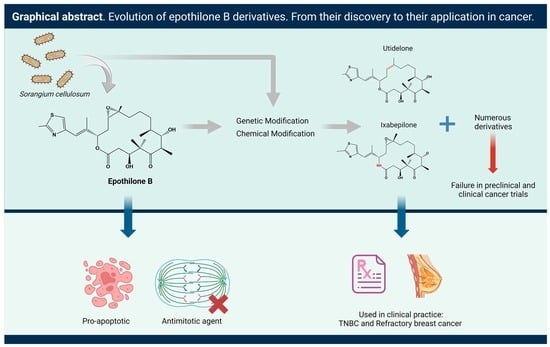
Epothilones as Natural Compounds for Novel Anticancer Drugs Development
Abstract
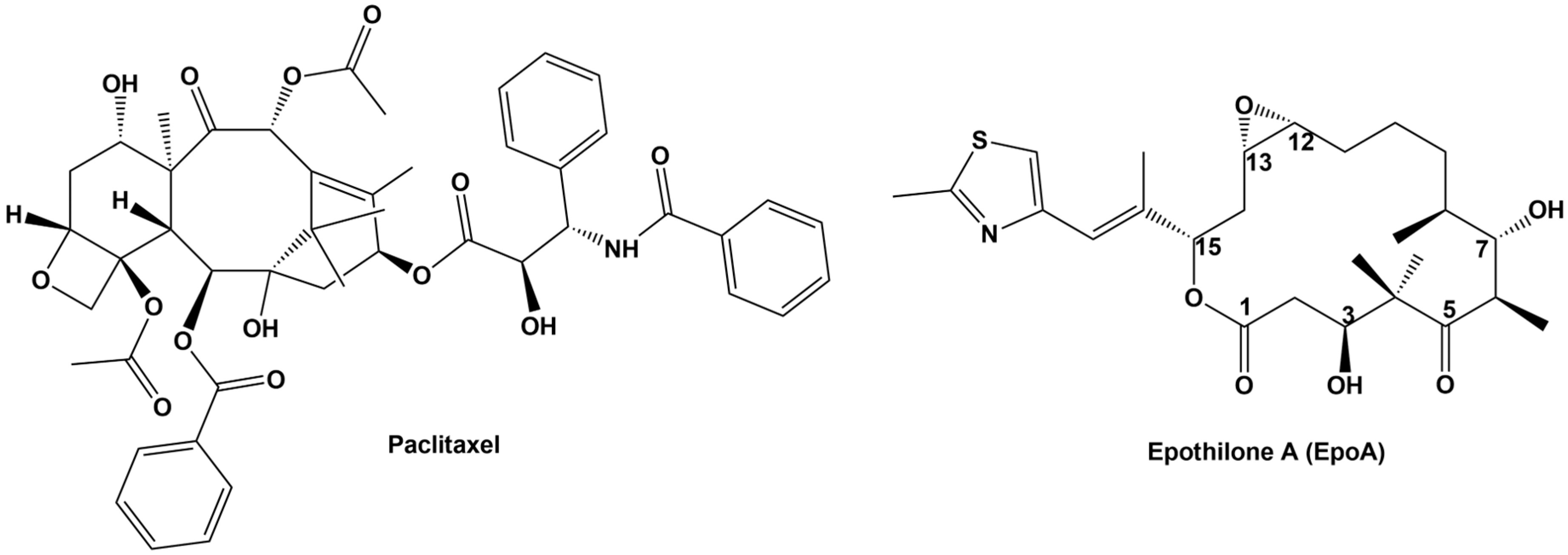
:1. Introduction
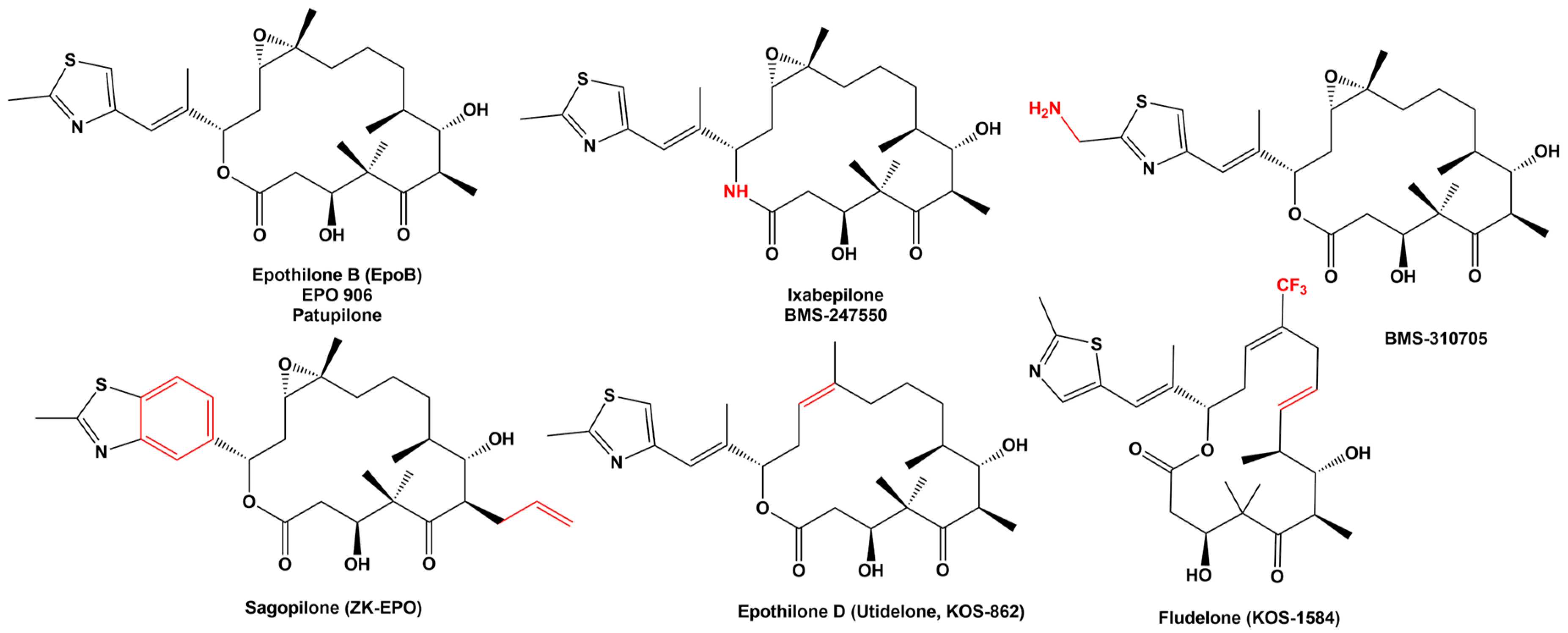
2. Epothilone Synthesis
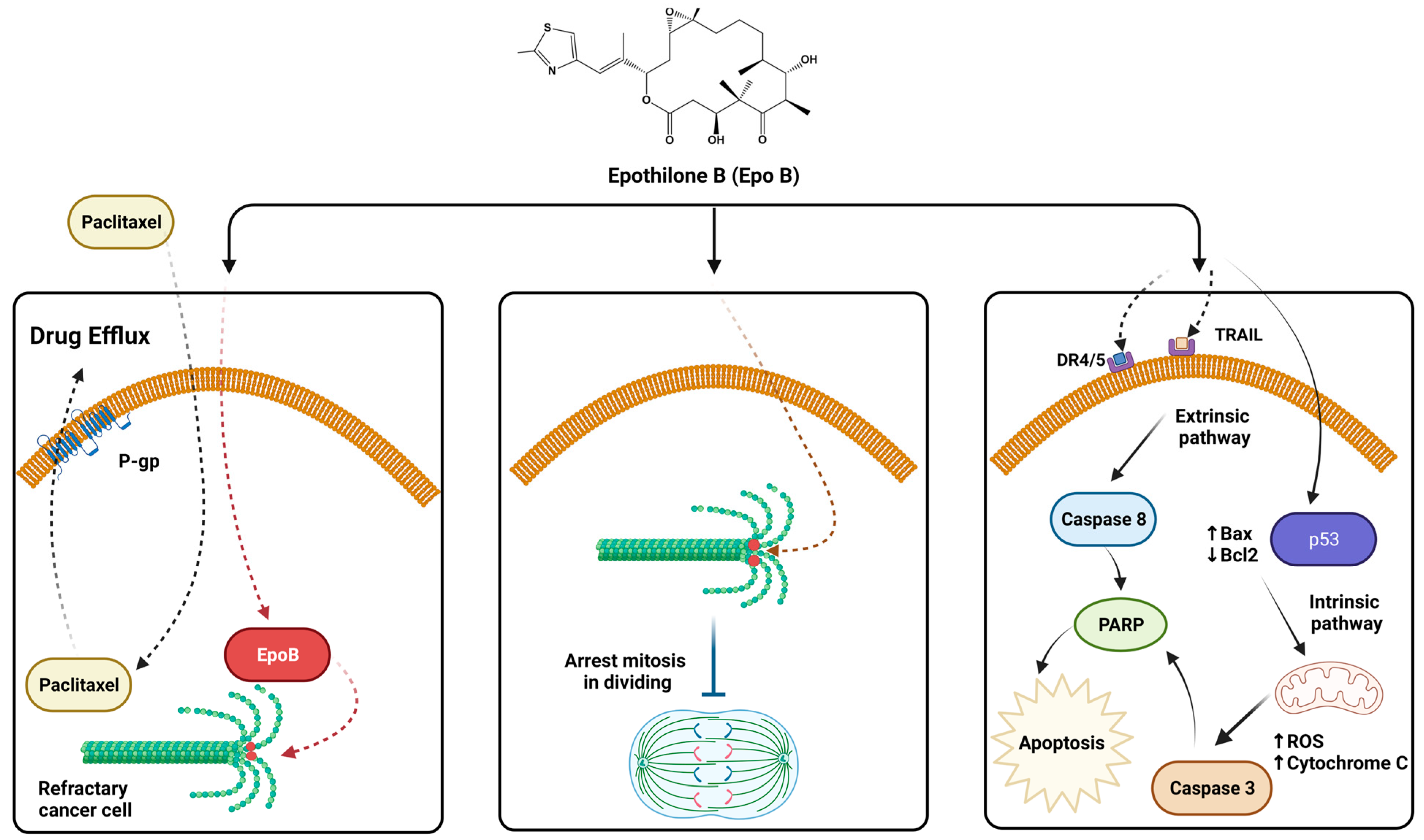
3. Epothilone Induce Stabilized Microtubule Assembly
4. Epothilones Induce Apoptosis in Multidrug-Resistant Cancer Cells
5. Epothilones with Clinical Significance in Cancer
5.1. Epothilone B
5.2. Ixabepilone
6. Toxicity and Safety Profile of Epothilones
7. New Epothilone Derivatives with Increased Cytotoxic Activity
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Höfle, G.R.H. Epothilone, a Myxobacterial Metabolite with Promising Antitumor Activity. In Anticancer Agents from Natural Products, 1st ed.; Cragg, G.M.K.D., Newman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 413–450. [Google Scholar]
- Gerth, K.; Bedorf, N.; Höfle, G.; Irschik, H.; Reichenbach, H. Epothilons A and B: Antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria). Production, physico-chemical and biological properties. J. Antibiot. 1996, 49, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Höfle, G.; Bedorf, N.; Steinmetz, H.; Schomburg, D.; Gerth, K.; Reichenbach, H. Epothilone A and B—Novel 16-Membered Macrolides with Cytotoxic Activity: Isolation, Crystal Structure, and Conformation in Solution. Angew. Chem. Int. Ed. 1996, 35, 1567–1569. [Google Scholar] [CrossRef]
- Hardt, I.H.; Steinmetz, H.; Gerth, K.; Sasse, F.; Reichenbach, H.; Höfle, G. New Natural Epothilones from Sorangium cellulosum, Strains So ce90/B2 and So ce90/D13: Isolation, Structure Elucidation, and SAR Studies. J. Nat. Prod. 2001, 64, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus(®)-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Lee, J.J.; Swain, S.M. The epothilones: Translating from the laboratory to the clinic. Clin. Cancer Res. 2008, 14, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Forli, S. Epothilones: From discovery to clinical trials. Curr. Top Med. Chem. 2014, 14, 2312–2321. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Huang, G. Synthesis & antitumor activity of epothilones B and D and their analogs. Futur. Med. Chem. 2018, 10, 1483–1496. [Google Scholar] [CrossRef]
- Goodin, S. Ixabepilone: A novel microtubule-stabilizing agent for the treatment of metastatic breast cancer. Am. J. Health Syst. Pharm. 2008, 65, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Brogdon, C.F.; Lee, F.Y.; Canetta, R.M. Development of other microtubule-stabilizer families: The epothilones and their derivatives. Anticancer Drugs 2014, 25, 599–609. [Google Scholar] [CrossRef]
- O’Reilly, T.; McSheehy, P.M.J.; Wenger, F.; Hattenberger, M.; Muller, M.; Vaxelaire, J.; Altmann, K.-H.; Wartmann, M. Patupilone (epothilone B, EPO906) inhibits growth and metastasis of experimental prostate tumors in vivo. Prostate 2005, 65, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Becquet, M.; Laborde, L.; Texier, C.; Sterker, D.; Gschwind, H.P.; Pfaar, U.; Wartmann, M.; O’Reilly, T.M.; McSheehy, P.M. Continuous low-dose infusion of patupilone increases the therapeutic index in mouse and rat tumour models. Anticancer Drugs 2018, 29, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X. Classic cytotoxic drugs: A narrow path for regulatory approval. Lancet Oncol. 2017, 18, 279–281. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhu, M.; Zhang, W.; Huang, Z.; Li, S.; Li, H.; Kong, Y.; Chen, Y. An Easy and Efficient Strategy for the Enhancement of Epothilone Production Mediated by TALE-TF and CRISPR/dcas9 Systems in Sorangium cellulosum. Front. Bioeng. Biotechnol. 2019, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Shindia, A.A.; Ali, G.S.; Yassin, M.A.; Hussein, H.; Awad, S.A.; Ammar, H.A. Production and bioprocess optimization of antitumor Epothilone B analogue from Aspergillus fumigatus, endophyte of Catharanthus roseus, with response surface methodology. Enzym. Microb. Technol. 2021, 143, 109718. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rhoades, D.; Wang, Y.; Totokotsopoulos, S.; Bai, R.; Hamel, E. Synthesis and Biological Evaluation of Novel Epothilone B Side Chain Analogues. ChemMedChem 2015, 10, 1974–1979. [Google Scholar] [CrossRef]
- Foley, C.N.; Chen, L.-A.; Sackett, D.L.; Leighton, J.L. Synthesis and Evaluation of a Linkable Functional Group-Equipped Analogue of the Epothilones. ACS Med. Chem. Lett. 2017, 8, 701–704. [Google Scholar] [CrossRef]
- Balog, A.; Meng, D.; Kamenecka, T.; Bertinato, P.; Su, D.S.; Sorensen, E.J.; Danishefsky, S.J. Totalsynthese von (—)-Epothilon A. Angew. Chem. 1996, 108, 2976–2978. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Sarabia, F.; Ninkovic, S.; Yang, Z. Totalsynthese von Epothilon A durch Makrolactonisierung. Angew. Chem. 1997, 109, 539–540. [Google Scholar] [CrossRef]
- Schinzer, D.; Limberg, A.; Bauer, A.; Böhm, O.M.; Cordes, M. Totalsynthese von (−)-Epothilon A. Angew. Chem. 1997, 109, 543–544. [Google Scholar] [CrossRef]
- Storer, R.I.; Takemoto, T.; Jackson, P.S.; Brown, D.S.; Baxendale, I.R.; Ley, S.V. Multi-step application of immobilized reagents and scavengers: A total synthesis of epothilone C. Chemistry 2004, 10, 2529–2547. [Google Scholar] [CrossRef] [PubMed]
- Storer, R.I.; Takemoto, T.; Jackson, P.S.; Ley, S.V. A total synthesis of epothilones using solid-supported reagents and scavengers. Angew. Chem. 2003, 115, 2625–2629. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Roschangar, F.; Vourloumis, D. Chemical Biology of Epothilones. Angew. Chem. Int. Ed. 1998, 37, 2014–2045. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Scheid, G.n.O.; Eichelberger, U.; Umbreen, S. Total synthesis of Epothilone D: The Nerol/Macroaldolization approach. J. Org. Chem. 2013, 78, 10588–10595. [Google Scholar] [CrossRef]
- Sawada, D.; Kanai, M.; Shibasaki, M. Enantioselective total synthesis of epothilones A and B using multifunctional asymmetric catalysis. J. Am. Chem. Soc. 2000, 122, 10521–10532. [Google Scholar] [CrossRef]
- Sinha, S.C.; Sun, J.; Miller, G.P.; Wartmann, M.; Lerner, R.A. Catalytic antibody route to the naturally occurring epothilones: Total synthesis of epothilones A–F. Chemistry 2001, 7, 1691–1702. [Google Scholar] [CrossRef]
- Sun, J.; Sinha, S.C. Stereoselective Total Synthesis of Epothilones by the Metathesis Approach Involving C9− C10 Bond Formation. Angew. Chem. Int. Ed. 2002, 41, 1381–1383. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.-F.; Cui, K.; Lin, G.-Q. An Efficient Total Synthesis of (−)-Epothilone B. Org. Lett. 2012, 14, 6354–6357. [Google Scholar] [CrossRef]
- Chen, H.; O’Connor, S.; Cane, D.E.; Walsh, C.T. Epothilone biosynthesis: Assembly of the methylthiazolylcarboxy starter unit on the EpoB subunit. Chem. Biol. 2001, 8, 899–912. [Google Scholar] [CrossRef] [Green Version]
- Beyer, S.; Kunze, B.; Silakowski, B.; Müller, R. Metabolic diversity in myxobacteria: Identification of the myxalamid and the stigmatellin biosynthetic gene cluster of Stigmatella aurantiaca Sg a15 and a combined polyketide-(poly) peptide gene cluster from the epothilone producing strain Sorangium cellulosum So ce90. Biochim. Biophys. Acta 1999, 1445, 185–195. [Google Scholar] [PubMed]
- Ye, W.; Liu, T.; Zhang, W.-M.; Zhang, W.; Li, S. The Improvement of Epothilone D Yield by the Disruption of epoK Gene in Sorangium cellulosum Using TALEN System. Mol. Biotechnol. 2022, 65, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, H.; Tang, B.; Liang, J.; Zhang, L.; Wang, H.; Bian, X.; Li, Y.-Z.; Zhang, Y.; Zhao, G.-P. Reassembly of the biosynthetic gene cluster enables high epothilone yield in engineered Schlegelella brevitalea. ACS Synth. Biol. 2020, 9, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Yang, W.; Huang, G. Optimization of fermentation conditions for the production of epothilone B. Chem. Biol. Drug Des. 2020, 96, 768–772. [Google Scholar] [CrossRef]
- Gao, H.; Huang, G. Synthesis, anticancer activity and cytotoxicity of galactosylated epothilone B. Bioorg. Med. Chem. 2018, 26, 5578–5581. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Tang, B.; Yu, Y.; Tu, Q.; Gross, F.; Wang, H.; Li, A.; Fu, J.; Shen, Y.; Li, Y.-Z. Heterologous production and yield improvement of epothilones in Burkholderiales strain DSM 7029. ACS Chem. Biol. 2017, 12, 1805–1812. [Google Scholar] [CrossRef]
- Gong, G.-L.; Huang, Y.-Y.; Liu, L.-L.; Chen, X.-F.; Liu, H. Enhanced production of epothilone by immobilized Sorangium cellulosum in porous ceramics. J. Microbiol. Biotechnol. 2015, 25, 1653–1659. [Google Scholar] [CrossRef] [Green Version]
- Bollag, D.M.; McQueney, P.A.; Zhu, J.; Hensens, O.; Koupal, L.; Liesch, J.; Goetz, M.; Lazarides, E.; Woods, C.M. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995, 55, 2325–2333. [Google Scholar]
- Meurer-Grob, P.; Kasparian, J.; Wade, R.H. Microtubule Structure at Improved Resolution. Biochemistry 2001, 40, 8000–8008. [Google Scholar] [CrossRef]
- Kamath, K.; Jordan, M.A. Suppression of Microtubule Dynamics by Epothilone B Is Associated with Mitotic Arrest1. Cancer Res. 2003, 63, 6026–6031. [Google Scholar]
- Kowalski, R.J.; Giannakakou, P.; Hamel, E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)). J. Biol. Chem. 1997, 272, 2534–2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buey, R.M.; Díaz, J.F.; Andreu, J.M.; O’Brate, A.; Giannakakou, P.; Nicolaou, K.C.; Sasmal, P.K.; Ritzén, A.; Namoto, K. Interaction of epothilone analogs with the paclitaxel binding site: Relationship between binding affinity, microtubule stabilization, and cytotoxicity. Chem. Biol. 2004, 11, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.H. Recent developments in the chemical biology of epothilones. Curr. Pharm. Des. 2005, 11, 1595–1613. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Díaz, J.F.; Altmann, K.-H.; Steinmetz, M.O. Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer Agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Xue, T.; Shuai, W.; Wu, C.; Zhang, Z.; Zhang, T.; Zeng, S.; Sun, B.; Wang, Y. High-resolution X-ray structure of three microtubule-stabilizing agents in complex with tubulin provide a rationale for drug design. Biochem. Biophys. Res. Commun. 2021, 534, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Ranade, A.R.; Higgins, L.; Markowski, T.W.; Glaser, N.; Kashin, D.; Bai, R.; Hong, K.H.; Hamel, E.; Höfle, G.; Georg, G.I. Characterizing the Epothilone Binding Site on β-Tubulin by Photoaffinity Labeling: Identification of β-Tubulin Peptides TARGSQQY and TSRGSQQY as Targets of an Epothilone Photoprobe for Polymerized Tubulin. J. Med. Chem. 2016, 59, 3499–3514. [Google Scholar] [CrossRef] [PubMed]
- Wartmann, M.; Altmann, K. The biology and medicinal chemistry of epothilones. Curr. Med. Chem. Anticancer Agents 2002, 2, 123–148. [Google Scholar] [CrossRef]
- Jantsch, A.; Nieto, L.; Gertsch, J.; Rodríguez-Salarichs, J.; Matesanz, R.; Jiménez-Barbero, J.; Díaz, J.F.; Canales, Á.; Altmann, K.-H. Synthesis, Biological Profiling and Determination of the Tubulin-Bound Conformation of 12-Aza-Epothilones (Azathilones). Molecules 2016, 21, 1010. [Google Scholar] [CrossRef] [Green Version]
- Bröker, L.E.; Huisman, C.; Ferreira, C.G.; Rodriguez, J.A.; Kruyt, F.A.E.; Giaccone, G. Late Activation of Apoptotic Pathways Plays a Negligible Role in Mediating the Cytotoxic Effects of Discodermolide and Epothilone B in Non-Small Cell Lung Cancer Cells. Cancer Res. 2002, 62, 4081–4088. [Google Scholar]
- Ioffe, M.L.; White, E.; Nelson, D.A.; Dvorzhinski, D.; DiPaola, R.S. Epothilone induced cytotoxicity is dependent on p53 status in prostate cells. Prostate 2004, 61, 243–247. [Google Scholar] [CrossRef]
- Baumgart, T.; Kriesen, S.; Neels, O.; Hildebrandt, G.; Manda, K. Investigation of Epothilone B-Induced Cell Death Mechanisms in Human Epithelial Cancer Cells –in Consideration of Combined Treatment With Ionizing Radiation. Cancer Investig. 2015, 33, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Son, S.M.; Son, D.J.; Kim, S.M.; Kim, T.J.; Song, S.; Moon, D.C.; Lee, H.W.; Ryu, J.C.; Yoon, D.Y.; et al. Epothilones induce human colon cancer SW620 cell apoptosis via the tubulin polymerization independent activation of the nuclear factor-kappaB/IkappaB kinase signal pathway. Mol. Cancer Ther. 2007, 6, 2786–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Huang, T.; Tang, Y.; Li, Q.; Wang, J.; Cheng, X.; Zhang, W.; Zhang, B.; Zhou, C.; Tu, S. Utidelone inhibits growth of colorectal cancer cells through ROS/JNK signaling pathway. Cell Death Dis. 2021, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Rogalska, A.; Marczak, A.; Gajek, A.; Szwed, M.; Śliwińska, A.; Drzewoski, J.; Jóźwiak, Z. Induction of apoptosis in human ovarian cancer cells by new anticancer compounds, epothilone A and B. Toxicol. Vitr. 2013, 27, 239–249. [Google Scholar] [CrossRef]
- Rogalska, A.; Gajek, A.; Marczak, A. Epothilone B induces extrinsic pathway of apoptosis in human SKOV-3 ovarian cancer cells. Toxicol. Vitr. 2014, 28, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, N.R.; Carré, M.; Kovacic, H.; Estève, M.A.; Braguer, D. Patupilone-induced apoptosis is mediated by mitochondrial reactive oxygen species through Bim relocalization to mitochondria. Mol. Pharmacol. 2008, 74, 1072–1083. [Google Scholar] [CrossRef] [Green Version]
- Rogalska, A.; Marczak, A. Epothilone B induces human ovarian cancer OV-90 cell apoptosis via external pathway. Environ. Toxicol. Pharmacol. 2015, 39, 700–712. [Google Scholar] [CrossRef]
- Stöhr, D.; Jeltsch, A.; Rehm, M. TRAIL receptor signaling: From the basics of canonical signal transduction toward its entanglement with ER stress and the unfolded protein response. Int. Rev. Cell Mol. Biol. 2020, 351, 57–99. [Google Scholar] [CrossRef]
- Griffin, D.; Wittmann, S.; Guo, F.; Nimmanapalli, R.; Bali, P.; Wang, H.G.; Bhalla, K. Molecular determinants of epothilone B derivative (BMS 247550) and Apo-2L/TRAIL-induced apoptosis of human ovarian cancer cells. Gynecol. Oncol. 2003, 89, 37–47. [Google Scholar] [CrossRef]
- Wolff, A.; Technau, A.; Brandner, G. Epothilone A induces apoptosis in neuroblastoma cells with multiple mechanisms of drug resistance. Int. J. Oncol. 1997, 11, 123–126. [Google Scholar] [CrossRef]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaramillo, A.C.; Saig, F.A.; Cloos, J.; Jansen, G.; Peters, G.J. How to overcome ATP-binding cassette drug efflux transporter-mediated drug resistance? Cancer Drug Resist 2018, 1, 6–29. [Google Scholar] [CrossRef] [Green Version]
- Galletti, E.; Magnani, M.; Renzulli, M.L.; Botta, M. Paclitaxel and docetaxel resistance: Molecular mechanisms and development of new generation taxanes. ChemMedChem 2007, 2, 920–942. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of taxane resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updat. 2021, 54, 100742. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Swain, S.M. Development of Novel Chemotherapeutic Agents to Evade the Mechanisms of Multidrug Resistance (MDR). Semin. Oncol. 2005, 32, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Dumontet, C. EPO-906 (Novartis). IDrugs 2003, 6, 1182–1187. [Google Scholar]
- Lin, B.; Catley, L.; LeBlanc, R.; Mitsiades, C.; Burger, R.; Tai, Y.T.; Podar, K.; Wartmann, M.; Chauhan, D.; Griffin, J.D.; et al. Patupilone (epothilone B) inhibits growth and survival of multiple myeloma cells in vitro and in vivo. Blood 2005, 105, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Smit, W.M.; Sufliarsky, J.; Werner, T.L.; Dizon, D.; Wagnerova, M.; Hirte, H.W.; Delaney, R.; Li, J.; Weber, D.; Schellens, J.H. A phase II study evaluating the safety and efficacy of patupilone in patients with platinum refractory/resistant ovarian, primary fallopian, or peritoneal cancer. J. Clin. Oncol. 2009, 27, 5563. [Google Scholar] [CrossRef]
- Hsin, K.W.; Boyer, M.; Ducreux, M.; Liu, M.; Soo, R.; Yeo, W.; Williams, K.J.; Johri, A. Efficacy of patupilone in advanced local or metastatic gastric cancer: A phase IIa trial. J. Clin. Oncol. 2006, 24, 4069. [Google Scholar] [CrossRef]
- Nayak, L.; DeAngelis, L.M.; Robins, H.I.; Govindan, R.; Gadgeel, S.; Kelly, K.; Rigas, J.R.; Peereboom, D.M.; Rosenfeld, S.S.; Muzikansky, A.; et al. Multicenter phase 2 study of patupilone for recurrent or progressive brain metastases from non-small cell lung cancer. Cancer 2015, 121, 4165–4172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorcraft, S.Y.; Chau, I.; Peckitt, C.; Cunningham, D.; Rao, S.; Yim, K.L.; Walther, A.; Jackson, C.G.; Stamp, G.; Webb, J.; et al. Patupilone in patients with pretreated metastatic/locally recurrent colorectal cancer: Results of the Phase II CINATRA trial. Investig. New Drugs 2013, 31, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Peereboom, D.M.; Murphy, C.; Ahluwalia, M.S.; Conlin, A.; Eichler, A.; Van Poznak, C.; Baar, J.; Elson, P.; Seidman, A.D. Phase II trial of patupilone in patients with brain metastases from breast cancer. Neuro Oncol. 2014, 16, 579–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; DiPaola, R.S.; Baron, A.D.; Higano, C.S.; Tchekmedyian, N.S.; Johri, A.R. Phase II trial of weekly patupilone in patients with castration-resistant prostate cancer. Ann. Oncol. 2009, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Beardsley, E.; Eigl, B.J.; Venner, P.; Hotte, S.J.; Winquist, E.; Ko, Y.J.; Sridhar, S.S.; Weber, D.; Saad, F. A phase 2 study of patupilone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel: Canadian Urologic Oncology Group study P07a. Ann. Oncol. 2012, 23, 53–58. [Google Scholar] [CrossRef]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T. NCCN guidelines insights: Prostate cancer, version 1.2021: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- De Souza, P.L.; Mellado, B.; Pfister, C.; Rosenthal, M.; Castellano, D.E.; Weber, D.; Ferrara, S.; Shaik, N.; Tan, E.; Patterson, S.G. Randomized phase II trial of patupilone plus prednisone versus docetaxel plus prednisone in patients with chemotherapy-naïve, metastatic, castrate-resistant prostate cancer (CRPC). J. Clin. Oncol. 2010, 28, 4553. [Google Scholar] [CrossRef]
- Colombo, N.; Kutarska, E.; Dimopoulos, M.; Bae, D.S.; Rzepka-Gorska, I.; Bidzinski, M.; Scambia, G.; Engelholm, S.A.; Joly, F.; Weber, D.; et al. Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J. Clin. Oncol. 2012, 30, 3841–3847. [Google Scholar] [CrossRef]
- Bystricky, B.; Chau, I. Patupilone in cancer treatment. Expert Opin. Investig. Drugs 2011, 20, 107–117. [Google Scholar] [CrossRef]
- Krause, W.; Klar, U. Differences and similarities of epothilones. Curr. Cancer Ther. Rev. 2011, 7, 10–36. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Marmiroli, P.; Cavaletti, G.; Kalofonos, H.P. Epothilone-induced peripheral neuropathy: A review of current knowledge. J. Pain Symptom Manag. 2011, 42, 931–940. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, T.; Wartmann, M.; Brueggen, J.; Allegrini, P.R.; Floersheimer, A.; Maira, M.; McSheehy, P.M.J. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother. Pharmacol. 2008, 62, 1045–1054. [Google Scholar] [CrossRef]
- Oehler, C.; von Bueren, A.O.; Furmanova, P.; Broggini-Tenzer, A.; Orlowski, K.; Rutkowski, S.; Frei, K.; Grotzer, M.A.; Pruschy, M. The microtubule stabilizer patupilone (epothilone B) is a potent radiosensitizer in medulloblastoma cells. Neuro Oncol. 2011, 13, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Oehler, C.; Frei, K.; Rushing, E.J.; McSheehy, P.M.; Weber, D.; Allegrini, P.R.; Weniger, D.; Lütolf, U.M.; Knuth, A.; Yonekawa, Y.; et al. Patupilone (epothilone B) for recurrent glioblastoma: Clinical outcome and translational analysis of a single-institution phase I/II trial. Oncology 2012, 83, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruschel, J.; Hellal, F.; Flynn, K.C.; Dupraz, S.; Elliott, D.A.; Tedeschi, A.; Bates, M.; Sliwinski, C.; Brook, G.; Dobrindt, K.; et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 2015, 348, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, J.T. Discovery of ixabepilone. Mol. Cancer Ther. 2009, 8, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandhare, A.; Biradar, S.; Gurule, A. Azaepothilone B and its derivatives: A patent review. Expert Opin. Ther. Pat. 2016, 26, 891–905. [Google Scholar] [CrossRef]
- Vahdat, L. Ixabepilone: A novel antineoplastic agent with low susceptibility to multiple tumor resistance mechanisms. Oncologist 2008, 13, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.Y.; Borzilleri, R.; Fairchild, C.R.; Kim, S.H.; Long, B.H.; Reventos-Suarez, C.; Vite, G.D.; Rose, W.C.; Kramer, R.A. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin. Cancer Res. 2001, 7, 1429–1437. [Google Scholar]
- Stover, E. In vitro cytotoxicity of the epothilone analog, BMS 247550, in pediatric malignacies. Cantaurus 2002, 10, 31–37. [Google Scholar]
- Dawson, N.A. Epothilones in prostate cancer: Review of clinical experience. Ann. Oncol. 2007, 18 (Suppl. S5), v22–v27. [Google Scholar] [CrossRef] [PubMed]
- Shipley, D.; Spigel, D.R.; Burris, H.A.; Waterhouse, D.M.; Webb, C.D.; Gian, V.; Hart, L.L.; Greco, F.A.; Hainsworth, J.D. Phase II trial of ixabepilone and carboplatin with or without bevacizumab in patients with previously untreated advanced non-small cell lung cancer. J. Clin. Oncol. 2010, 28, 7601. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Lara, P.N.; Le Chevalier, T.; Breton, J.-L.; Bonomi, P.; Sandler, A.B.; Socinski, M.A.; Delbaldo, C.; McHenry, B.; Lebwohl, D. Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non-small-cell lung cancer whose tumors have failed first-line platinum-based chemotherapy. J. Clin. Oncol. 2007, 25, 3448–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Menefee, M.; Edgerly, M.; Zhuang, S.; Kotz, H.; Poruchynsky, M.; Huff, L.M.; Bates, S.; Fojo, T. A phase II clinical trial of ixabepilone (Ixempra; BMS-247550; NSC 710428), an epothilone B analog, in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 2010, 16, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Rocha Lima, C.M.; Lin, E.H.; Kim, G.P.; Giguere, J.K.; Marshall, J.; Zalupski, M.; Papageorgio, C.; Auber, M.L.; Kaleta, R.; McHenry, M.B.; et al. A phase 2 trial of ixabepilone plus cetuximab in first-line treatment of metastatic pancreatic cancer. Gastrointest. Cancer Res. 2012, 5, 155–160. [Google Scholar]
- McCourt, C.K.; Deng, W.; Dizon, D.S.; Lankes, H.A.; Birrer, M.J.; Lomme, M.M.; Powell, M.A.; Kendrick, J.E.; Saltzman, J.N.; Warshal, D.; et al. A phase II evaluation of ixabepilone in the treatment of recurrent/persistent carcinosarcoma of the uterus, an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017, 144, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Roque, D.M.; Siegel, E.R.; Buza, N.; Bellone, S.; Silasi, D.-A.; Huang, G.S.; Andikyan, V.; Clark, M.; Azodi, M.; Schwartz, P.E.; et al. Randomised phase II trial of weekly ixabepilone ± biweekly bevacizumab for platinum-resistant or refractory ovarian/fallopian tube/primary peritoneal cancer. Br. J. Cancer 2022, 126, 1695–1703. [Google Scholar] [CrossRef]
- Thomas, E.S.; Gomez, H.L.; Li, R.K.; Chung, H.-C.; Fein, L.E.; Chan, V.F.; Jassem, J.; Pivot, X.B.; Klimovsky, J.V.; de Mendoza, F.H. Ixabepilone Plus Capecitabine for Metastatic Breast Cancer Progressing After Anthracycline and Taxane Treatment. J. Clin. Oncol. 2007, 25, 5210–5217. [Google Scholar] [CrossRef] [Green Version]
- Sparano, J.A.; Vrdoljak, E.; Rixe, O.; Xu, B.; Manikhas, A.; Medina, C.; Da Costa, S.C.; Ro, J.; Rubio, G.; Rondinon, M.; et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2010, 28, 3256–3263. [Google Scholar] [CrossRef]
- Rugo, H.S.; Roche, H.; Thomas, E.; Chung, H.C.; Lerzo, G.L.; Vasyutin, I.; Patel, A.; Vahdat, L. Efficacy and Safety of Ixabepilone and Capecitabine in Patients With Advanced Triple-negative Breast Cancer: A Pooled Analysis From Two Large Phase III, Randomized Clinical Trials. Clin. Breast Cancer 2018, 18, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Patel, T.; Moreno-Aspitia, A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res. Treat. 2010, 121, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Trédan, O.; Campone, M.; Jassem, J.; Vyzula, R.; Coudert, B.; Pacilio, C.; Prausova, J.; Hardy-Bessard, A.-C.; Arance, A.; Mukhopadhyay, P.; et al. Ixabepilone Alone or With Cetuximab as First-Line Treatment for Advanced/Metastatic Triple-Negative Breast Cancer. Clin. Breast Cancer 2015, 15, 8–15. [Google Scholar] [CrossRef]
- Yardley, D.A.; Arrowsmith, E.R.; Daniel, B.R.; Eakle, J.; Brufsky, A.; Drosick, D.R.; Kudrik, F.; Bosserman, L.D.; Keaton, M.R.; Goble, S.A.; et al. TITAN: Phase III study of doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early-stage triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 164, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, M.; Qiu, R.; Tang, L.; Dou, G.; Xu, B. Phase I clinical and pharmacokinetic study of UTD1, a genetically engineered epothilone analog in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2011, 68, 971–978. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Z.; Tian, F.; Wang, Y.; Yang, J.; Li, W.; Di, L.; Liu, W.; Tang, L.; Qiu, R. Phase II trial of utidelone as monotherapy or in combination with capecitabine in heavily pretreated metastatic breast cancer patients. J. Hematol. Oncol. 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Lv, H.; Niu, L.; Zhang, M.; Zeng, H.; Zhao, S.; Wang, J.; Sun, H.; Chen, S. Anti-HER2 antibody inetetamab plus camrelizumab and utidelone for pretreated HER2-positive advanced breast cancer: A single-arm, multicenter, phase 2 study. J. Clin. Oncol. 2022, 40, e13030. [Google Scholar] [CrossRef]
- Xu, B.; Sun, T.; Zhang, Q.; Zhang, P.; Yuan, Z.; Jiang, Z.; Wang, X.; Cui, S.; Teng, Y.; Hu, X.C.; et al. Efficacy of utidelone plus capecitabine versus capecitabine for heavily pretreated, anthracycline- and taxane-refractory metastatic breast cancer: Final analysis of overall survival in a phase III randomised controlled trial. Ann. Oncol. 2021, 32, 218–228. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Chen, J.; Liu, Y.; Wang, K.; Nie, J.; Wang, X.; Hao, C.; Yin, Y.; Wang, S. Chinese society of clinical oncology (CSCO) breast cancer guidelines 2022. Transl Breast Cancer Res 2022, 3, 13. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Zhao, Y.; Zhao, J.; Lin, L. 31P Efficacy and safety of utidelone in treatment-refractory advanced non-small cell lung cancer. Ann. Oncol. 2022, 33, S45–S46. [Google Scholar] [CrossRef]
- de Jonge, M.; Verweij, J. The epothilone dilemma. J. Clin. Oncol. 2005, 23, 9048–9050. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Dufresne, A.; Villanueva, C. Efficacy and safety of ixabepilone, a novel epothilone analogue. Clin. Breast Cancer 2007, 7, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Durando, X.; Dalenc, F.; Abrial, C.; Mouret-Reynier, M.A.; Herviou, P.; Kwiatkowski, F.; Chollet, P.; Roche, H.; Thivat, E. Neurotoxicity as a prognostic factor in patients with metastatic breast cancer treated with ixabepilone as a first-line therapy. Oncology 2015, 88, 180–188. [Google Scholar] [CrossRef]
- Ibrahim, N.K. Ixabepilone: Overview of Effectiveness, Safety, and Tolerability in Metastatic Breast Cancer. Front. Oncol. 2021, 11, 617874. [Google Scholar] [CrossRef]
- Roché, H.; Yelle, L.; Cognetti, F.; Mauriac, L.; Bunnell, C.; Sparano, J.; Kerbrat, P.; Delord, J.P.; Vahdat, L.; Peck, R.; et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J. Clin. Oncol. 2007, 25, 3415–3420. [Google Scholar] [CrossRef]
- Cavaletti, G.; Bogliun, G.; Marzorati, L.; Zincone, A.; Marzola, M.; Colombo, N.; Tredici, G. Peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer 1995, 75, 1141–1150. [Google Scholar] [CrossRef]
- Yardley, D.A.; Peacock, N.W.; Shastry, M.; Burris, H.A., 3rd; Bechhold, R.G.; Hendricks, C.B.; Yoshizawa, C.N.; Sing, A.P.; Hainsworth, J.D. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: Correlation of pathologic complete response with the 21-gene recurrence score. Breast Cancer Res. Treat. 2015, 154, 299–308. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rhoades, D.; Wang, Y.; Bai, R.; Hamel, E.; Aujay, M.; Sandoval, J.; Gavrilyuk, J. 12, 13-Aziridinyl epothilones. stereoselective synthesis of trisubstituted olefinic bonds from methyl ketones and heteroaromatic phosphonates and design, synthesis, and biological evaluation of potent antitumor agents. J. Am. Chem. Soc. 2017, 139, 7318–7334. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Sun, W. Systematic review of ixabepilone for treating metastatic breast cancer. Breast Cancer 2017, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Shelke, Y.G.; Dherange, B.D.; Kempema, A.; Lin, B.; Gu, C.; Sandoval, J.; Hammond, M.; Aujay, M.; Gavrilyuk, J. Design, synthesis, and biological investigation of epothilone b analogues featuring lactone, lactam, and carbocyclic macrocycles, epoxide, aziridine, and 1, 1-difluorocyclopropane and other fluorine residues. J. Org. Chem. 2020, 85, 2865–2917. [Google Scholar] [CrossRef] [PubMed]

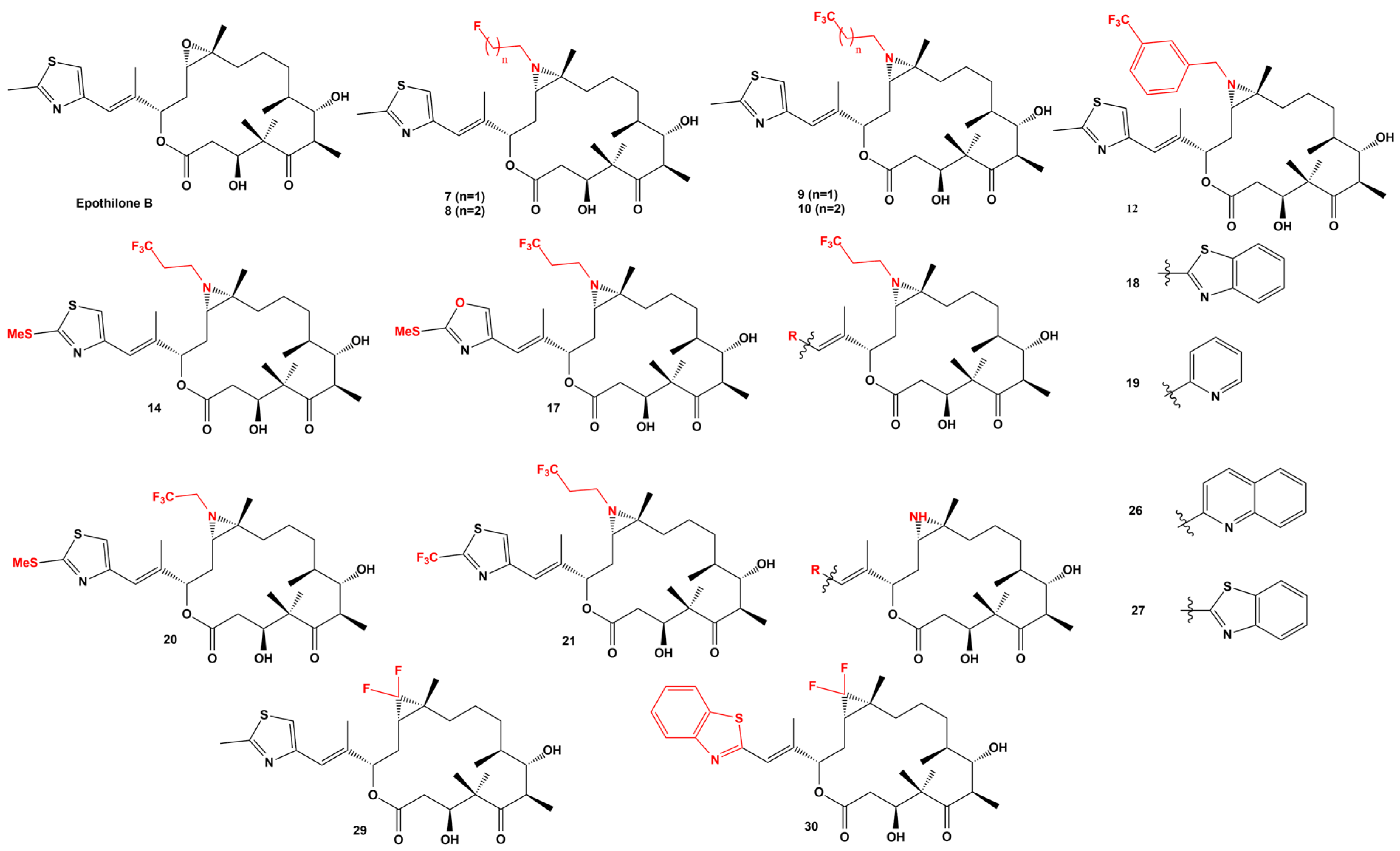
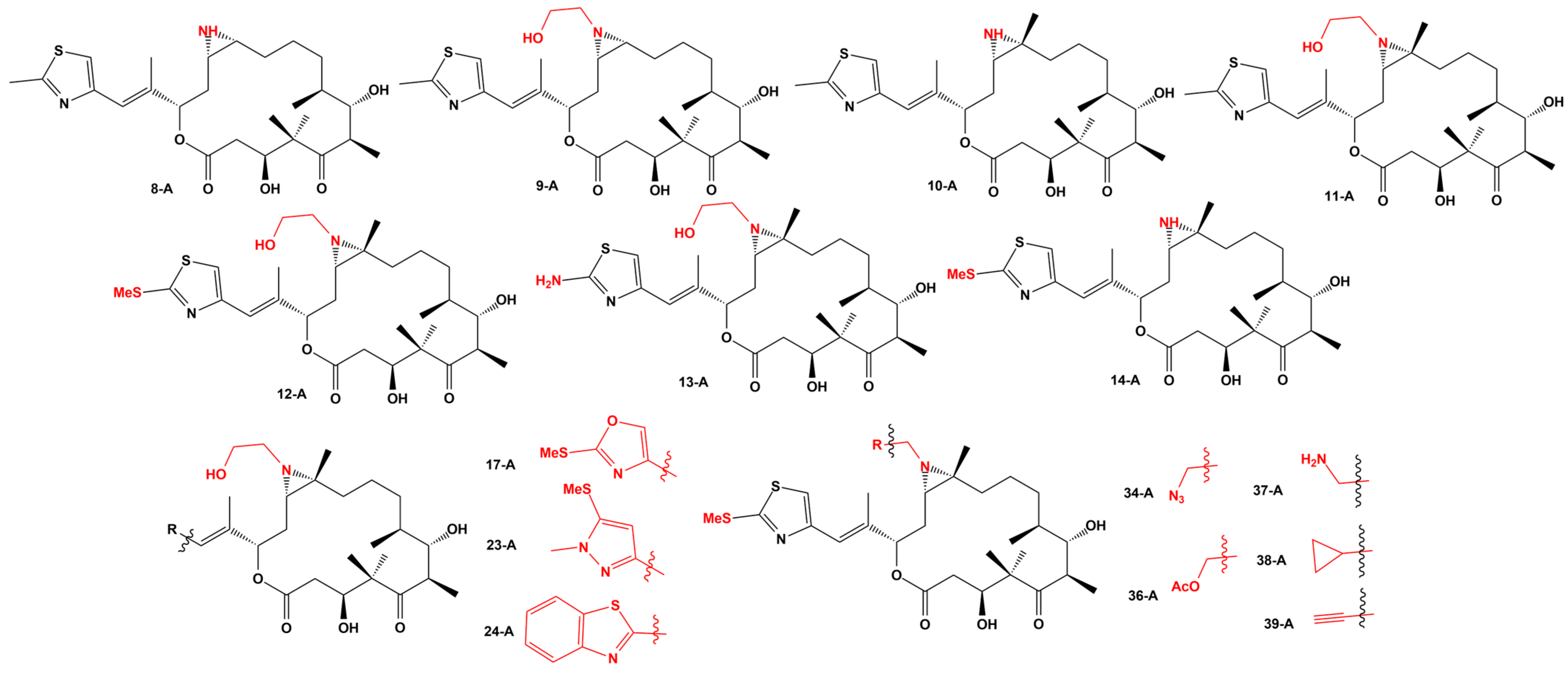
| Strains | Method | Results | Reference |
|---|---|---|---|
| S. cellulosum | Inactivation of the epoK gene by TALEN gene knockout system | Epothilone D yield increased to 34.9% and Epothilone B decreased to 34.2% | [33] |
| Schlegella brevitalea DSM 7029 | Heterologous expression of different plasmids created by BioBricksTM and SSRTA methods | Enhancement of Epothilone B production to 82 mg/L in 6 days of fermentation | [34] |
| S. cellulosum | Optimization of parameters to 30 °C, initial pH = 7.4, speed of 200 r/min, inoculation of 10%, loading amount of 50/250 mL, fermentation 6 days, seed age of 60 h. | Increasing epothilone B production to 39.76 mg/L | [35] |
| S. cellulosum | Enhance the epothilone gene cluster with a novel promoter P3 by TALE-TF and CRISPR/dCas9 | Epothilone B yield increased by 2.89- and 1.53-fold. Epothilone D yield improvement by 1.12- and 2.18-fold | [16] |
| S. cellulosum | Fermentation of S. cellulosum modified with plasmids pR6K-Amp-H.a-f-Ptet-H.a-r and pR6K-H.a-f-PBAD-H.a-r | Increasing the Epothilone B production to 93 mg/L | [36] |
| Burkholderiales strain DSM 7029 | Electroporation of epothilone gene cluster 56 kb to DSM 7029, plus methylmalonyl-CoA and overexpression of tRNA genes | Increase the yields of epothilones production by 75-fold to 307 μg/L | [37] |
| S. cellulosum | Fermentation of immobilized S. cellulosum into porous ceramics | Increasing by 4-Folds the epothilone production to 90.2 mg/L | [38] |
| Epothilone | Cell Line | Mechanism | Reference |
|---|---|---|---|
| Epothilone A |
|
| [61] |
| Epothilones A and B |
|
| [55] |
| Epothilone B |
|
| [50] |
| Epothilone B |
|
| [56] |
| Epothilone B |
|
| [58] |
| Iaxabepilone |
|
| [60] |
| Patupilone (EpoB) |
|
| [57] |
| Utidelone (UTD1) |
|
| [54] |
| Cell Line | Ixabepilone IC50 (nM) | Cell Line | Ixabepilone IC50 (nM) | Cell Line | Ixabepilone IC50 (nM) |
|---|---|---|---|---|---|
| A2780/DDP-S | 2.8 | A2780/DDP-R | 1.8 | A2780/TAX-S | 2.6 |
| A2780/TAX-R | 4.9 | OVCAR-3 | 1.8 | MCF-7 | 2.7 |
| SKBR3 | 2.3 | LNCAP | 1.5 | PC3 | 4.6 |
| HCT116 | 2.6 | HCT116/VM46 | 24.5 | HCT116/VP35 | 2.0 |
| LS174T | 5.8 | MIP | 24.8 | A549 | 5.2 |
| LX-1 | 3.1 | A431 | 1.4 | CCRF-CEM | 6.0 |
| K562 | 2.9 | M109 | 2.9 | MLF | 34.5 |
| Compound | MES SA DXE | MES SA DX | HEK 293T | SKBR3 | SKOV3 | HeLa |
|---|---|---|---|---|---|---|
| 7 | 0.33 | 0.55 | 0.02 | 0.60 | 0.10 | 0.61 |
| 8 | 0.36 | 0.91 | 0.05 | 0.94 | 0.17 | 0.78 |
| 9 | 0.01 | 0.03 | 0.001 | 0.02 | 0.01 | 0.02 |
| 10 | 0.03 | 0.61 | 0.05 | 0.59 | 0.18 | 0.40 |
| 12 | 0.99 | 2.30 | 0.35 | |||
| 14 | 0.48 | 0.51 | 0.05 | 0.85 | 0.16 | 0.52 |
| 17 | 0.35 | 0.63 | 0.05 | 1.03 | 0.12 | 0.86 |
| 18 | 0.43 | 0.46 | 0.05 | 0.72 | 0.08 | 0.43 |
| 19 | 0.44 | 0.66 | 0.06 | 1.35 | 0.30 | 0.80 |
| 20 | 0.52 | 0.44 | 0.04 | 1.57 | 0.26 | 1.26 |
| 21 | 0.28 | 0.65 | 0.05 | 0.49 | 0.13 | 0.28 |
| 26 | 0.92 | 20.62 | 0.52 | 2.01 | 2.02 | 2.46 |
| 27 | 0.78 | 3.01 | 0.30 | |||
| 29 | 0.10 | 0.19 | 0.02 | 0.19 | 0.04 | 0.10 |
| 30 | 0.33 | 0.28 | 0.03 | 0.53 | 0.14 | 0.54 |
| MMAE | 0.46 | 113.7 | 0.10 | 0.10 | 0.09 | 1.17 |
| EpoB | 1.49 | 3.63 | 0.33 | 2.32 | 1.27 | 1.87 |
| Ixabepilone | 7.72 | 278.4 | 2.72 | 9.29 | 8.41 | 9.75 |
| Compound | MCF-7 | OVCAR-8 | NCI/ADR-RES | MDA-MB-435 | SNB-75 | MES SA | MES SA DX | HEK 293 |
|---|---|---|---|---|---|---|---|---|
| 8-A | 2.5 | 5.5 | 38 | - | - | 1.14 | 16.95 | 0.95 |
| 9-A | 2.0 | 3.0 | 8.3 | - | - | 8.01 | 15.73 | 0.67 |
| 10-A | 2.0 | 1.5 | 35 | - | - | 0.04 | 0.51 | 0.02 |
| 11-A | 3.0 | 4.5 | 55 | - | - | 0.94 | 13.71 | 0.17 |
| 12-A | 28 | 75 | 55 | 42 | 60 | 0.13 | 0.66 | 0.03 |
| 13-A | 65 | 93 | 2800 | 20 | 130 | 0.078 | 0.85 | 0.058 |
| 14-A | 4.0 | 16 | 8.8 | 4.5 | 11 | 0.28 | 5.66 | 017 |
| 17-A | 7.5 | 25 | 6.5 | 3.5 | 13 | 0.02 | 1.11 | 0.05 |
| 23-A | 78 | 10 | 7.5 | 12 | 10 | 0.02 | 37.76 | 0.24 |
| 24-A | 11 | 23 | 630 | 3.5 | 12 | 0.18 | 1.32 | 0.06 |
| 34-A | 13 | 15 | 3.2 | 7.3 | 22 | 0.24 | 0.52 | 0.10 |
| 36-A | 5.5 | 18 | 7.0 | 3.5 | 23 | 0.29 | 0.86 | 0.07 |
| 37-A | 14 | 63 | 70 | 15 | 23 | 0.108 | 11.98 | 0.079 |
| 38-A | 18 | 15 | 18 | 9.5 | 16 | 0.056 | 1.257 | 0.051 |
| 39-A | 30 | 18 | 7.0 | 3.5 | 31 | 0.23 | 0.45 | 0.09 |
| Paclitaxel | 7.8 | 26 | 4800 | 5.0 | 15 | 2.47 | >400 | 1.76 |
| MMAE | - | - | - | - | - | 0.096 | 88.19 | 0.068 |
| NAC | - | - | - | - | - | 0.364 | 15.31 | 0.166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, C.; González-Chavarría, I.; Burgos, V.; Iturra-Beiza, H.; Ulrich, H.; Paz, C. Epothilones as Natural Compounds for Novel Anticancer Drugs Development. Int. J. Mol. Sci. 2023, 24, 6063. https://doi.org/10.3390/ijms24076063
Villegas C, González-Chavarría I, Burgos V, Iturra-Beiza H, Ulrich H, Paz C. Epothilones as Natural Compounds for Novel Anticancer Drugs Development. International Journal of Molecular Sciences. 2023; 24(7):6063. https://doi.org/10.3390/ijms24076063
Chicago/Turabian StyleVillegas, Cecilia, Iván González-Chavarría, Viviana Burgos, Héctor Iturra-Beiza, Henning Ulrich, and Cristian Paz. 2023. "Epothilones as Natural Compounds for Novel Anticancer Drugs Development" International Journal of Molecular Sciences 24, no. 7: 6063. https://doi.org/10.3390/ijms24076063
APA StyleVillegas, C., González-Chavarría, I., Burgos, V., Iturra-Beiza, H., Ulrich, H., & Paz, C. (2023). Epothilones as Natural Compounds for Novel Anticancer Drugs Development. International Journal of Molecular Sciences, 24(7), 6063. https://doi.org/10.3390/ijms24076063