X.; Kast, W.M. Expression of LIGHT/TNFSF14 combined with vaccination against human papillomavirus Type 16 E7 induces signifi-cant tumor regression. Cancer Res. 2010, 70, 3955–3964. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Hernandez, M.D.L.L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: A candidate antigen for treating prostate cancer. Cancer Res. 2007, 67, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Hernandez, M.D.L.L.; Gray, A.; Hubby, B.; Klinger, O.J.; Kast, W.M. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008, 68, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R.; Wang, X.; Maughan, M.F.; Talarico, T.L.; Olmsted, R.A.; et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2007, 13, 3999–4008. [Google Scholar] [CrossRef] [Green Version]
- Nelson, E.L.; Prieto, D.; Alexander, T.G.; Pushko, P.; Lofts, L.A.; Rayner, J.O.; Kamrud, K.I.; Fralish, B.; Smith, J.F. Venezuelan equine encephalitis replicon immunization overcomes intrinsic tolerance and elicits effective anti-tumor immunity to the ‘self’ tumor-associated antigen, neu in a rat mammary tumor model. Breast Cancer Res. Treat. 2003, 82, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, J.-P.; Maughan, M.F.; Lachman, L.B. Alphavirus replicon particles containing the gene for HER2/neuinhibit breast cancer growth and tumorigenesis. Breast Cancer Res. 2004, 7, R145. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.A.; Hart, M.K. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J. Virol. 2001, 75, 2660–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velders, M.P.; McElhiney, S.; Cassetti, M.C.; Eiben, G.L.; Higgins, T.; Kovacs, G.R.; Elmishad, A.G.; Kast, W.M.; Smith, L.R. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001, 61, 7861–7867. [Google Scholar] [PubMed]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational re-search. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanlan, M.J.; Simpson, A.J.G.; Old, L.J. The cancer/testis genes: Review, standardization, and commentary. Cancer Immun. 2004, 4, 1. [Google Scholar]
- Simpson, A.J.G.; Caballero, O.L.; Jungbluth, A.; Chen, Y.-T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 2005, 5, 615–625. [Google Scholar] [CrossRef]
- Kuşoğlu, A.; Avcı, Ç.B. Cancer stem cells: A brief review of the current status. Gene 2018, 681, 80–85. [Google Scholar] [CrossRef]
- Asano, T.; Hirohashi, Y.; Torigoe, T.; Mariya, T.; Horibe, R.; Kuroda, T.; Tabuchi, Y.; Saijo, H.; Yasuda, K.; Mizuuchi, M.; et al. Brother of the regulator of the imprinted site (BORIS) variant subfamily 6 is involved in cervical cancer stemness and can be a target of immunotherapy. Oncotarget 2016, 7, 11223–11237. [Google Scholar] [CrossRef] [Green Version]
- Horibe, R.; Hirohashi, Y.; Asano, T.; Mariya, T.; Suzuki, T.; Takaya, A.; Saijo, H.; Shionoya, Y.; Kubo, T.; Nakatsugawa, M.; et al. Brother of the regulator of the imprinted site (BORIS) variant subfamily 6 is a novel target of lung cancer stem-like cell immunotherapy. PLoS ONE 2017, 12, e0171460. [Google Scholar] [CrossRef]
- Zhao, Y.; Baldin, A.; Isayev, O.; Werner, J.; Zamyatnin, A.; Bazhin, A. Cancer Vaccines: Antigen Selection Strategy. Vaccines 2021, 9, 85. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.C.; Wang, W.; Zhang, L.; Wang, X. A tour of 3D genome with a focus on CTCF. Semin. Cell Dev. Biol. 2018, 90, 4–11. [Google Scholar] [CrossRef]
- D’Arcy, V.; Pore, N.; Docquier, F.; Abdullaev, Z.K.; Chernukhin, I.; Kita, G.-X.; Rai, S.; Smart, M.; Farrar, D.; Pack, S.; et al. BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br. J. Cancer 2008, 98, 571–579. [Google Scholar] [CrossRef]
- Hong, J.A.; Kang, Y.; Abdullaev, Z.; Flanagan, P.T.; Pack, S.D.; Fischette, M.R.; Adnani, M.T.; Loukinov, D.I.; Vatolin, S.; Risinger, J.I.; et al. Reciprocal Binding of CTCF and BORIS to the NY-ESO-1 Promoter Coincides with Derepression of this Cancer-Testis Gene in Lung Cancer Cells. Cancer Res. 2005, 65, 7763–7774. [Google Scholar] [CrossRef] [Green Version]
- Kouprina, N.; Noskov, V.N.; Pavlicek, A.; Collins, N.K.; Bortz, P.D.S.; Ottolenghi, C.; Loukinov, D.; Goldsmith, P.; Risinger, J.I.; Kim, J.-H.; et al. Evolutionary diversification of SPANX-N sperm protein gene structure and expression. PLoS ONE 2007, 2, e359. [Google Scholar] [CrossRef] [Green Version]
- Vatolin, S.; Abdullaev, Z.; Pack, S.D.; Flanagan, P.T.; Custer, M.; Loukinov, D.I.; Pugacheva, E.; Hong, J.A.; Morse, H.; Schrump, D.S.; et al. Conditional Expression of the CTCF-Paralogous Transcriptional Factor BORIS in Normal Cells Results in Demethylation and Derepression of MAGE-A1 and Reactivation of Other Cancer-Testis Genes. Cancer Res. 2005, 65, 7751–7762. [Google Scholar] [CrossRef] [Green Version]
- Woloszynska-Read, A.; James, S.R.; Link, P.A.; Yu, J.; Odunsi, K.; Karpf, A.R. DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun. 2007, 7, 21. [Google Scholar]
- Kalaora, S.; Nagler, A.; Wargo, J.A.; Samuels, Y. Mechanisms of immune activation and regulation: Lessons from melanoma. Nat. Rev. Cancer 2022, 22, 195–207. [Google Scholar] [CrossRef]
- Rouf, N.Z.; Biswas, S.; Tarannum, N.; Oishee, L.M.; Muna, M.M. Demystifying mRNA vaccines: An emerging platform at the forefront of cryptic diseases. RNA Biol. 2022, 19, 386–410. [Google Scholar] [CrossRef]
- Nishimoto, K.P.; Laust, A.K.; Wang, K.; Kamrud, K.I.; Hubby, B.; Smith, J.F.; Nelson, E.L. Restricted and selective tropism of a Venezuelan equine encephalitis virus-derived replicon vector for human dendritic cells. Viral Immunol. 2007, 20, 88–104. [Google Scholar] [CrossRef]
- Tonkin, D.R.; Whitmore, A.; Johnston, R.E.; Barro, M. Infected dendritic cells are sufficient to mediate the adjuvant activity generated by Venezuelan equine encephalitis virus replicon particles. Vaccine 2012, 30, 4532–4542. [Google Scholar] [CrossRef] [Green Version]
- Pushko, P.; Parker, M.; Ludwig, G.V.; Davis, N.L.; Johnston, R.E.; Smith, J.F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: Expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 1997, 239, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.A.; Deome, K.B.; Weiss, D.W. Immunology Of Spontaneous Mammary Carcinomas in Mice: II. Resistance To A Rapidly And A Slowly Developing Tumor. Cancer Res. 1965, 25, 451–457. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukinov, D.; Anderson, A.L.; Mkrtichyan, M.; Ghochikyan, A.; Rivero-Hinojosa, S.; Tucker, J.; Lobanenkov, V.; Agadjanyan, M.G.; Nelson, E.L. A Therapeutic Vaccine Targeting Rat BORIS (CTCFL) for the Treatment of Rat Breast Cancer Tumors. Int. J. Mol. Sci. 2023, 24, 5976. https://doi.org/10.3390/ijms24065976
Loukinov D, Anderson AL, Mkrtichyan M, Ghochikyan A, Rivero-Hinojosa S, Tucker J, Lobanenkov V, Agadjanyan MG, Nelson EL. A Therapeutic Vaccine Targeting Rat BORIS (CTCFL) for the Treatment of Rat Breast Cancer Tumors. International Journal of Molecular Sciences. 2023; 24(6):5976. https://doi.org/10.3390/ijms24065976
Chicago/Turabian StyleLoukinov, Dmitri, Amanda Laust Anderson, Mikayel Mkrtichyan, Anahit Ghochikyan, Samuel Rivero-Hinojosa, Jo Tucker, Victor Lobanenkov, Michael G. Agadjanyan, and Edward L. Nelson. 2023. "A Therapeutic Vaccine Targeting Rat BORIS (CTCFL) for the Treatment of Rat Breast Cancer Tumors" International Journal of Molecular Sciences 24, no. 6: 5976. https://doi.org/10.3390/ijms24065976
APA StyleLoukinov, D., Anderson, A. L., Mkrtichyan, M., Ghochikyan, A., Rivero-Hinojosa, S., Tucker, J., Lobanenkov, V., Agadjanyan, M. G., & Nelson, E. L. (2023). A Therapeutic Vaccine Targeting Rat BORIS (CTCFL) for the Treatment of Rat Breast Cancer Tumors. International Journal of Molecular Sciences, 24(6), 5976. https://doi.org/10.3390/ijms24065976





