Insecticide Resistance and Its Management in Two Invasive Cryptic Species of Bemisia tabaci in China
Abstract
:1. Introduction: The Whitefly Bemisia tabaci
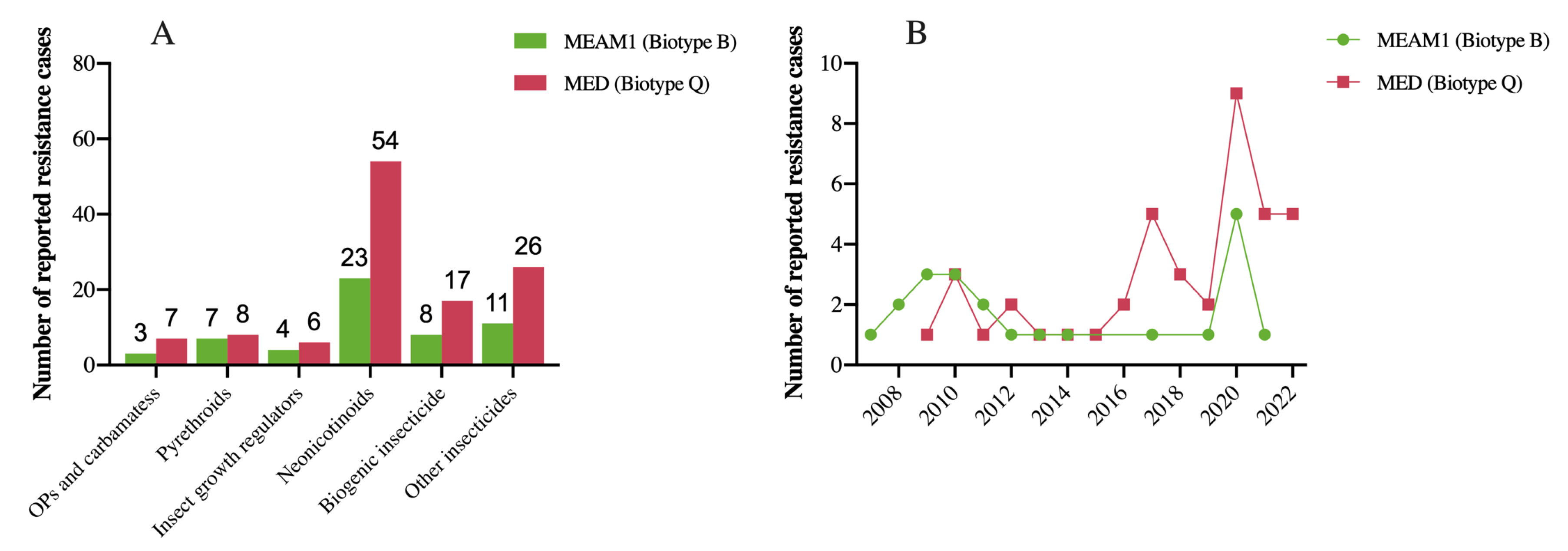
2. B. tabaci Resistance to Pesticides of Different Classes
2.1. Organophosphates (OPs) and Carbamates
2.2. Pyrethroids
2.3. Insect Growth Regulators (IGRs)
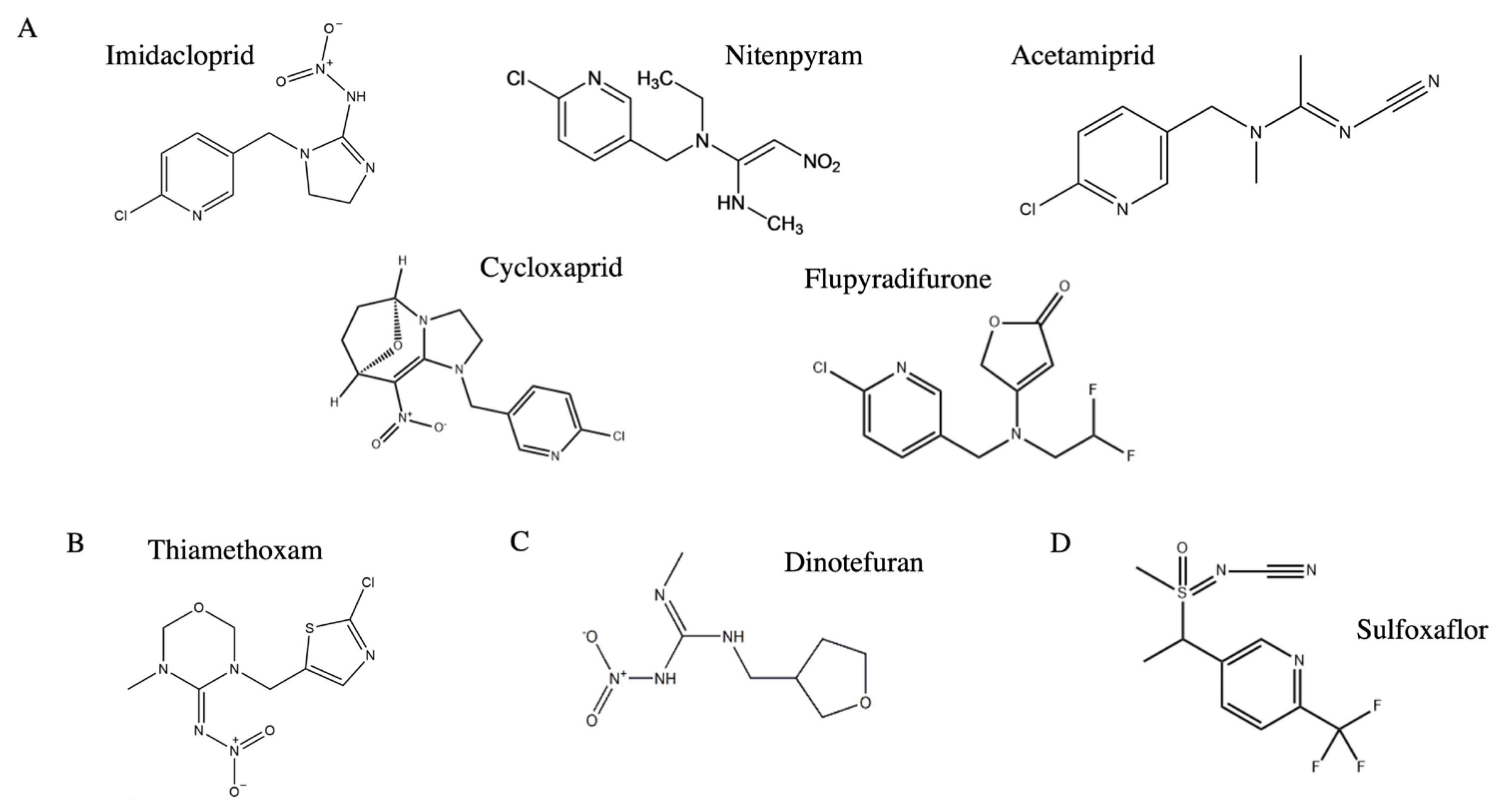
2.4. Neonicotinoids
2.5. Biogenic Insecticide
2.6. Other Insecticides
2.7. Cross-Resistance
| Insecticide | Resistance Mechanism | Associated Gene | References |
|---|---|---|---|
| Organophosphates (ops) Methamidophos Phoxim Chlorpyrifos Profenofos Acephate Malathion | Target resistance | Acetylcholinesterase ace1 gene | [14,18,20,21] |
| Pyrethroids Bifenthrin Cypermethrin | Target resistance | Para-type voltage-gated sodium channel gene | [14,18] |
| Neonicotinoids Dinotefuran Imidacloprid Thiamethoxam Acetamiprid Sulfoxaflor Flupyradifurone Acetamiprid | Metabolic resistance | Cytochrome P450 gene; glutathione-S-transferase gene (GST); nicotinic acetylcholine receptor β1 subunit gene (Btβ1); and ATP-binding cassette subfamily G member 3 gene (ABCG3) | [8,9,18,28,29,32,33,34,35,39,47] |
| Biogenic insecticides Abamectin Afidopyropen | Metabolic resistance | Cytochrome P450 gene and glutathione-S-transferase gene (GST) | [40,48] |
| Other insecticides Pymetrozine Cyantraniliprole | Metabolic resistance | Cytochrome P450 gene | [28] |
3. Management of B. tabaci Resistance
3.1. Insecticide Rotation
3.2. Improvement in Insecticide Efficiency
3.3. Synergists
3.4. Bacteriostatic and Insecticidal Compounds
3.5. New Prevention and Control Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Liu, S. Whitefly interactions with plants. Curr. Opin. Insect Sci. 2017, 19, 70–75. [Google Scholar] [CrossRef]
- Zhou, Y. Directory of whiteflies in China. Chin. J. Entomol. 1949, 3, 1–18. [Google Scholar]
- Luo, C.; Yao, Y.; Wang, R.; Yan, F.; Hu, D.; Zhang, Z. The use of mitochondrial cytochrome oxidaseI (mtCOI) gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius)in China. Acta Entomol. Sin. 2002, 45, 759–763. [Google Scholar]
- Chu, D.; Zhang, Y.-J.; Brown, J.K.; Cong, B.; Xu, B.-Y.; Wu, Q.-J.; Zhu, G.-R. The Introduction Of The Exotic Q Biotype Of Bemisia Tabaci From The Mediterranean Region Into China On Ornamental Crops. Fla. Entomol. 2006, 89, 168–194. [Google Scholar] [CrossRef]
- Pan, H.; Chu, D.; Ge, D.; Wang, S.; Wu, Q.; Xie, W.; Jiao, X.; Liu, B.; Yang, X.; Yang, N.; et al. Further spread of and domination by Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q on field crops in China. J. Econ. Entomol. 2011, 104, 978–985. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, W.; Wang, S.; Wu, Q.; Zhou, X.; Zhang, Y. Dynamic monitoring (B versus Q) and further resistance status of Q-type Bemisia tabaci in China. Crop Prot. 2017, 94, 115–122. [Google Scholar] [CrossRef]
- Yang, X.; Xie, W.; Wang, S.L.; Wu, Q.J.; Pan, H.P.; Li, R.M.; Yang, N.N.; Liu, B.M.; Xu, B.Y.; Zhou, X.; et al. Two cytochrome P450 genes are involved in imidacloprid resistance in field populations of the whitefly, Bemisia tabaci, in China. Pestic. Biochem. Physiol. 2013, 107, 343–350. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Jia, Z.; Ahmat, T.; Xie, L.; Jiang, W. Resistance to neonicotinoid insecticides and expression changes of eighteen cytochrome P450 genes in field populations of Bemisia tabaci from Xinjiang, China. Entomol. Res. 2020, 50, 205–211. [Google Scholar] [CrossRef]
- Guo, L.; Lv, H.; Tan, D.; Liang, N.; Guo, C.; Chu, D. Resistance to insecticides in the field and baseline susceptibility to cyclaniliprole of whitefly Bemisia tabaci (Gennadius) in China. Crop Prot. 2020, 130, 105065. [Google Scholar] [CrossRef]
- Yao, F.; Zheng, Y.; Huang, X.; Ding, X.; Zhao, J.; Desneux, N.; He, Y.; Weng, Q. Dynamics of Bemisia tabaci biotypes and insecticide resistance in Fujian province in China during 2005–2014. Sci. Rep. 2017, 7, 40803. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, S.; Zhou, J.; Du, Y.; Zhang, Y.; Wang, J. Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot. 2012, 31, 67–71. [Google Scholar] [CrossRef]
- Kang, C.Y.; Wu, G.; Miyata, T. Synergism of enzyme inhibitors and mechanisms of insecticide resistance in Bemisia tabaci (Gennadius) (Hom., Aleyrodidae). J. Appl. Entomol. 2006, 130, 377–385. [Google Scholar] [CrossRef]
- Qiu, B.; Liu, L.; Li, X.; Mathur, V.; Qin, Z.; Ren, S. Genetic mutations associated with chemical resistance in the cytochrome P450 genes of invasive and native Bemisia tabaci (Hemiptera: Aleyrodidae) populations in China. Insect Sci. 2009, 16, 237–245. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Zheng, Y.; Weng, Q.; Biondi, A.; Desneux, N.; Wu, K. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int. J. Biol. Sci. 2013, 9, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Che, W.; Wang, J.; Luo, C. Monitoring insecticide resistance and diagnostics of resistance mechanisms in Bemisia tabaci Mediterranean (Q biotype) in China. Pestic. Biochem. Physiol. 2020, 163, 117–122. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Chen, P.; Li, H.; Ma, J.; Liu, Y.; Wang, K. Bemisia tabaci (Hemiptera: Aleyrodidae) Insecticide Resistance in Shandong Province, China. J. Econ. Entomol. 2019, 113, 911–917. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Yang, F.; Dong, S.; Han, Z. Resistance mechanisms to chlorpyrifos and F392W mutation frequencies in the acetylcholine esterase ace1 allele of field populations of the tobacco whitefly, Bemisia tabaci in China. J. Insect Sci. 2011, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Alon, M.; Alon, F.; Nauen, R.; Morin, S. Organophosphates’ resistance in the B-biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1-type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem. Mol. Biol. 2008, 38, 940–949. [Google Scholar] [CrossRef]
- Ma, D.; Gorman, K.; Devine, G.; Luo, W.; Denholm, I. The biotype and insecticide-resistance status of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), invading cropping systems in Xinjiang Uygur Autonomous Region, northwestern China. Crop Prot. 2007, 26, 612–617. [Google Scholar] [CrossRef]
- Luo, C.; Jones, C.M.; Devine, G.; Zhang, F.; Denholm, I.; Gorman, K. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 2010, 29, 429–434. [Google Scholar] [CrossRef]
- Wei, Y.; Guan, F.; Wang, R.; Qu, C.; Luo, C. Amplicon sequencing detects mutations associated with pyrethroid resistance in Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2021, 77, 2914–2923. [Google Scholar] [CrossRef]
- Xie, W.; Liu, Y.; Wang, S.; Wu, Q.; Pan, H.; Yang, X.; Guo, L.; Zhang, Y. Sensitivity of Bemisia tabaci (Hemiptera: Aleyrodidae) to several new insecticides in China: Effects of insecticide type and whitefly species, strain, and stage. J. Insect Sci. 2014, 14, 261. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, W.; Fu, B.; Xiao, S.; Tan, X.; Ji, Y.; Cheng, J.; Wang, R.; Liu, B.; Yang, X.; et al. Annual analysis of field-evolved insecticide resistance in Bemisia tabaci across China. Pest Manag. Sci. 2021, 77, 2990–3001. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, Q.; Xu, B.; Wang, S.; Chang, X.; Xie, W.; Zhang, Y. Fitness costs and morphological change of laboratory-selected thiamethoxam resistance in the B-type Bemisia tabaci (Hemiptera: Aleyrodidae). J. Appl. Entomol. 2009, 133, 466–472. [Google Scholar] [CrossRef]
- Rao, Q.; Xu, Y.-h.; Luo, C.; Zhang, H.-y.; Jones, C.M.; Devine, G.J.; Gorman, K.; Denholm, I. Characterisation of Neonicotinoid and Pymetrozine Resistance in Strains of Bemisia tabaci (Hemiptera: Aleyrodidae) from China. J. Integr. Agric. 2012, 11, 321–326. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Han, G.; Du, Y.; Wang, J. Lack of cross-resistance between neonicotinoids and sulfoxaflor in field strains of Q-biotype of whitefly, Bemisia tabaci, from eastern China. Pestic. Biochem. Physiol. 2017, 136, 46–51. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Che, W.; Wang, J.; Luo, C. Genetics and fitness costs of resistance to flupyradifurone in Bemisia tabaci from China. J. Integr. Agric. 2022, 21, 1436–1443. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Wu, Y. Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Cao, Q.; Li, G.; Ma, D. Role of several cytochrome P450s in the resistance and cross-resistance against imidacloprid and acetamiprid of Bemisia tabaci (Hemiptera: Aleyrodidae) MEAM1 cryptic species in Xinjiang, China. Pestic. Biochem. Physiol. 2020, 163, 209–215. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, C.; Feng, Y.; Li, W.; Shao, X.; Xu, Z.; Cheng, J.; Li, Z. Computational Insights into the Different Resistance Mechanism of Imidacloprid versus Dinotefuran in Bemisia tabaci. J. Agric. Food Chem. 2016, 64, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Zhang, J.; Che, W.; Feng, H.; Luo, C. Characterization of flupyradifurone resistance in the whitefly Bemisia tabaci Mediterranean (Q biotype). Pest Manag. Sci. 2020, 76, 4286–4292. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef]
- He, C.; Xie, W.; Yang, X.; Wang, S.; Wu, Q.; Zhang, Y. Identification of glutathione S-transferases in Bemisia tabaci (Hemiptera: Aleyrodidae) and evidence that GSTd7 helps explain the difference in insecticide susceptibility between B. tabaci Middle East-Minor Asia 1 and Mediterranean. Insect Mol. Biol. 2018, 27, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, X.; Yang, J.; Du, T.; Yin, C.; Fu, B.; Huang, M.; Liang, J.; Gong, P.; Liu, S.; et al. Epitranscriptomic regulation of insecticide resistance. Sci. Adv. 2021, 7, eabe5903. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, Q.; Wang, S.; Chang, X.; Xie, W.; Xu, B.; Zhang, Y. Cross-resistance study and biochemical mechanisms of thiamethoxam resistance in B-biotype Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. Former. Pestic. Sci. 2010, 66, 313–318. [Google Scholar] [CrossRef]
- Yang, X.; He, C.; Xie, W.; Liu, Y.; Xia, J.; Yang, Z.; Guo, L.; Wen, Y.; Wang, S.; Wu, Q.; et al. Glutathione S-transferases are involved in thiamethoxam resistance in the field whitefly Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2016, 134, 73–78. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y. Cross-resistance and biochemical mechanisms of abamectin resistance in the B-type Bemisia tabaci. J. Appl. Entomol. 2007, 131, 98–103. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Yang, X.; Xie, W.; Wu, Q. Resistance Monitoring for Eight Insecticides on the Sweetpotato Whitefly (Hemiptera: Aleyrodidae) in China. J. Econ. Entomol. 2017, 110, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, H.; Xu, W.; Liu, J.; Geng, Z.; Chu, D.; Guo, L. Pymetrozine-resistant whitefly Bemisia tabaci (Gennadius) populations in China remain susceptible to afidopyropen. Crop Prot. 2021, 149, 105757. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Zhou, X.; Zhang, M.; Yang, Q.; Su, Q.; Luo, C. Characterization of Field-Evolved Resistance to Afidopyropen, a Novel Insecticidal Toxin Developed from Microbial Secondary Metabolites, in Bemisia tabaci. Toxins 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Che, W.; Wang, J.; Qu, C.; Luo, C. Cross-resistance and biochemical mechanism of resistance to cyantraniliprole in a near-isogenic line of whitefly Bemisia tabaci Mediterranean (Q biotype). Pestic. Biochem. Physiol. 2020, 167, 104590. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Shuai, S.; Miao, C.; Chi, B.; Chen, P.; Wang, K.; Li, H.; Liu, Y. Resistance of Bemisia tabaci Mediterranean (Q-biotype) to pymetrozine: Resistance risk assessment, cross-resistance to six other insecticides and detoxification enzyme assay. Pest Manag. Sci. 2021, 77, 2114–2121. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Che, W.; Sun, Y.; Li, W.; Luo, C. Characterization of field-evolved resistance to cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 2019, 18, 2571–2578. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Che, W.; Fang, Y.; Luo, C. Baseline susceptibility and biochemical mechanism of resistance to flupyradifurone in Bemisia tabaci. Crop Protect. 2020, 132, 105132. [Google Scholar] [CrossRef]
- Wang, R.; Fang, Y.; Che, W.; Zhang, Q.; Wang, J.; Luo, C. Metabolic Resistance in Abamectin-Resistant Bemisia tabaci Mediterranean from Northern China. Toxins 2022, 14, 424. [Google Scholar] [CrossRef]
- Xie, W.; Meng, Q.S.; Wu, Q.J.; Wang, S.L.; Yang, X.; Yang, N.N.; Li, R.M.; Jiao, X.G.; Pan, H.P.; Liu, B.M.; et al. Pyrosequencing the Bemisia tabaci transcriptome reveals a highly diverse bacterial community and a robust system for insecticide resistance. PLoS ONE 2012, 7, e35181. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cai, T.; Ren, Z.; Liu, Y.; Yuan, M.; Cai, Y.; Yu, C.; Shu, R.; He, S.; Li, J.; et al. Decline in symbiont-dependent host detoxification metabolism contributes to increased insecticide susceptibility of insects under high temperature. ISME J. 2021, 15, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhang, Y.; Cai, T.; Deng, X.; Liu, C.; Li, J.; He, S.; Li, J.; Wan, H. Antibiotics increased host insecticide susceptibility via collapsed bacterial symbionts reducing detoxification metabolism in the brown planthopper, Nilaparvata lugens. J. Pest Sci. 2020, 94, 757–767. [Google Scholar] [CrossRef]
- Brumin, M.; Lebedev, G.; Kontsedalov, S.; Ghanim, M. Levels of the endosymbiont Rickettsia in the whitefly Bemisia tabaci are influenced by the expression of vitellogenin. Insect Mol. Biol. 2020, 29, 241–255. [Google Scholar] [CrossRef]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Yuan, M.; Yang, K.; Guo, L.; Chu, D. Influences of endosymbiont Cardinium on the insecticide tolerance of Bemisia tabaci MED (Hemiptera: Aleyrodidae). Acta Entomol. Sin. 2021, 64, 1168–1175. [Google Scholar]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-Generation Insect-Resistant Plants: RNAi-Mediated Crop Protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.; Ma, D.; Zhong, S.; Liu, X.; Xin, Z. Nucleic acid pesticides—The new plant protection products with great potential. Chin. J. Pestic. Sci. 2019, 21, 681–691. [Google Scholar]
- Gong, C.; Yang, Z.; Hu, Y.; Wu, Q.; Wang, S.; Guo, Z.; Zhang, Y. Silencing of the BtTPS genes by transgenic plant-mediated RNAi to control Bemisia tabaci MED. Pest Manag. Sci 2022, 78, 1128–1137. [Google Scholar] [CrossRef]
- He, C.; Liu, S.; Liang, J.; Zeng, Y.; Wang, S.; Wu, Q.; Xie, W.; Zhang, Y. Genome-wide identification and analysis of nuclear receptors genes for lethal screening against Bemisia tabaci Q. Pest Manag. Sci 2020, 76, 2040–2048. [Google Scholar] [CrossRef]
- Wang, R.; Gao, B.; Zhang, Q.; Qu, C.; Luo, C. Knockdown of TRPV gene Nanchung decreases resistance to the novel pyropene insecticide, afidopyropen, in Bemisia tabaci. Int. J. Biol. Macromol. 2022, 224, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Wang, Y.; Wang, C.; Cui, B.; Sun, C.; Zhao, X.; Zeng, Z.; Yao, J.; Liu, G.; Cui, H. Research progress on nanocapsules formulations of pesticides. J. Agric. Sci. Technol. 2018, 20, 10–18. [Google Scholar]
- Yan, S.; Jiang, Q.; Shen, J. Research actuality on synergistic mechanism of nanopesticides and their carriers. J. Plant Prot. 2022, Online. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Luo, C.; Wang, R. Insecticide Resistance and Its Management in Two Invasive Cryptic Species of Bemisia tabaci in China. Int. J. Mol. Sci. 2023, 24, 6048. https://doi.org/10.3390/ijms24076048
Wang Q, Luo C, Wang R. Insecticide Resistance and Its Management in Two Invasive Cryptic Species of Bemisia tabaci in China. International Journal of Molecular Sciences. 2023; 24(7):6048. https://doi.org/10.3390/ijms24076048
Chicago/Turabian StyleWang, Qian, Chen Luo, and Ran Wang. 2023. "Insecticide Resistance and Its Management in Two Invasive Cryptic Species of Bemisia tabaci in China" International Journal of Molecular Sciences 24, no. 7: 6048. https://doi.org/10.3390/ijms24076048




