Kisspeptin-10 Mitigates α-Synuclein-Mediated Mitochondrial Apoptosis in SH-SY5Y-Derived Neurons via a Kisspeptin Receptor-Independent Manner
Abstract
1. Introduction
2. Results
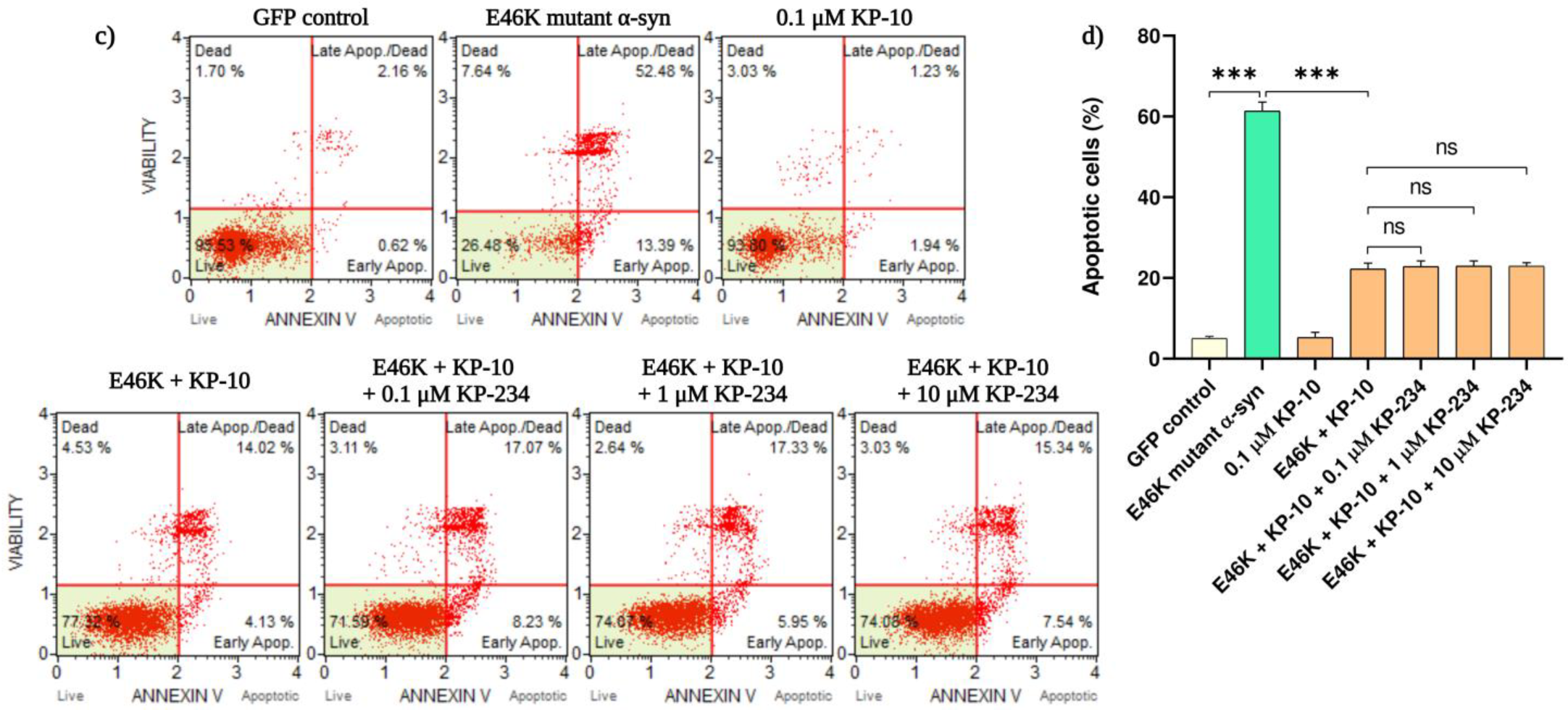
2.1. KP-10 Attenuates α-Syn-Mediated Apoptotic Death in Cholinergic-like Neurons via a GPR54-Independent Manner
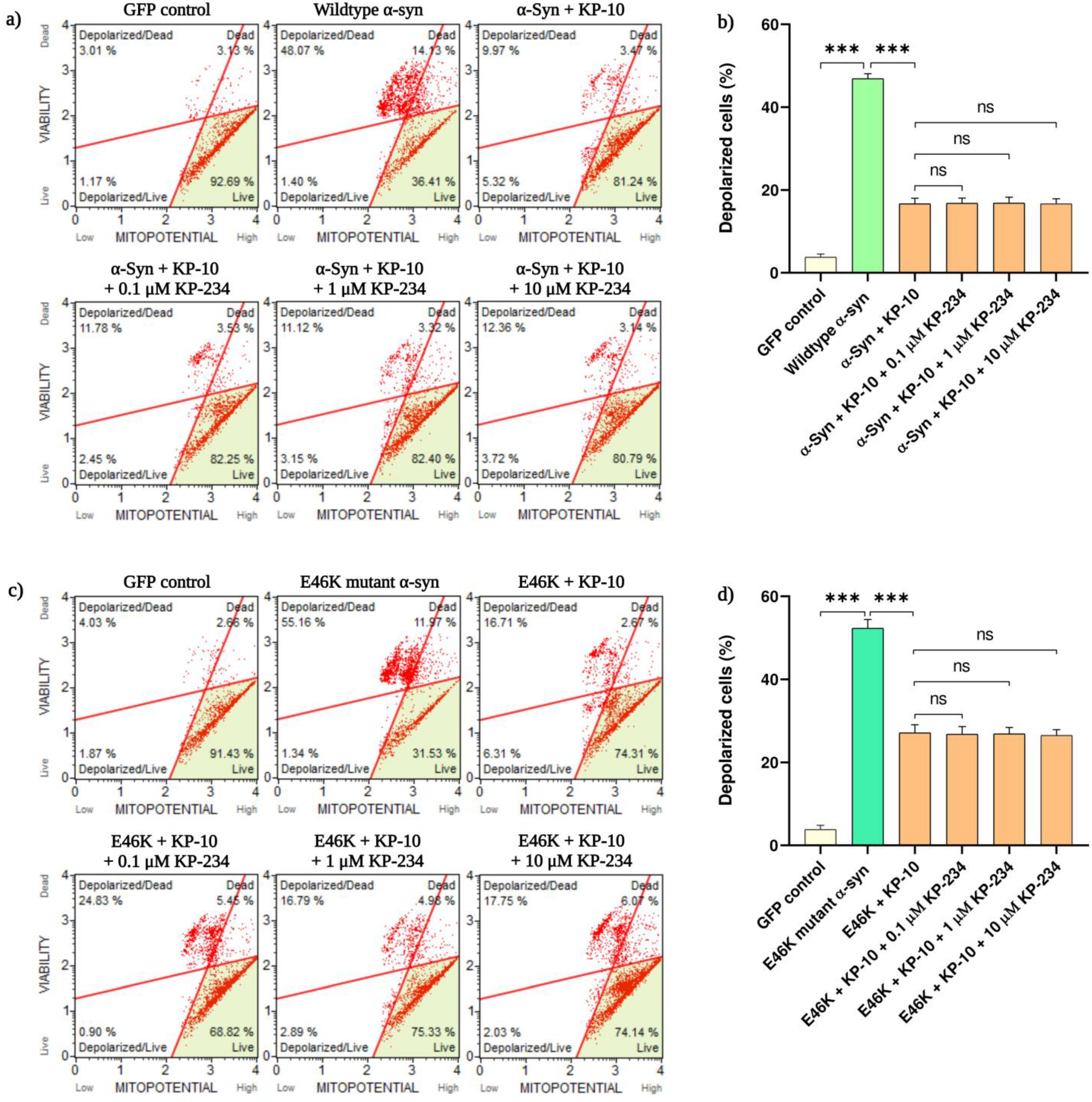
2.2. KP-10 Rescues α-Syn-Induced Mitochondrial Depolarization in Cholinergic-like Neurons through a GPR54 Dispensable Mechanism
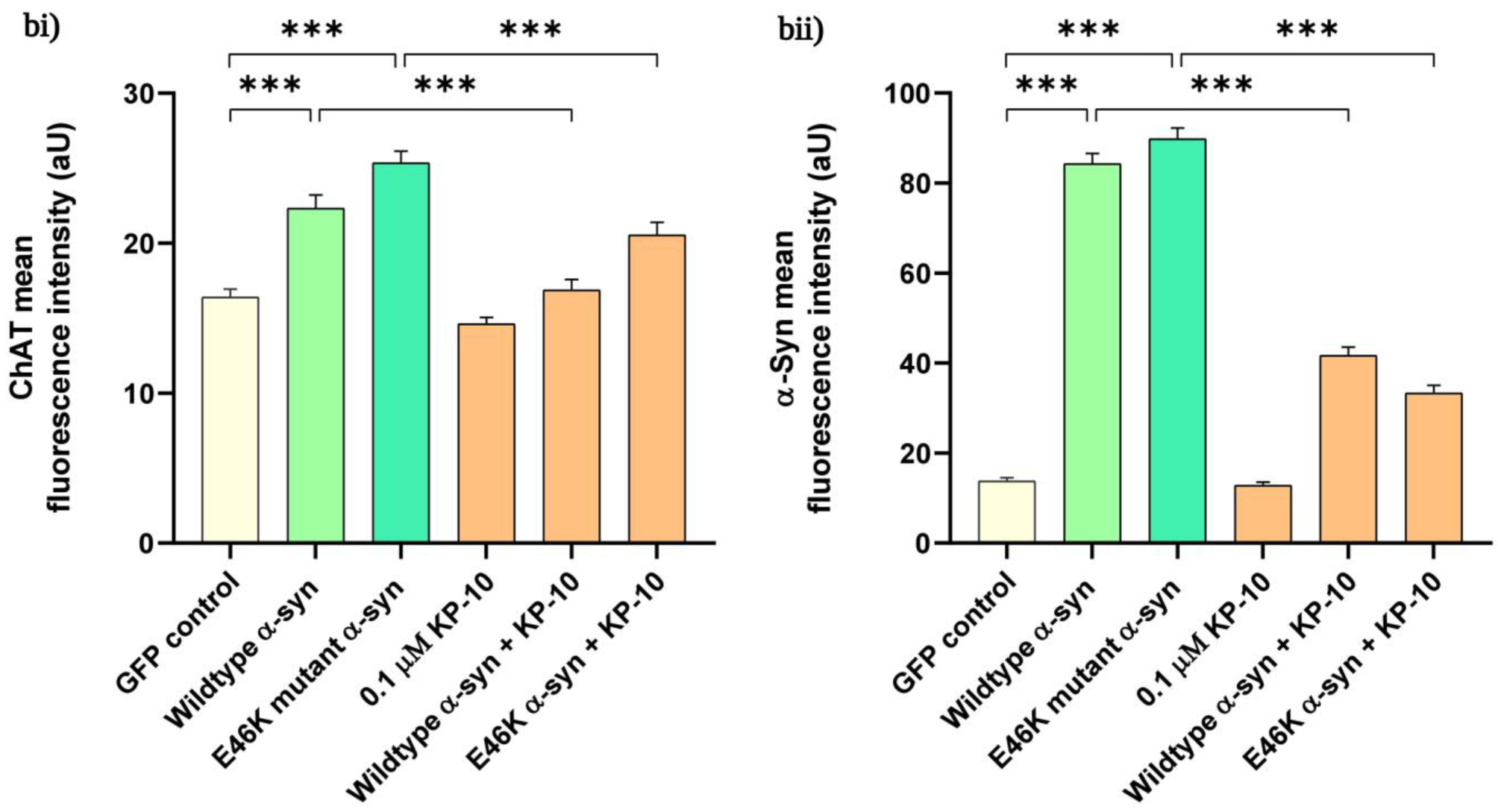
2.3. KP-10 Diminishes α-Syn and ChAT Immunofluorescence Intensity in Cholinergic-like Neurons Overexpressing Human Wild-Type or E46K Mutant α-Syn
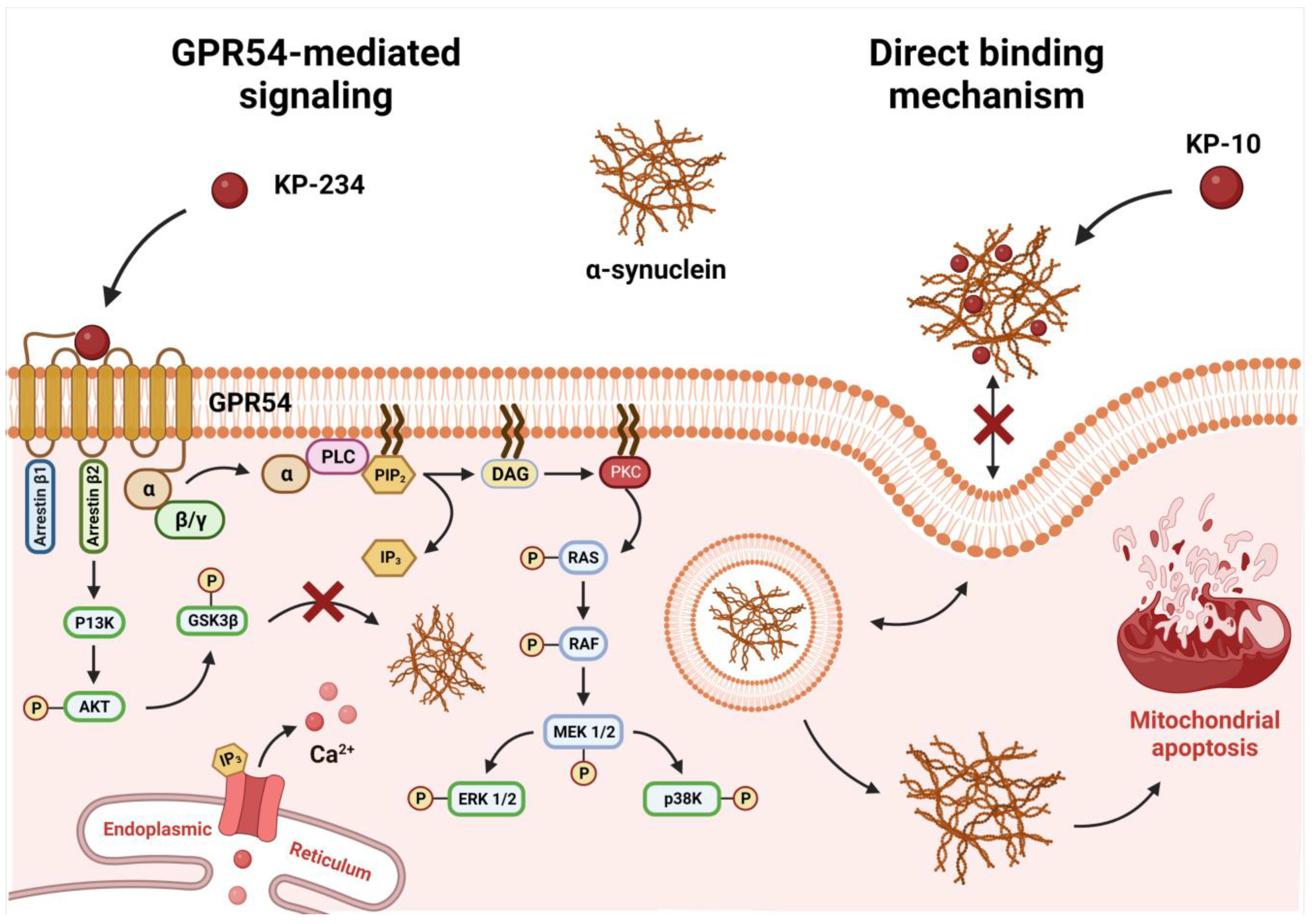
3. Discussion
4. Materials and Methods
4.1. Kisspeptin Peptides
4.2. α-Synuclein Plasmids
4.3. Cell Culture
4.4. Differentiation into Cholinergic-like Neurons
4.5. Transient Transfections
4.6. Quantification of Apoptosis by Annexin-V Labeling
4.7. Mitochondrial Membrane Potential
4.8. Double Label Immunofluorescence
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, C.; Ziabreva, I.; Perry, R.; Larsen, J.; O’brien, J.; McKeith, I.; Perry, E.; Aarsland, D. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 2006, 67, 1931–1934. [Google Scholar] [CrossRef]
- Simon, C.; Soga, T.; Okano, H.J.; Parhar, I. α-Synuclein-mediated neurodegeneration in Dementia with Lewy bodies: The pathobiology of a paradox. Cell Biosci. 2021, 11, 196. [Google Scholar] [CrossRef]
- Tagliafierro, L.; Chiba-Falek, O. Up-regulation of SNCA gene expression: Implications to synucleinopathies. Neurogenetics 2016, 17, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, J.J.; Alegre, J.; Gómez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atarés, B. The new mutation, E46K, of α-synuclein causes parkinson and Lewy body dementia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2004, 55, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L.; Emmanouilidou, E.; Pantazopoulou, M.; Kirik, D.; Vekrellis, K.; Tofaris, G.K. How is alpha-synuclein cleared from the cell? J. Neurochem. 2019, 150, 577–590. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Baba, M.; Nakajo, S.; Tu, P.-H.; Tomita, T.; Nakaya, K.; Lee, V.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998, 152, 879. [Google Scholar]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Lloyd, G.M.; Dhillon, J.-K.S.; Gorion, K.-M.M.; Riffe, C.; Fromholt, S.E.; Xia, Y.; Giasson, B.I.; Borchelt, D.R. Collusion of α-Synuclein and Aβ aggravating co-morbidities in a novel prion-type mouse model. Mol. Neurodegener. 2021, 16, 63. [Google Scholar] [CrossRef]
- Tanaka, Y.; Engelender, S.; Igarashi, S.; Rao, R.K.; Wanner, T.; Tanzi, R.E.; Sawa, A.; Dawson, V.L.; Dawson, T.M.; Ross, C.A. Inducible expression of mutant α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 2001, 10, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. α-Synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000, 157, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Cell type specific sequestration of choline acetyltransferase and tyrosine hydroxylase within Lewy bodies. Acta Neuropathol. 2010, 120, 633–639. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Ikonomovic, M.D.; Styren, S.D.; Beckett, L.; Wisniewski, S.; Bennett, D.A.; Cochran, E.J.; Kordower, J.H.; Mufson, E.J. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2002, 51, 145–155. [Google Scholar] [CrossRef]
- Perry, E.K.; Gibson, P.H.; Blessed, G.; Perry, R.H.; Tomlinson, B.E. Neurotransmitter enzyme abnormalities in senile dementia: Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977, 34, 247–265. [Google Scholar] [CrossRef]
- Chilumuri, A.; Ashioti, M.; Nercessian, A.N.; Milton, N.G. Immunolocalization of kisspeptin associated with amyloid-β deposits in the pons of an Alzheimer’s disease patient. J. Neurodegener. Dis. 2013, 2013, 879710. [Google Scholar] [CrossRef]
- Mills, E.G.; O’Byrne, K.T.; Comninos, A.N. Kisspeptin as a Behavioral Hormone. In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2019; pp. 56–63. [Google Scholar]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin signaling in the brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef]
- Milton, N.G.; Chilumuri, A.; Rocha-Ferreira, E.; Nercessian, A.N.; Ashioti, M. Kisspeptin prevention of amyloid-β peptide neurotoxicity in vitro. ACS Chem. Neurosci. 2012, 3, 706–719. [Google Scholar] [CrossRef]
- Chilumuri, A.; Milton, N.G. The role of neurotransmitters in protection against amyloid-β toxicity by KiSS-1 overexpression in SH-SY5Y neurons. Int. Sch. Res. Not. 2013, 2013, 253210. [Google Scholar] [CrossRef]
- Simon, C.; Soga, T.; Ahemad, N.; Bhuvanendran, S.; Parhar, I. Kisspeptin-10 Rescues Cholinergic Differentiated SHSY-5Y Cells from α-Synuclein-Induced Toxicity In Vitro. Int. J. Mol. Sci. 2022, 23, 5193. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Atika, B.; Shahab, M.; Behr, R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat. Rev. Urol. 2016, 13, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M. Kisspeptin signaling in the brain: Recent developments and future challenges. Mol. Cell. Endocrinol. 2010, 314, 164–169. [Google Scholar] [CrossRef] [PubMed]
- D’Anglemont de Tassigny, X.; Colledge, W.H. The role of kisspeptin signaling in reproduction. Physiology 2010, 25, 207–217. [Google Scholar] [CrossRef]
- Tena-Sempere, M. GPR54 and kisspeptin in reproduction. Hum. Reprod. Update 2006, 12, 631–639. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.-M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef]
- Abbara, A.; Clarke, S.A.; Dhillo, W.S. Clinical potential of Kisspeptin in reproductive health. Trends Mol. Med. 2021, 27, 807–823. [Google Scholar] [CrossRef]
- Pampillo, M.; Camuso, N.; Taylor, J.E.; Szereszewski, J.M.; Ahow, M.R.; Zajac, M.; Millar, R.P.; Bhattacharya, M.; Babwah, A.V. Regulation of GPR54 signaling by GRK2 and β-arrestin. Mol. Endocrinol. 2009, 23, 2060–2074. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Huang, L.; Fang, Y.; Li, D.; Liu, R.; Lu, Q.; Ren, R.; Tang, L.; Lian, L. Kisspeptin-54 attenuates oxidative stress and neuronal apoptosis in early brain injury after subarachnoid hemorrhage in rats via GPR54/ARRB2/AKT/GSK3β signaling pathway. Free Radic. Biol. Med. 2021, 171, 99–111. [Google Scholar] [CrossRef]
- Gonzalo-Ruiz, A.; Sanz, J. Alteration of cholinergic, excitatory amino acid and neuropeptide markers in the septum-diagonal band complex following injections of fibrillar?-amyloid protein into the retrosplenial granular cortex of the rat. Eur. J. Anat. 2021, 6, 95–107. [Google Scholar]
- Szegő, É.M.; Gerhardt, E.; Outeiro, T.F.; Kermer, P. Dopamine-depletion and increased α-synuclein load induce degeneration of cortical cholinergic fibers in mice. J. Neurol. Sci. 2011, 310, 90–95. [Google Scholar] [CrossRef] [PubMed]
- König, M.; Berlin, B.; Schwab, K.; Frahm, S.; Theuring, F.; Wischik, C.M.; Harrington, C.R.; Riedel, G.; Klein, J. Increased cholinergic response in α-synuclein transgenic mice (h-α-synL62). ACS Chem. Neurosci. 2018, 10, 1915–1922. [Google Scholar]
- Taylor, J.-P.; Collerton, D.; LeBeau, F.; Perry, E. Cholinergic Pathology in Dementia with Lewy Bodies. In Dementia with Lewy Bodies; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–39. [Google Scholar]
- Pepeu, G.; Giovannini, M.G. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 2017, 1670, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.N.; Flower, T.R. α-Synuclein, oxidative stress and apoptosis from the perspective of a yeast model of Parkinson’s disease. FEMS Yeast Res. 2006, 6, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hersh, L.B. Choline acetyltransferase: Celebrating its fiftieth year. J. Neurochem. 1994, 62, 1653–1663. [Google Scholar] [CrossRef]
- Wilcock, G.; Esiri, M.; Bowen, D.; Smith, C. Alzheimer’s disease: Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J. Neurol. Sci. 1982, 57, 407–417. [Google Scholar] [CrossRef]
- Oda, Y. Choline acetyltransferase: The structure, distribution and pathologic changes in the central nervous system. Pathol. Int. 1999, 49, 921–937. [Google Scholar] [CrossRef]
- Brundin, P.; Dave, K.D.; Kordower, J.H. Therapeutic approaches to target alpha-synuclein pathology. Exp. Neurol. 2017, 298, 225–235. [Google Scholar] [CrossRef]
- Kingwell, K. Zeroing in on neurodegenerative [alpha]-synuclein. Nat. Rev. Drug Discov. 2017, 16, 371–374. [Google Scholar] [CrossRef]
- Albers-Wolthers, C.; De Gier, J.; Walen, M.; Van Kooten, P.; Lambalk, C.; Leegwater, P.; Roelen, B.; Schaefers-Okkens, A.; Rutten, V.P.; Millar, R. In vitro and in vivo effects of kisspeptin antagonists p234, p271, p354, and p356 on GPR54 activation. PLoS ONE 2017, 12, e0179156. [Google Scholar] [CrossRef] [PubMed]
- Roseweir, A.K.; Millar, R.P. Kisspeptin antagonists. In Kisspeptin Signaling in Reproductive Biology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 159–186. [Google Scholar]
- Millar, R.P.; Roseweir, A.K.; Tello, J.A.; Anderson, R.A.; George, J.T.; Morgan, K.; Pawson, A.J. Kisspeptin antagonists: Unraveling the role of kisspeptin in reproductive physiology. Brain Res. 2010, 1364, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Roseweir, A.K.; Kauffman, A.S.; Smith, J.T.; Guerriero, K.A.; Morgan, K.; Pielecka-Fortuna, J.; Pineda, R.; Gottsch, M.L.; Tena-Sempere, M.; Moenter, S.M. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J. Neurosci. 2009, 29, 3920–3929. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Kakish, J.; Lee, D.; Lee, J.S. Drugs that bind to α-synuclein: Neuroprotective or neurotoxic? ACS Chem. Neurosci. 2015, 6, 1930–1940. [Google Scholar] [CrossRef]
- Tavassoly, O.; Kakish, J.; Nokhrin, S.; Dmitriev, O.; Lee, J.S. The use of nanopore analysis for discovering drugs which bind to α-synuclein for treatment of Parkinson’s disease. Eur. J. Med. Chem. 2014, 88, 42–54. [Google Scholar] [CrossRef]
- Ruzza, P.; Siligardi, G.; Hussain, R.; Marchiani, A.; Islami, M.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sechi, M. Ceftriaxone blocks the polymerization of α-synuclein and exerts neuroprotective effects in vitro. ACS Chem. Neurosci. 2014, 5, 30–38. [Google Scholar] [CrossRef]
- Jia, C.; Ma, X.; Liu, Z.; Gu, J.; Zhang, X.; Li, D.; Zhang, S. Different heat shock proteins bind α-Synuclein with distinct mechanisms and synergistically prevent its amyloid aggregation. Front. Neurosci. 2019, 13, 1124. [Google Scholar] [CrossRef]
- Cox, D.; Whiten, D.R.; Brown, J.W.; Horrocks, M.H.; San Gil, R.; Dobson, C.M.; Klenerman, D.; Van Oijen, A.M.; Ecroyd, H. The small heat shock protein Hsp27 binds α-synuclein fibrils, preventing elongation and cytotoxicity. J. Biol. Chem. 2018, 293, 4486–4497. [Google Scholar] [CrossRef]
- Zhu, M.; Rajamani, S.; Kaylor, J.; Han, S.; Zhou, F.; Fink, A.L. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004, 279, 26846–26857. [Google Scholar] [CrossRef]
- Jiang, M.; Porat-Shliom, Y.; Pei, Z.; Cheng, Y.; Xiang, L.; Sommers, K.; Li, Q.; Gillardon, F.; Hengerer, B.; Berlinicke, C. Baicalein reduces E46K α-synuclein aggregation in vitro and protects cells against E46K α-synuclein toxicity in cell models of familiar Parkinsonism. J. Neurochem. 2010, 114, 419–429. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, C.; Soga, T.; Parhar, I. Kisspeptin-10 Mitigates α-Synuclein-Mediated Mitochondrial Apoptosis in SH-SY5Y-Derived Neurons via a Kisspeptin Receptor-Independent Manner. Int. J. Mol. Sci. 2023, 24, 6056. https://doi.org/10.3390/ijms24076056
Simon C, Soga T, Parhar I. Kisspeptin-10 Mitigates α-Synuclein-Mediated Mitochondrial Apoptosis in SH-SY5Y-Derived Neurons via a Kisspeptin Receptor-Independent Manner. International Journal of Molecular Sciences. 2023; 24(7):6056. https://doi.org/10.3390/ijms24076056
Chicago/Turabian StyleSimon, Christopher, Tomoko Soga, and Ishwar Parhar. 2023. "Kisspeptin-10 Mitigates α-Synuclein-Mediated Mitochondrial Apoptosis in SH-SY5Y-Derived Neurons via a Kisspeptin Receptor-Independent Manner" International Journal of Molecular Sciences 24, no. 7: 6056. https://doi.org/10.3390/ijms24076056
APA StyleSimon, C., Soga, T., & Parhar, I. (2023). Kisspeptin-10 Mitigates α-Synuclein-Mediated Mitochondrial Apoptosis in SH-SY5Y-Derived Neurons via a Kisspeptin Receptor-Independent Manner. International Journal of Molecular Sciences, 24(7), 6056. https://doi.org/10.3390/ijms24076056





