Silicon-Induced Morphological, Biochemical and Molecular Regulation in Phoenix dactylifera L. under Low-Temperature Stress
Abstract
1. Introduction
2. Results
2.1. Morphological and Physiological Impact of Silicon on Date Palm Seedlings under Low-Temperature Stress
2.2. Influence of Silicon on Photosynthetic Pigments under Low-Temperature Stress
2.3. Silicon Influences Anti-Oxidative System of Date Palm Seedlings under Low-Temperature Stress
2.4. Silicon Inhibits Lipid Peroxidation and O2•– Generation under Low-Temperature Stress
2.5. Determination of Endogenous Hormones
2.6. Determination of Organic Acid Levels
2.7. Influence of Si on Date Palm Morphology under Stress
2.8. ICP-Elemental Analysis for Silicon (Si) and Nutrients Accumulation
2.9. Si influences the Regulation of Abiotic Stress-Associated Genes
2.10. Histogram-Correlation Analysis and Principal Component Analysis (PCA)
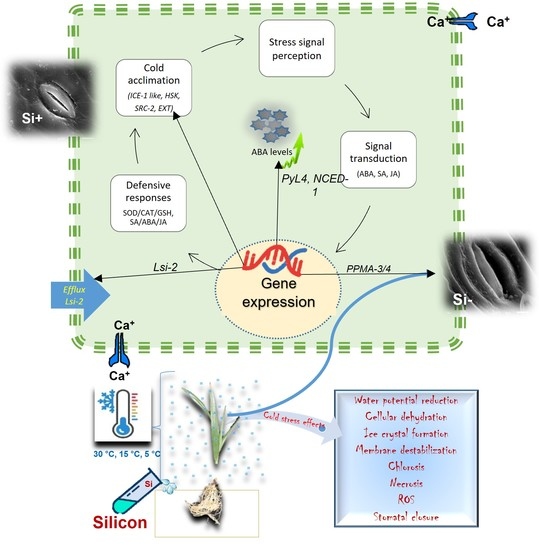
3. Discussion
4. Materials and Methods
4.1. Plant Growth under Si Supplementation and Low-Temperature Stress
4.2. Root Phenotypic Data Collection
4.3. Determination of Chlorophyll Contents and Carotenoids
4.4. Leaf Relative Water Content (LRWC) of Date Palm Seedlings
4.5. Quantification of Total Protein Content and Anti-Oxidant Enzymes
4.6. Measurement of Lipid Peroxidation
4.7. Superoxide Anion (O2•−) Activity
4.8. Elemental Uptake and Accumulation in Date Palm Seedlings
4.9. SEM analysis of Date Palm Shoot and Root
4.10. Phytohormones Extraction and Quantification
4.11. Organic Acid Quantification
4.12. RNA extraction and Quantification
4.13. cDNA Synthesis and qRT-PCR
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tigchelaar, M.; Battisti, D.S.; Naylor, R.L.; Ray, D.K. Future warming increases probability of globally synchronized maize production shocks. Proc. Natl. Acad. Sci. USA 2018, 115, 6644–6649. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef] [PubMed]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted role of salicylic acid in combating cold stress in plants: A review. J. Plant Growth Regul. 2021, 40, 464–485. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Gil-Muñoz, F.; Forner-Giner, M.A. Physiological characterization and proline route genes quantification under long-term cold stress in Carrizo citrange. Sci. Hortic. 2021, 276, 109744. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.-M.; Lin, H.-X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Liu, L.; Tang, L.; Cao, W.; Zhu, Y. Testing the responses of four wheat crop models to heat stress at anthesis and grain filling. Glob. Chang. Biol. 2016, 22, 1890–1903. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, Y.-M.; Lee, J.; Lee, K.-I.; Piao, R.; Han, L.; Shin, J.-C.; Jin, R.-D.; Cao, T.; Pan, H.-Y. Quantitative trait loci for cold tolerance of rice recombinant inbred lines in low temperature environments. Mol. Cells 2011, 32, 579–587. [Google Scholar] [CrossRef]
- Adhikari, L.; Makaju, S.O.; Lindstrom, O.M.; Missaoui, A.M. Mapping freezing tolerance QTL in alfalfa: Based on indoor phenotyping. BMC Plant Biol. 2021, 21, 403. [Google Scholar] [CrossRef] [PubMed]
- Goering, R.; Larsen, S.; Tan, J.; Whelan, J.; Makarevitch, I. QTL mapping of seedling tolerance to exposure to low temperature in the maize IBM RIL population. PLoS ONE 2021, 16, e0254437. [Google Scholar] [CrossRef]
- Sowiński, P.; Rudzińska-Langwald, A.; Adamczyk, J.; Kubica, I.; Fronk, J. Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. J. Plant Physiol. 2005, 162, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kumar, S.; Nayyar, H.; Upadhyaya, H. Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative components of seeds. J. Agron. Crop Sci. 2008, 194, 457–464. [Google Scholar]
- Kumar, S.; Malik, J.; Thakur, P.; Kaistha, S.; Sharma, K.D.; Upadhyaya, H.; Berger, J.; Nayyar, H. Growth and metabolic responses of contrasting chickpea (Cicer arietinum L.) genotypes to chilling stress at reproductive phase. Acta Physiol. Plant. 2011, 33, 779–787. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Y.; Bai, L.; Zhang, L.; Chen, L.; Li, H.; Yin, Y.; Yan, W.; Yi, Y.; Guo, Z. The ICE-CBF-COR pathway in cold acclimation and AFPs in plants. Middle East J. Sci. Res. 2011, 8, 493–498. [Google Scholar]
- Sun, X.; Zhu, Z.; Zhang, L.; Fang, L.; Zhang, J.; Wang, Q.; Li, S.; Liang, Z.; Xin, H. Overexpression of ethylene response factors VaERF080 and VaERF087 from Vitis amurensis enhances cold tolerance in Arabidopsis. Sci. Hortic. 2019, 243, 320–326. [Google Scholar] [CrossRef]
- Thakur, P.; Nayyar, H. Facing the cold stress by plants in the changing environment: Sensing, signaling, and defending mechanisms. In Plant Acclimation to Environmental Stress; Springer: Berlin/Heidelberg, Germany, 2013; pp. 29–69. [Google Scholar]
- Londo, J.P.; Kovaleski, A.P.; Lillis, J.A. Divergence in the transcriptional landscape between low temperature and freeze shock in cultivated grapevine (Vitis vinifera). Hortic. Res. 2018, 5, 10. [Google Scholar] [CrossRef]
- Mathur, S.; Sunoj, V.S.J.; Elsheery, N.I.; Reddy, V.R.; Jajoo, A.; Cao, K.-F. Regulation of Photosystem II heterogeneity and Photochemistry in two cultivars of C4 crop Sugarcane under Chilling stress. Front. Plant Sci. 2021, 12, 627012. [Google Scholar] [CrossRef]
- Savitch, L.V.; Ivanov, A.G.; Gudynaite-Savitch, L.; Huner, N.P.; Simmonds, J. Cold stress effects on PSI photochemistry in Zea mays: Differential increase of FQR-dependent cyclic electron flow and functional implications. Plant Cell Physiol. 2011, 52, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Shahzad, R.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Azhri, M.; Mohanta, T.K.; Lee, I.-J. Silicon and gibberellins: Synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Habibi, G. Effects of soil-and foliar-applied silicon on the resistance of grapevine plants to freezing stress. Acta Biol. Szeged. 2015, 59, 109–117. [Google Scholar]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Z.; Song, Z.; Wang, N.; Guo, N.; Niu, J.; Liu, A.; Bai, B.; Ahammed, G.J.; Chen, S. Silicon enhances plant resistance to Fusarium wilt by promoting antioxidant potential and photosynthetic capacity in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 604514. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Lee, I.-J. Silicon: A duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit. Rev. Biotechnol. 2016, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xiao, X.; Dong, Z.; Chen, Y. Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol. Plant. 2014, 36, 3063–3069. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef]
- Manna, M.C.; Rahman, M.M.; Naidu, R.; Sahu, A.; Bhattacharjya, S.; Wanjari, R.; Patra, A.K.; Chaudhari, S.; Majumdar, K.; Khanna, S. Bio-waste management in subtropical soils of India: Future challenges and opportunities in agriculture. Adv. Agron. 2018, 152, 87–148. [Google Scholar]
- Hall, C.R.; Waterman, J.M.; Vandegeer, R.K.; Hartley, S.E.; Johnson, S.N. The role of silicon in antiherbivore phytohormonal signalling. Front. Plant Sci. 2019, 10, 1132. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; Steppe, K.; Bauweraerts, I.; Kikuchi, S.; Asano, T.; Höfte, M.; De Vleesschauwer, D. Primary metabolism plays a central role in moulding silicon-inducible brown spot resistance in rice. Mol. Plant Pathol. 2015, 16, 811–824. [Google Scholar] [CrossRef]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Yaish, M.W.; Kumar, P.P. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 2015, 6, 348. [Google Scholar] [CrossRef]
- El-Juhany, L.I. Degradation of date palm trees and date production in Arab countries: Causes and potential rehabilitation. Aust. J. Basic Appl. Sci. 2010, 4, 3998–4010. [Google Scholar]
- Solanke, A.U.; Sharma, A.K. Signal transduction during cold stress in plants. Physiol. Mol. Biol. Plants 2008, 14, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, N.; Deswal, R. The molecular biology of the low-temperature response in plants. Bioessays 2005, 27, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Li, M.; Welti, R. Profiling lipid changes in plant response to low temperatures. Physiol. Plant. 2006, 126, 90–96. [Google Scholar] [CrossRef]
- Sonobe, K.; Hattori, T.; An, P.; Tsuji, W.; Eneji, A.E.; Kobayashi, S.; Kawamura, Y.; Tanaka, K.; Inanaga, S. Effect of silicon application on sorghum root responses to water stress. J. Plant Nutr. 2010, 34, 71–82. [Google Scholar] [CrossRef]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef]
- Salinas, J. Molecular mechanisms of signal transduction in cold acclimation. In Plant Signal Transduction; Oxford University Press: Oxford, UK, 2002; pp. 116–139. [Google Scholar]
- El Rabey, H.A.; Al-Malki, A.L.; Abulnaja, K.O. Proteome analysis of date palm (Phoenix dactylifera L.) under severe drought and salt stress. Int. J. Genom. 2016, 2016, 7840759. [Google Scholar]
- Abbas, T.; Balal, R.M.; Shahid, M.A.; Pervez, M.A.; Ayyub, C.M.; Aqueel, M.A.; Javaid, M.M. Silicon-induced alleviation of NaCl toxicity in okra (Abelmoschus esculentus) is associated with enhanced photosynthesis, osmoprotectants and antioxidant metabolism. Acta Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Xia, X.J.; Fang, P.P.; Guo, X.; Qian, X.J.; Zhou, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid-mediated apoplastic H2O2-glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.; Shin, D.; Park, S.; Jang, S.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 4. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic acid in plant abiotic stress tolerance and interaction with abscisic acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of CBF expression–c-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Wang, H.; Yoo, H.Y.; Park, J.; Ki, J.-S. Low temperature and cold stress significantly increase saxitoxins (STXs) and expression of STX biosynthesis genes sxtA4 and sxtG in the dinoflagellate Alexandrium catenella. Mar. Drugs 2021, 19, 291. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012, 169, 567–576. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life 2022, 12, 886. [Google Scholar] [CrossRef]
- Dong, C.-J.; Li, L.; Shang, Q.-M.; Liu, X.-Y.; Zhang, Z.-G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Muzi, C.; Camoni, L.; Visconti, S.; Aducci, P. Cold stress affects H+-ATPase and phospholipase D activity in Arabidopsis. Plant Physiol. Biochem. 2016, 108, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Janicka-Russak, M.; Kabała, K.; Wdowikowska, A.; Kłobus, G. Response of plasma membrane H+-ATPase to low temperature in cucumber roots. J. Plant Res. 2012, 125, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56. [Google Scholar] [CrossRef]
- Yu, S.; Sun, Q.; Wu, J.; Zhao, P.; Sun, Y.; Guo, Z. Genome-wide identification and characterization of short-chain dehydrogenase/reductase (SDR) gene family in medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9498. [Google Scholar] [CrossRef]
- Kumar, M.; Kambham, M.R.; Reddy, D.C.L.; Sriram, S.; Singh, T.H. Identification of molecular marker linked to resistance gene loci against Indian isolate of Phytophthora capsici L. causing root rot in chilli (Capsicum annuum L.). Australas. Plant Pathol. 2022, 51, 211–220. [Google Scholar] [CrossRef]
- Adhab, M.; Angel, C.; Leisner, S.; Schoelz, J.E. The P1 gene of Cauliflower mosaic virus is responsible for breaking resistance in Arabidopsis thaliana ecotype Enkheim (En-2). Virology 2018, 523, 15–21. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606X. [Google Scholar]
- Cao, Y.-Y.; Yang, M.-T.; Chen, S.-Y.; Zhou, Z.-Q.; Li, X.; Wang, X.-J.; Bai, J.-G. Exogenous sucrose influences antioxidant enzyme activities and reduces lipid peroxidation in water-stressed cucumber leaves. Biol. Plant. 2015, 59, 147–153. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Lee, I.-J. Synergistic interaction of fungal endophytes, Paecilomyces formosus LHL10 and Penicillium funiculosum LHL06, in alleviating multi-metal toxicity stress in Glycine max L. Environ. Sci. Pollut. Res. 2021, 28, 67429–67444. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Khan, A.L.; Numan, M.; Bilal, S.; Asaf, S.; Crafword, K.; Imran, M.; Al-Harrasi, A.; Al-Sabahi, J.N.; ur Rehman, N.; Ahmed, A. Mangrove’s rhizospheric engineering with bacterial inoculation improve degradation of diesel contamination. J. Hazard. Mater. 2022, 423, 127046. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Peckys, D.B.; Baudoin, J.-P.; Eder, M.; Werner, U.; De Jonge, N. Epidermal growth factor receptor subunit locations determined in hydrated cells with environmental scanning electron microscopy. Sci. Rep. 2013, 3, 2626. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Waqas, M.; Ullah, I.; Bilal, S.; Kim, Y.-H.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Metabolic and proteomic alteration in phytohormone-producing endophytic Bacillus amyloliquefaciens RWL-1 during methanol utilization. Metabolomics 2019, 15, 16. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Endophytic Paecilomyces formosus LHL10 augments Glycine max L. adaptation to Ni-contamination through affecting endogenous phytohormones and oxidative stress. Front. Plant Sci. 2017, 8, 870. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Asaf, S.; Imran, M.; Al-Harrasi, A.; Lee, I.-J. Efficacy of endophytic SB10 and glycine betaine duo in alleviating phytotoxic impact of combined heat and salinity in Glycine max L. via regulation of redox homeostasis and physiological and molecular responses. Environ. Pollut. 2023, 316, 120658. [Google Scholar] [CrossRef]
- Liu, L.; Han, R.; Yu, N.; Zhang, W.; Xing, L.; Xie, D.; Peng, D. A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS ONE 2018, 13, e0196592. [Google Scholar] [CrossRef]
- Khan, T.; Bilal, S.; Asaf, S.; Alamri, S.S.; Imran, M.; Khan, A.L.; Al-Rawahi, A.; Lee, I.-J.; Al-Harrasi, A. Silicon-Induced Tolerance against Arsenic Toxicity by Activating Physiological, Anatomical and Biochemical Regulation in Phoenix dactylifera (Date Palm). Plants 2022, 11, 2263. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, S.; Khan, T.; Asaf, S.; Khan, N.A.; Saad Jan, S.; Imran, M.; Al-Rawahi, A.; Khan, A.L.; Lee, I.-J.; Al-Harrasi, A. Silicon-Induced Morphological, Biochemical and Molecular Regulation in Phoenix dactylifera L. under Low-Temperature Stress. Int. J. Mol. Sci. 2023, 24, 6036. https://doi.org/10.3390/ijms24076036
Bilal S, Khan T, Asaf S, Khan NA, Saad Jan S, Imran M, Al-Rawahi A, Khan AL, Lee I-J, Al-Harrasi A. Silicon-Induced Morphological, Biochemical and Molecular Regulation in Phoenix dactylifera L. under Low-Temperature Stress. International Journal of Molecular Sciences. 2023; 24(7):6036. https://doi.org/10.3390/ijms24076036
Chicago/Turabian StyleBilal, Saqib, Taimoor Khan, Sajjad Asaf, Nasir Ali Khan, Syed Saad Jan, Muhammad Imran, Ahmed Al-Rawahi, Abdul Latif Khan, In-Jung Lee, and Ahmed Al-Harrasi. 2023. "Silicon-Induced Morphological, Biochemical and Molecular Regulation in Phoenix dactylifera L. under Low-Temperature Stress" International Journal of Molecular Sciences 24, no. 7: 6036. https://doi.org/10.3390/ijms24076036
APA StyleBilal, S., Khan, T., Asaf, S., Khan, N. A., Saad Jan, S., Imran, M., Al-Rawahi, A., Khan, A. L., Lee, I.-J., & Al-Harrasi, A. (2023). Silicon-Induced Morphological, Biochemical and Molecular Regulation in Phoenix dactylifera L. under Low-Temperature Stress. International Journal of Molecular Sciences, 24(7), 6036. https://doi.org/10.3390/ijms24076036