Butein Inhibits Cell Growth by Blocking the IL-6/IL-6Rα Interaction in Human Ovarian Cancer and by Regulation of the IL-6/STAT3/FoxO3a Pathway
Abstract
1. Introduction
2. Results
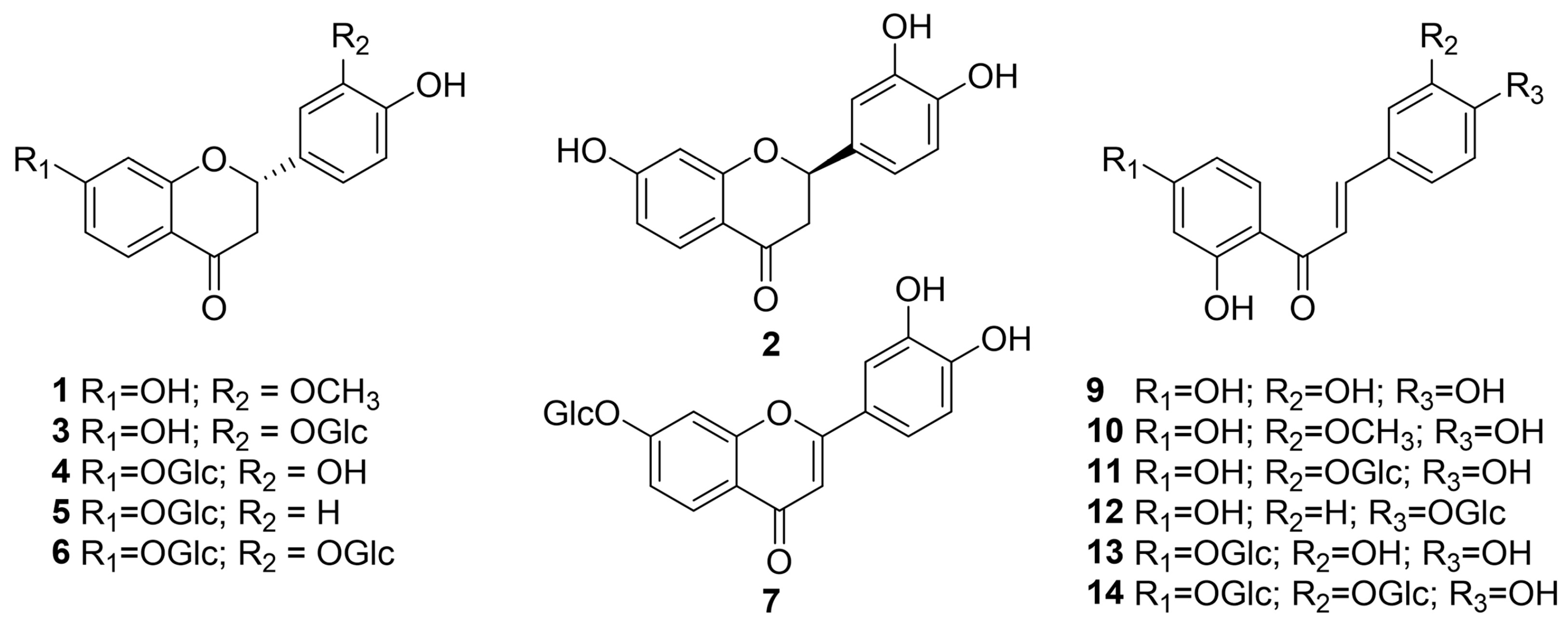
2.1. Identification of Compounds 1–14 from Butea monosperma Flowers
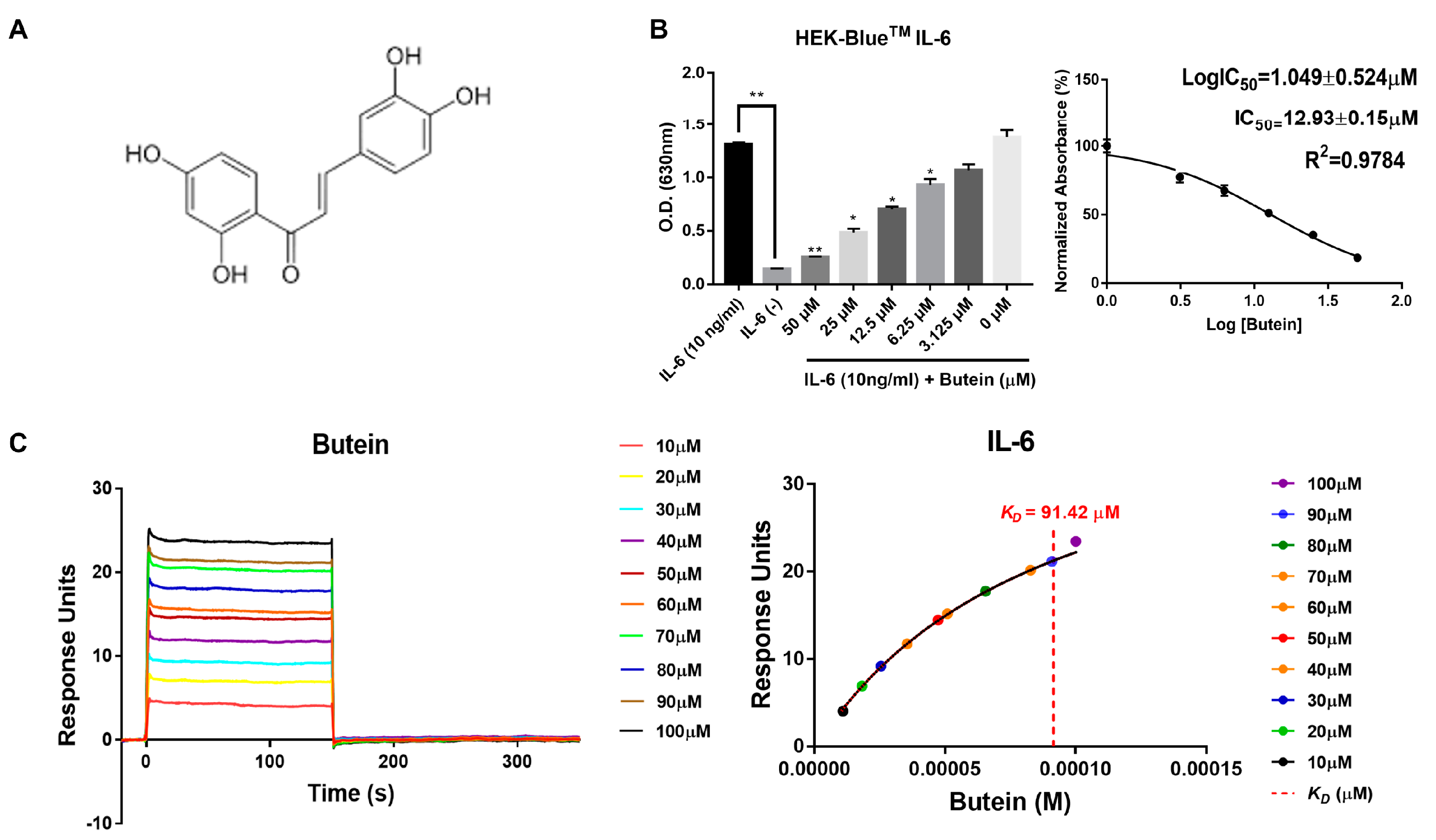
2.2. Characterization of Butein and Anti-IL-6 Activity In Vitro
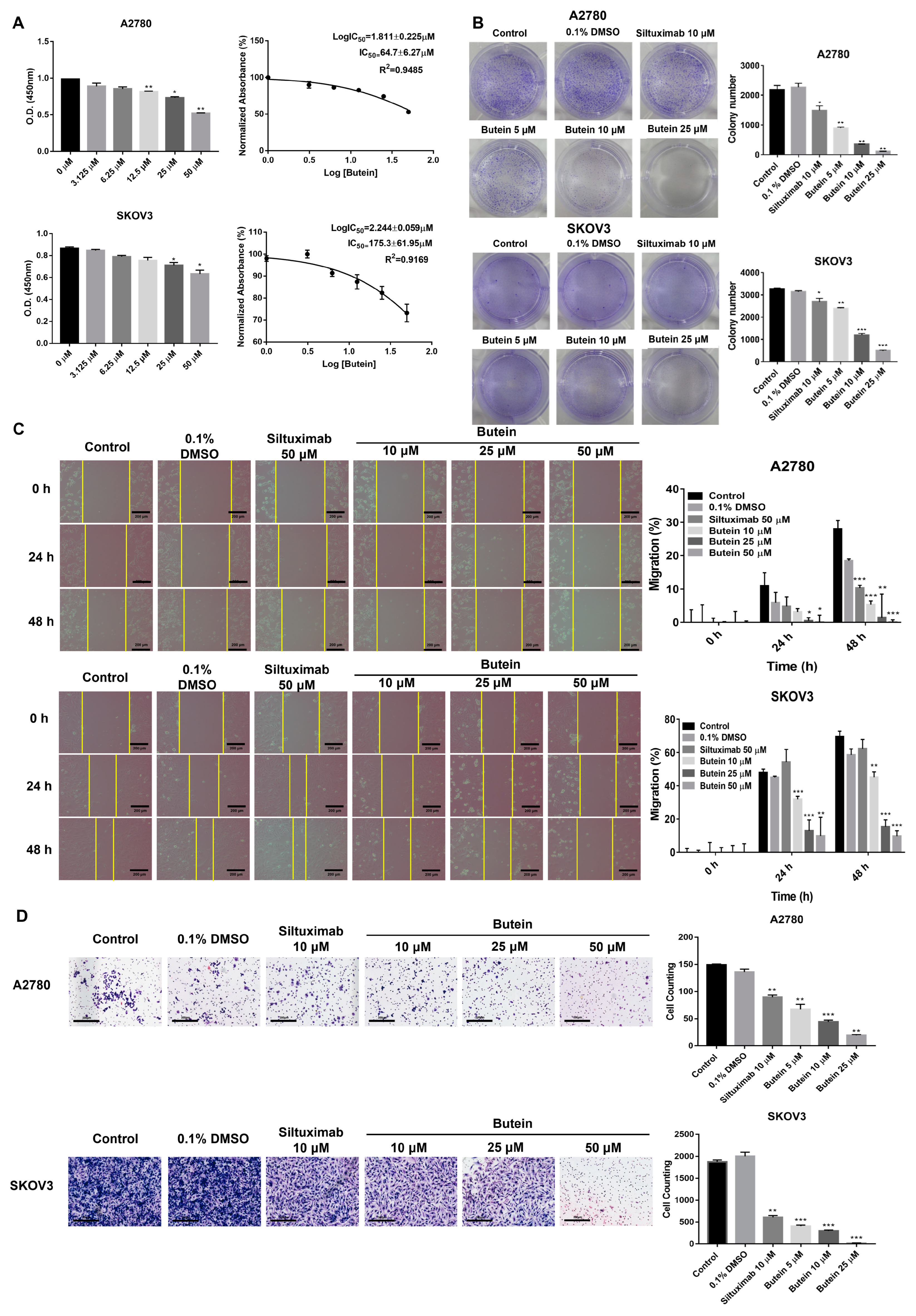
2.3. Butein Suppresses the Cell Viability, Migration, and Invasion of Ovarian Cancer Cells
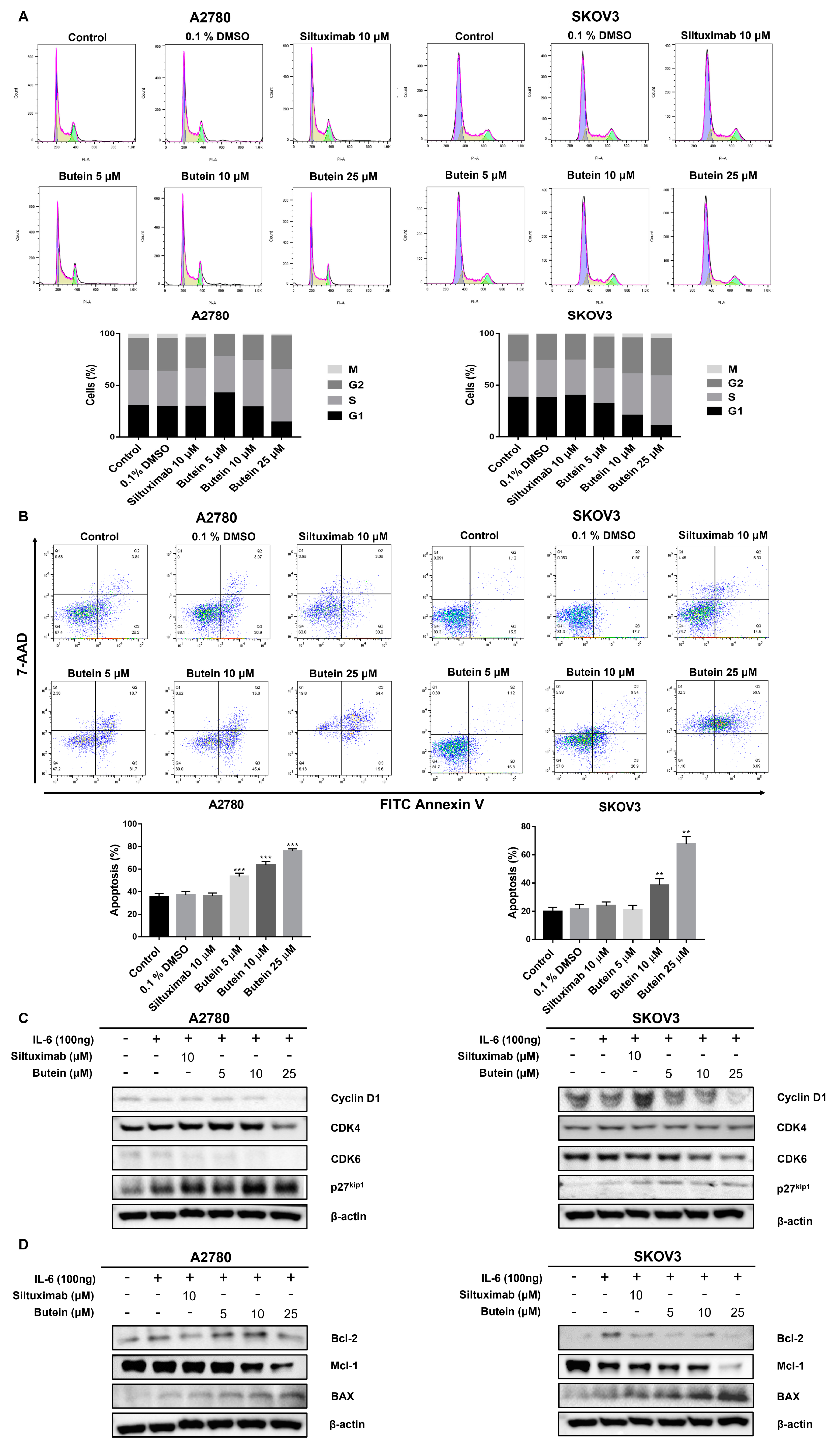
2.4. Butein Induced Ovarian Cancer Cell Cycle Arrest and Cell Apoptosis
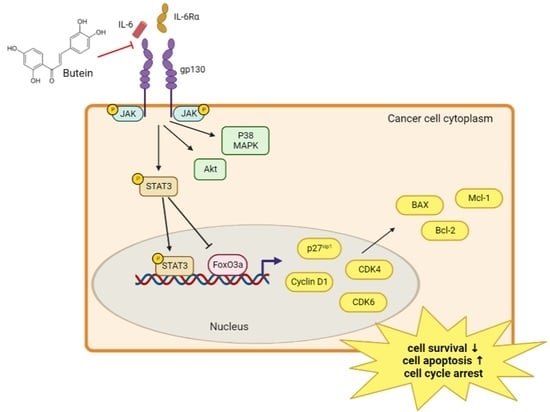
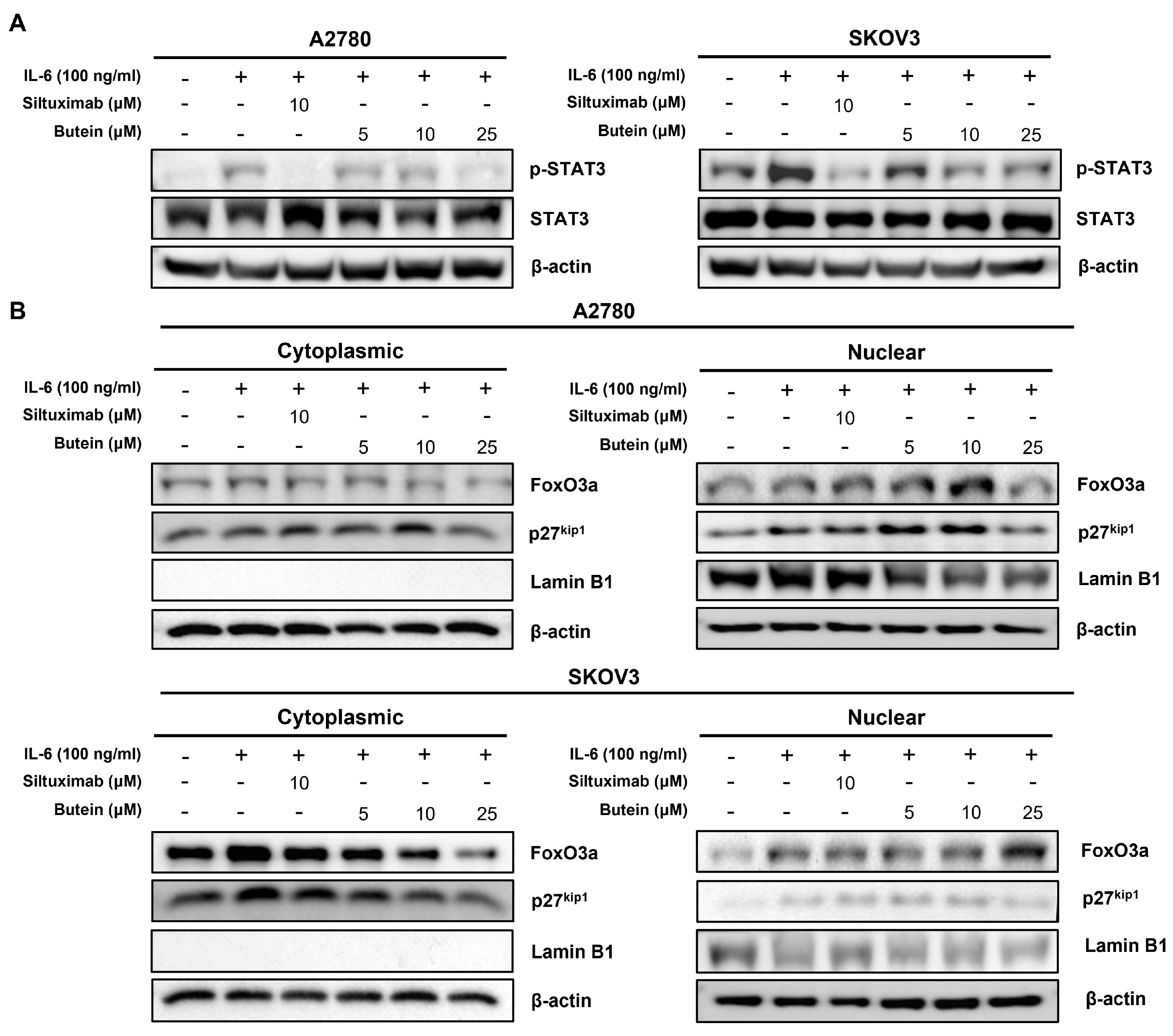
2.5. Butein Inhibited STAT3 Phosphorylation and Induced Intranuclear Accumulation of FoxO3a through Inhibition of IL-6 Signaling
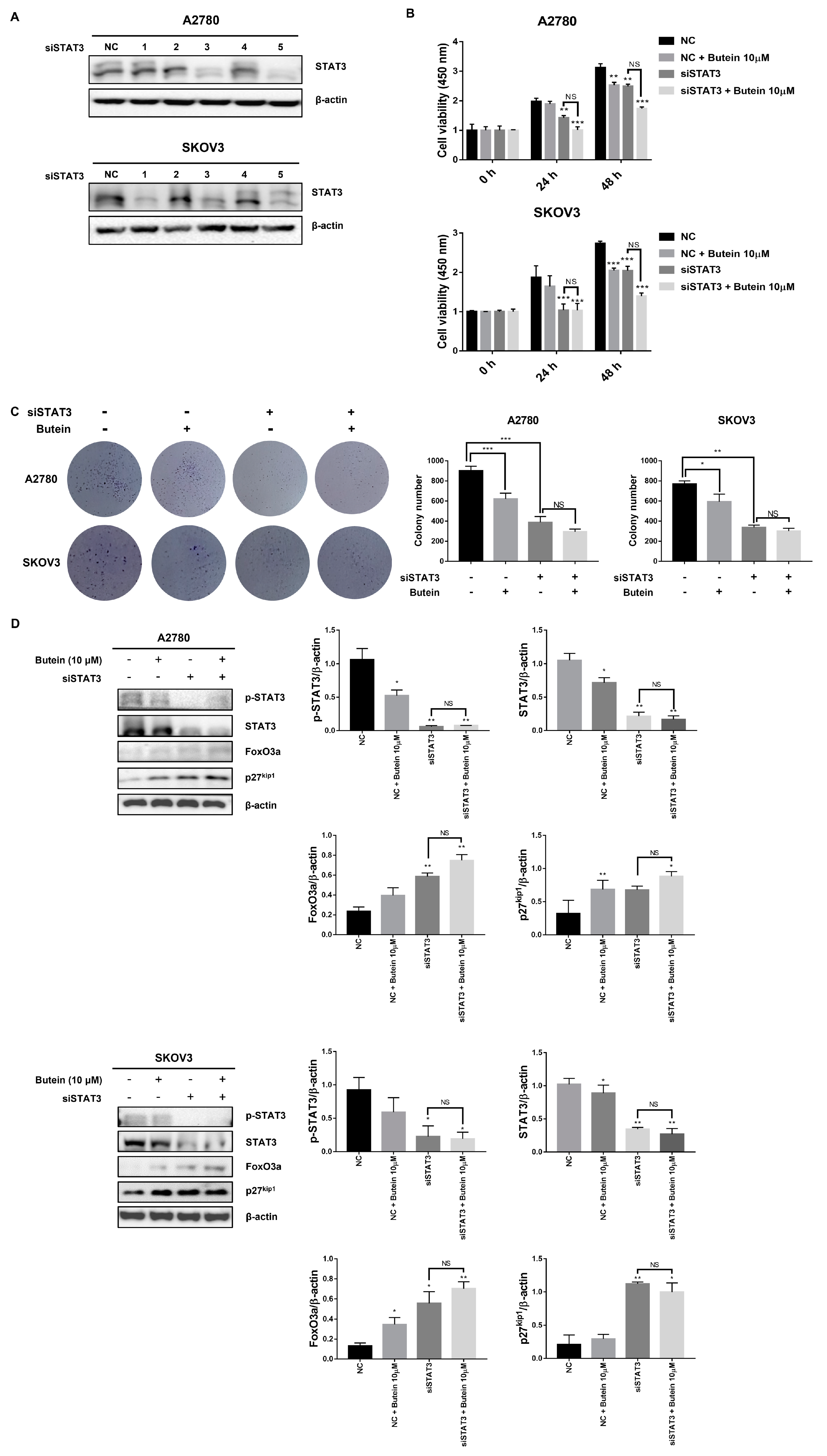
2.6. Butein Increased Protein Expression of FoxO3a and p27kip1 through Inactivation of STAT3
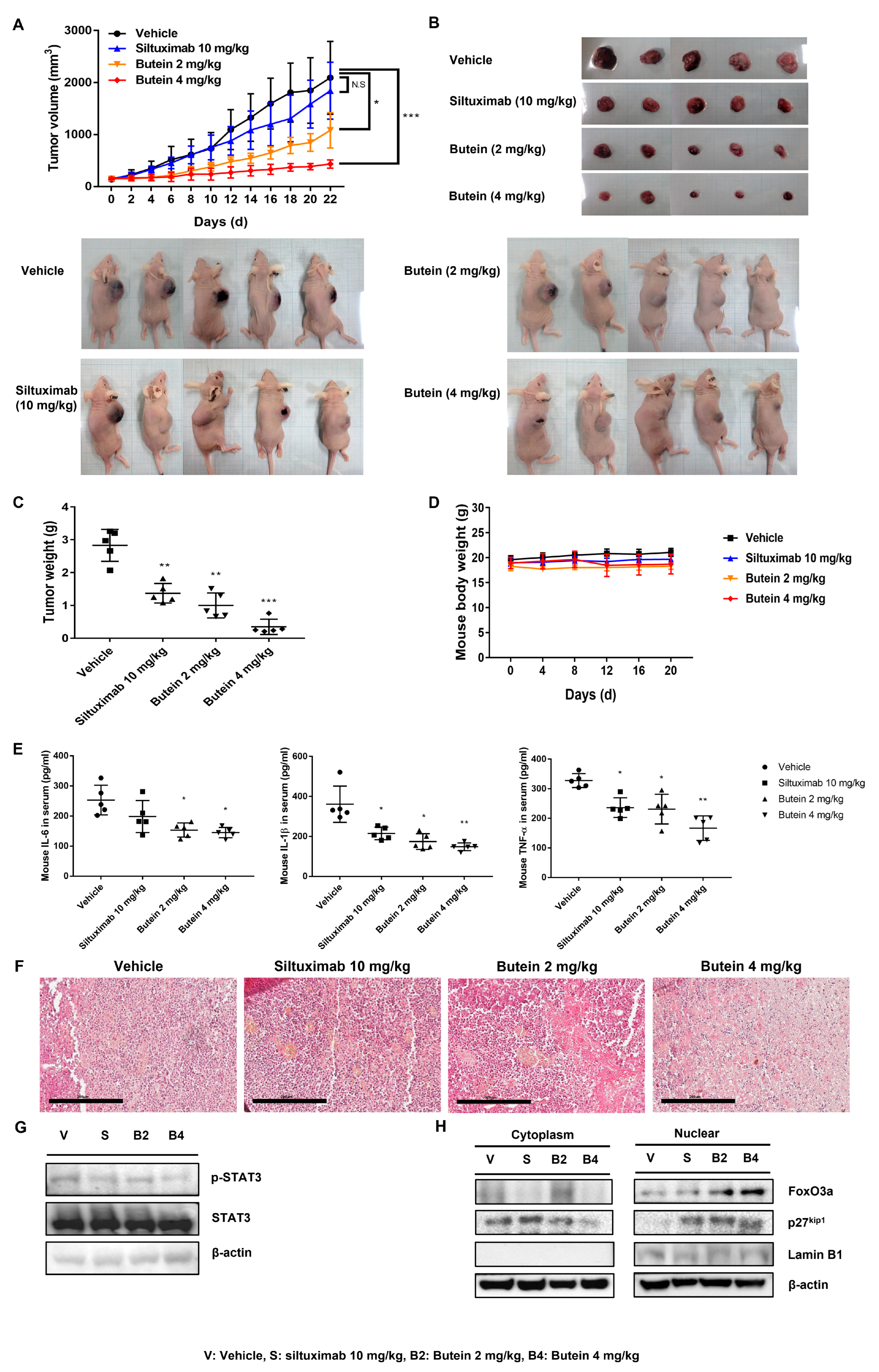
2.7. Butein Exerts an Antitumor Effect In Vivo on Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. IL-6 Inhibitory Bioassay with HEK-Blue™ IL-6 Cells
4.5. Surface Plasmon Resonance Assay
4.6. Analysis of Cell Viability by the MTT Assay
4.7. Clonogenic Formation Assay
4.8. Wound-Healing Assay
4.9. Matrigel Invasion Assay
4.10. Flow Cytometric Analysis of Cell Cycle and Apoptosis
4.11. Small Interfering RNA (siRNA) Transfection
4.12. Western Blotting
4.13. Mouse Xenografts
4.14. Hematoxylin and Eosin (H&E) Staining in Mouse Tumor Tissues
4.15. Enzyme-Linked Immunosorbent Assay (ELISA)
4.16. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kim, S.; Han, Y.; Kim, S.I.; Kim, H.-S.; Kim, S.J.; Song, Y.S. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis. Oncol. 2018, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Woodman, M.; Ribeiro, J.R. Prognostic immunologic signatures in epithelial ovarian cancer. Oncogene 2022, 41, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, B.K.; Saraf, M.N. A comprehensive review on pharmacological profile of Butea monosperma (Lam.) Taub. J. Appl. Pharm. Sci. 2015, 5, 159–166. [Google Scholar] [CrossRef]
- Somani, R.; Kasture, S.; Singhai, A.K. Antidiabetic potential of Butea monosperma in rats. Fitoterapia 2006, 77, 86–90. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.; Gaur, K.; Singh, A.; Kumar, L. A review on pharmacognostic study of Butea monosperma. Int. J. Res. Ayurveda Pharm. 2017, 8, 196–199. [Google Scholar] [CrossRef]
- Chokchaisiri, R.; Suaisom, C.; Sriphota, S.; Chindaduang, A.; Chuprajob, T.; Suksamrarn, A. Bioactive flavonoids of the flowers of Butea monosperma. Chem. Pharm. Bull. 2009, 57, 428–432. [Google Scholar] [CrossRef]
- Yang, P.Y.; Hu, D.N.; Kao, Y.H.; Lin, I.; Liu, F.S. Butein induces apoptotic cell death of human cervical cancer cells. Oncol. Lett. 2018, 16, 6615–6623. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Hu, D.-N.; Lin, I.-C.; Liu, F.-S. Butein shows cytotoxic effects and induces apoptosis in human ovarian cancer cells. Am. J. Chin. Med. 2015, 43, 769–782. [Google Scholar] [CrossRef]
- Bai, X.; Ma, Y.; Zhang, G. Butein suppresses cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol. Rep. 2015, 33, 3085–3092. [Google Scholar] [CrossRef]
- Seo, W.Y.; Youn, G.S.; Choi, S.Y.; Park, J. Butein, a tetrahydroxychalcone, suppresses pro-inflammatory responses in HaCaT keratinocytes. BMB Rep. 2015, 48, 495. [Google Scholar] [CrossRef]
- Cioce, M.; Sacconi, A.; Pass, H.I.; Canino, C.; Strano, S.; Blandino, G.; Fazio, V.M. Insights into intra-tumoral heterogeneity: Transcriptional profiling of chemoresistant MPM cell subpopulations reveals involvement of NFkB and DNA repair pathways and contributes a prognostic signature. Int. J. Mol. Sci. 2021, 22, 12071. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Choe, J.W.; Lee, B.J.; Kang, M.H.; Joo, M.K.; Kim, J.H.; Yeon, J.E.; Park, J.-J.; Kim, J.S.; Bak, Y.-T. Butein effects in colitis and interleukin-6/signal transducer and activator of transcription 3 expression. World J. Gastroenterol. WJG 2015, 21, 465. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Butein activates autophagy through AMPK/TSC2/ULK1/mTOR pathway to inhibit IL-6 expression in IL-1β stimulated human chondrocytes. Cell. Physiol. Biochem. 2018, 49, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.Y.; Davies, D.M.; Maher, J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem. Soc. Trans. 2018, 46, 1449–1462. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Browning, L.; Patel, M.; Bring Horvath, E.; Tawara, K.; Jorcyk, C. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018, 10, 6685–6693. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Huang, L.-B.; Lin, W.-H.; Wang, L.-N.; Tian, Y.; Shi, D.; Wang, J.; Qin, G.; Li, A.; Liang, Y.-N. Butein inhibits cell proliferation and induces cell cycle arrest in acute lymphoblastic leukemia via FOXO3a/p27kip1 pathway. Oncotarget 2016, 7, 18651. [Google Scholar] [CrossRef]
- Oh, H.-M.; Yu, C.-R.; Golestaneh, N.; Amadi-Obi, A.; Lee, Y.S.; Eseonu, A.; Mahdi, R.M.; Egwuagu, C.E. STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J. Biol. Chem. 2011, 286, 30888–30897. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.-N.; Zhou, X.-Q.; Zhang, Y.-Y.; Zhu, X.-Y.; Yue, R.-M.; Hui, Y.; Chen, W.-H. Chemical Constituents of Psychotria hainanensis. Chem. Nat. Compd. 2020, 56, 533–534. [Google Scholar] [CrossRef]
- Junior, G.M.V.; de M. Sousa, C.M.; Cavalheiro, A.J.; Lago, J.H.G.; Chaves, M.H. Phenolic derivatives from fruits of Dipteryx lacunifera Ducke and evaluation of their antiradical activities. Helv. Chim. Acta 2008, 91, 2159–2167. [Google Scholar] [CrossRef]
- Than, N.N.; Yangon, M.; Fiebig, H.-H.; Laatsch, H. Investigation of Bioactive Constituents and the Antitumour principle from Butea monosperma. Head Neck 2016, 4, 21. [Google Scholar]
- Jin, Q.; Lee, C.; Lee, J.-W.; Lee, I.-S.; Lee, M.-K.; Jeon, W.-K.; Hwang, B.-Y. Chemical constituents from the fruits of Prunus mume. Nat. Prod. Sci. 2012, 18, 200–203. [Google Scholar]
- Jassbi, A.R.; Singh, P.; Krishna, V.; Gupta, P.K.; Tahara, S. Antioxidant study and assignments of NMR spectral data for 3′, 4′, 7-trihydroxyflavanone 3′, 7-di-O-β-D-glucopyranoside (butrin) and its hydrolyzed product. Chem. Nat. Compd. 2004, 40, 250–253. [Google Scholar] [CrossRef]
- Do Nascimento, A.M.; de Oliveira, D.C. 5-deoxyflavone glycoside from Calea uniflora L. (Asteraceae). Biochem. Syst. Ecol. 2004, 32, 1079–1081. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Kim, J.H.; Park, S.-J.; Chang, J.S.; Rho, M.-C.; Bae, K.-H.; Park, K.H.; Lee, W.S. Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorganic Med. Chem. Lett. 2010, 20, 971–974. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Buraparuangsang, P.; Worachartcheewan, A.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar] [CrossRef]
- Roh, K.; Lee, J.-h.; Kang, H.; Park, K.W.; Song, Y.; Lee, S.; Ku, J.-M. Synthesis and evaluation of butein derivatives for in vitro and in vivo inflammatory response suppression in lymphedema. Eur. J. Med. Chem. 2020, 197, 112280. [Google Scholar] [CrossRef]
- Lima, T.C.; Souza, R.J.; Santos, A.D.; Moraes, M.H.; Biondo, N.E.; Barison, A.; Steindel, M.; Biavatti, M.W. Evaluation of leishmanicidal and trypanocidal activities of phenolic compounds from Calea uniflora Less. Nat. Prod. Res. 2016, 30, 551–557. [Google Scholar] [CrossRef]
- Heo, T.-H.; Wahler, J.; Suh, N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget 2016, 7, 15460. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Cioce, M.; Rutigliano, D.; Puglielli, A.; Fazio, V.M. Butein-instigated miR-186-5p-dependent modulation of TWIST1 affects resistance to cisplatin and bioenergetics of Malignant Pleural Mesothelioma cells. Cancer Drug Resist. 2022, 5, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, M.K.; Choi, Y.K.; Shin, Y.C.; Cho, S.-G.; Ko, S.-G. Rhus verniciflua Stokes (RVS) and butein induce apoptosis of paclitaxel-resistant SKOV-3/PAX ovarian cancer cells through inhibition of AKT phosphorylation. BMC Complement. Altern. Med. 2016, 16, 122. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, X.; Li, C.; Zhao, L.; Zhou, Y.; Hou, H. Butein sensitizes HeLa cells to cisplatin through the AKT and ERK/p38 MAPK pathways by targeting FoxO3a. Int. J. Mol. Med. 2015, 36, 957–966. [Google Scholar] [CrossRef]
- Wang, J.; Sun, T.; Meng, Z.; Wang, L.; Li, M.; Chen, J.; Qin, T.; Yu, J.; Zhang, M.; Bie, Z. XPO1 inhibition synergizes with PARP1 inhibition in small cell lung cancer by targeting nuclear transport of FOXO3a. Cancer Lett. 2021, 503, 197–212. [Google Scholar] [CrossRef]
- Hui, R.C.; Francis, R.E.; Guest, S.K.; Costa, J.R.; Gomes, A.R.; Myatt, S.S.; Brosens, J.J.; Lam, E.W. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol. Cancer Ther. 2008, 7, 670–678. [Google Scholar] [CrossRef]
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS ONE 2015, 10, e0144245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-A.; Seo, Y.J.; Kim, L.K.; Kim, H.J.; Yoon, K.D.; Heo, T.-H. Butein Inhibits Cell Growth by Blocking the IL-6/IL-6Rα Interaction in Human Ovarian Cancer and by Regulation of the IL-6/STAT3/FoxO3a Pathway. Int. J. Mol. Sci. 2023, 24, 6038. https://doi.org/10.3390/ijms24076038
Park S-A, Seo YJ, Kim LK, Kim HJ, Yoon KD, Heo T-H. Butein Inhibits Cell Growth by Blocking the IL-6/IL-6Rα Interaction in Human Ovarian Cancer and by Regulation of the IL-6/STAT3/FoxO3a Pathway. International Journal of Molecular Sciences. 2023; 24(7):6038. https://doi.org/10.3390/ijms24076038
Chicago/Turabian StylePark, Sun-Ae, Young Ju Seo, Lee Kyung Kim, Hee Jung Kim, Kee Dong Yoon, and Tae-Hwe Heo. 2023. "Butein Inhibits Cell Growth by Blocking the IL-6/IL-6Rα Interaction in Human Ovarian Cancer and by Regulation of the IL-6/STAT3/FoxO3a Pathway" International Journal of Molecular Sciences 24, no. 7: 6038. https://doi.org/10.3390/ijms24076038
APA StylePark, S.-A., Seo, Y. J., Kim, L. K., Kim, H. J., Yoon, K. D., & Heo, T.-H. (2023). Butein Inhibits Cell Growth by Blocking the IL-6/IL-6Rα Interaction in Human Ovarian Cancer and by Regulation of the IL-6/STAT3/FoxO3a Pathway. International Journal of Molecular Sciences, 24(7), 6038. https://doi.org/10.3390/ijms24076038