Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Cholinesterase Inhibitory Activity
2.2. α-Amylase Inhibitory Activity
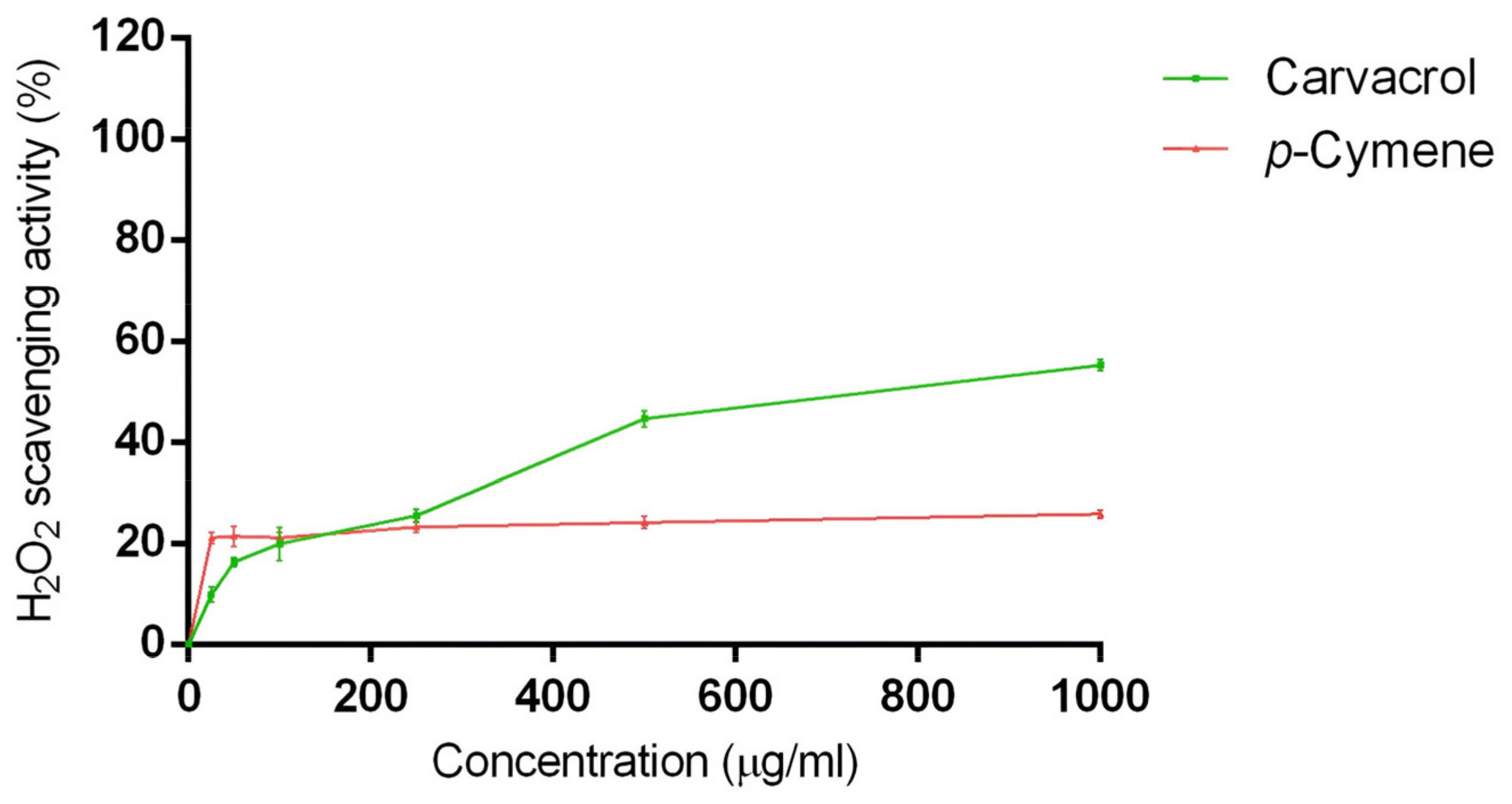
2.3. Hydrogen Peroxide Scavenging Activity
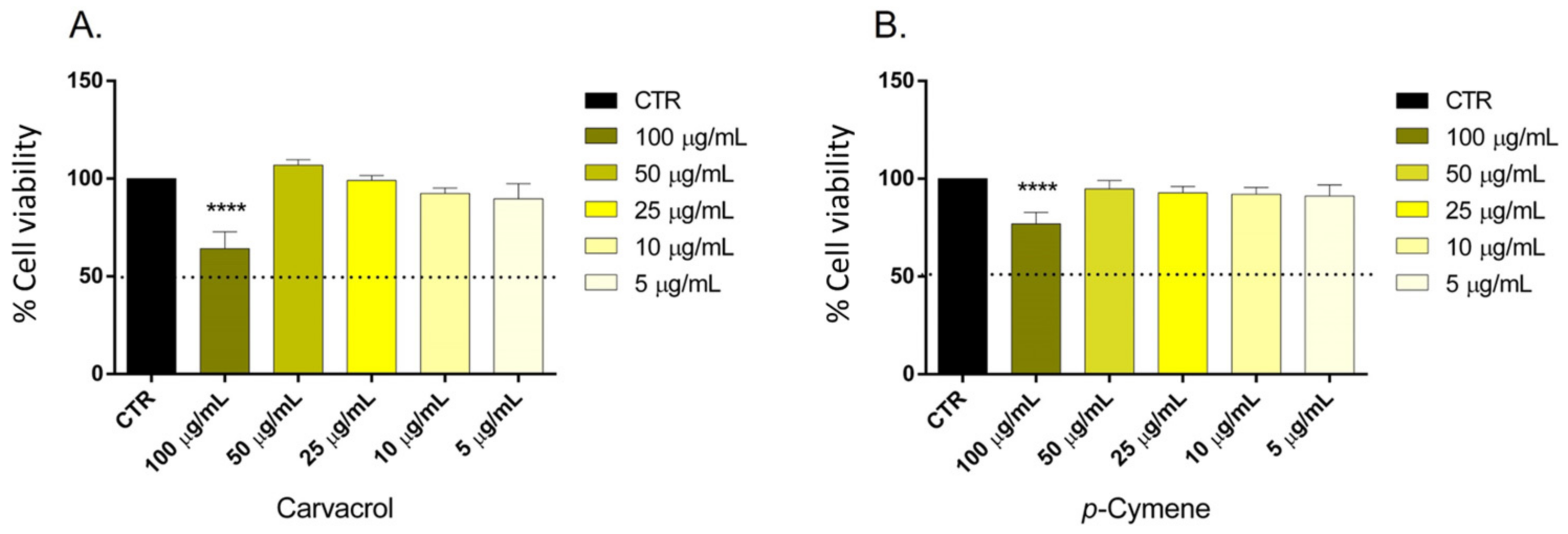
2.4. Determination of the Maximum Non-Toxic Dose (MNDT)
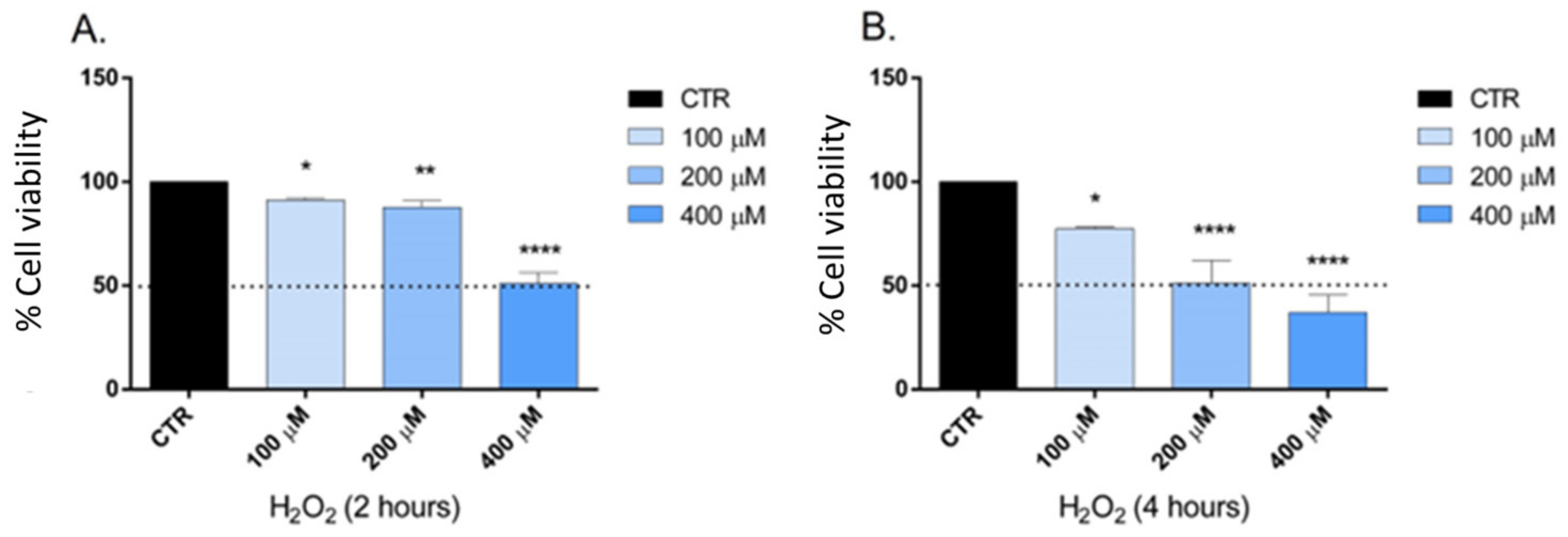
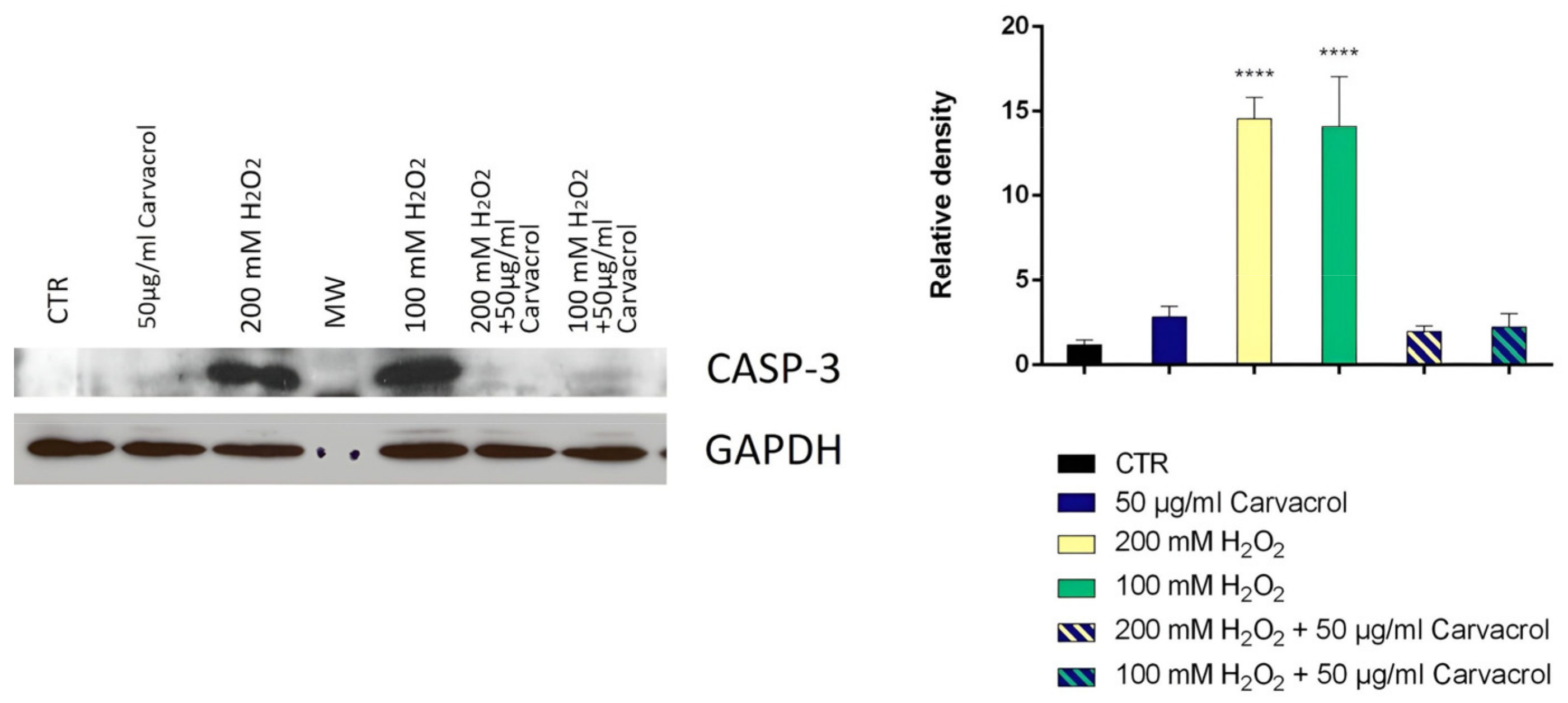
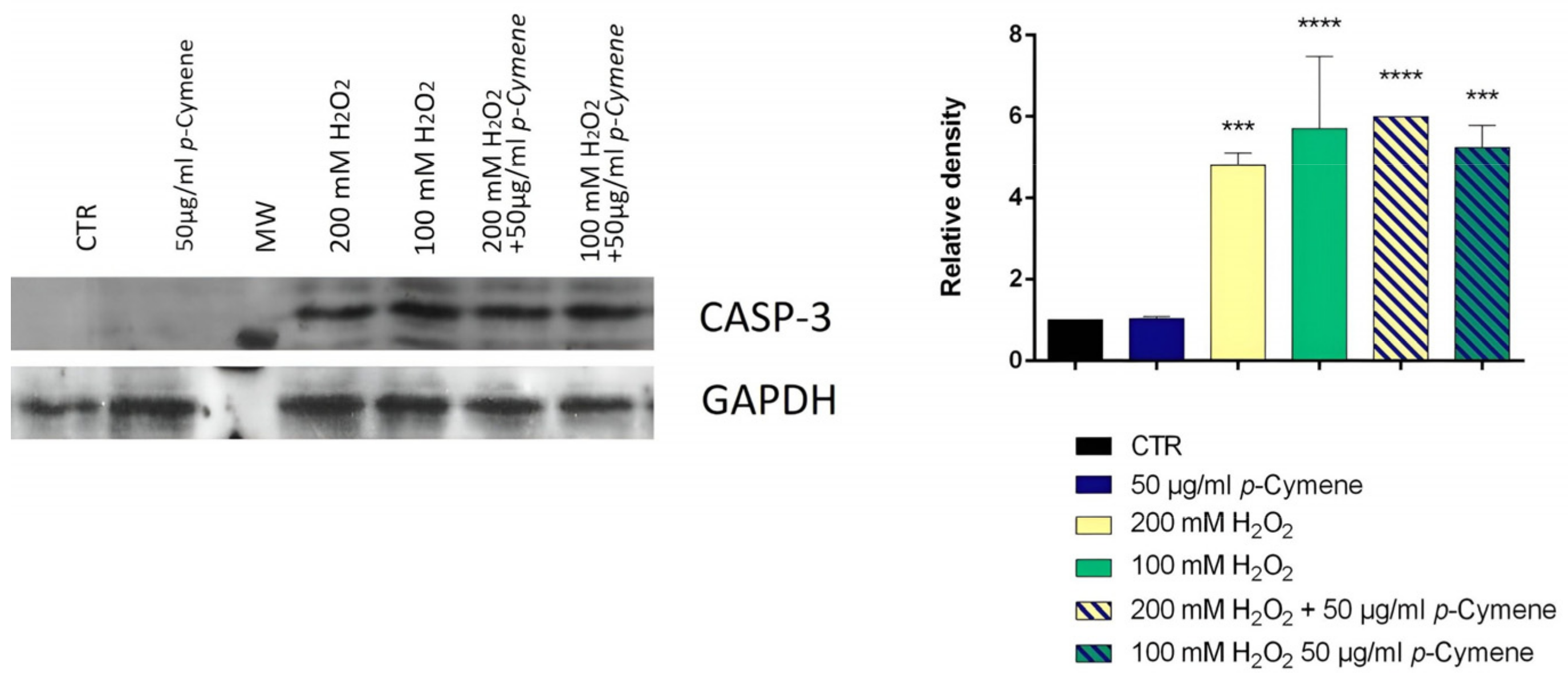
2.5. Determination of the Optimal Concentration of Hydrogen Peroxide and Caspase 3 Expression
3. Discussion
3.1. Cholinesterase Inhibitory Activity
3.2. α-Amylase Inhibitory Activity
3.3. Hydrogen Peroxide Scavenging Activity
3.4. Determination of the Maximum Non-Toxic Dose (MNDT)
4. Materials and Methods
4.1. Reagents
4.2. Cholinesterase Inhibition
4.3. α-Amylase Inhibition
4.4. Enzyme Activity
4.5. Hydrogen Peroxide Scavenging Assay
4.6. Cell Cultures
4.7. Determination of the Maximum Non-Toxic Dose (MNTD)
4.8. Determination of the Optimal Concentration of H2O2
4.9. Determination of Neuroprotective Effects of Carvacrol and p-Cymene
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manoharan, S.; Essa, M.M.; Vinoth, A.; Kowsalya, R.; Manimaran, A.; Selvasundaram, R. Alzheimer’s disease and medicinal plants: An overview. Adv. Neurobiol. 2016, 12, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Small, D.H.; Avila, J.; Sáez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with p-tau and β-amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef]
- Darvesh, S. Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 1173–1177. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Guillozet, A.L.; Mesulam, M.M.; Smiley, J.F.; Mash, D.C. Butyrylcholinesterase in the Life Cycle of Amyloid Plaques. Ann. Neurol. 1997, 42, 909–918. [Google Scholar] [CrossRef]
- Li, S.; Li, A.J.; Travers, J.; Xu, T.; Sakamuru, S.; Klumpp-Thomas, C.; Huang, R.; Xia, M. Identification of compounds for butyrylcholinesterase inhibition. SLAS Discov. 2021, 26, 1355–1364. [Google Scholar] [CrossRef]
- Kim, H.G.; Oh, M.S. Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 57–75. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Faqih, N.T.; Ashoor, A.F.; Alshaikh, S.A.; Maglan, A.F.; Jastaniah, N. Association of Alzheimer’s disease and insulin resistance in King Abdulaziz Medical City, Jeddah. Cureus 2021, 13, 19811. [Google Scholar] [CrossRef]
- Blencowe, M.; Furterer, A.; Wang, Q.; Gao, F.; Rosenberger, M.; Pei, L.; Nomoto, H.; Mawla, A.M.; Huising, M.O.; Coppola, G.; et al. IAPP-induced beta cell stress recapitulates the islet transcriptome in type 2 diabetes. Diabetologia 2022, 65, 173–187. [Google Scholar] [CrossRef]
- De Nazareth, A.M. Type 2 diabetes mellitus in the pathophysiology of Alzheimer’s disease. Dement. Neuropsychol. 2017, 11, 105–113. [Google Scholar] [CrossRef]
- Mushtaq, G.H.; Greig, N.; Khan, J.; Kamal, M. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Agarwal, P.; Gupta, R. Alpha-amylase inhibition can treat diabetes mellitus. Res. Rev. J. Med. Health Sci. 2016, 5, 1–8. [Google Scholar]
- Byman, E.; Schultz, N.; Netherlands Brain Bank; Fex, M.; Wennström, M. Brain alpha-amylase: A novel energy regulator important in Alzheimer disease? Brain Pathol. 2018, 28, 920–932. [Google Scholar] [CrossRef]
- Bnouham, M.; Ziyya, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990–2000). Int. J. Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Hutchings, D.; Vanoli, A.; Mckeith, I.; Brotherton, S.; Mcnamee, P.; Bond, J. Cholinesterase inhibitors and Alzheimer’s disease: Patient, carer and professional factors influencing the use of drugs for Alzheimer’s disease in the United Kingdom. Dementia 2010, 9, 427–443. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The antioxidant activity of limonene counteracts neurotoxicity triggered by Aβ1–42 oligomers in primary cortical neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Heghes essential oil-bearing plants from Balkan peninsula: Promising sources for new drug candidates for the prevention and treatment of diabetes mellitus and dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Reguilon, M.D.; Mińarro, J.; De Feo, V.; Rodriguez-Arias, M. Lavandula angustifolia Essential Oil and Linalool Counteract Social Aversion Induced by Social Defeat. Molecules 2018, 23, 2694. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Piccialli, I.; Ciccone, R.; de Caprariis, P.; Massa, A.; De Feo, V.; Pannaccione, A. Lavander and Coriander essential oils and their main contistuent linalool exert a protective effect against Amyloid β neurotoxicity. Phytother. Res. 2021, 35, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.; Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Chun, H.S. p-Cymene and its derivatives exhibit antiaflatoxigenic activities against Aspergillus flavus through multiple modes of action. Appl. Biol. Chem. 2018, 61, 489–497. [Google Scholar] [CrossRef]
- Xie, G.; Chen, N.; Soromou, L.W.; Liu, F.; Xiong, Y.; Wu, Q.; Li, H.; Feng, H.; Liu, G. p-Cymene protects mice against lipopolysaccharide-induced acute lung injury by inhibiting inflammatory cell activation. Molecules 2012, 17, 8159–8173. [Google Scholar] [CrossRef]
- De Oliveira, T.M.; de Carvalho, R.B.; da Costa, I.H.; de Oliveira, G.A.; de Souza, A.A.; de Lima, S.G.; de Freitas, R.M. Evaluation of p-cymene, a natural antioxidant. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’Errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the therapeutic potential of phytochemicals in Alzheimer’s disease: Focus on polyphenols and monoterpenes. Front. Pharmacol. 2022, 13, 876614. [Google Scholar] [CrossRef]
- Dos Santos Cardoso, A.; Santos, E.G.G.; da Silva Lima, A.; Temeyer, K.B.; de Leon, A.A.P.; Junior, L.M.C.; dos Santos Soares, A.M. Terpenes on Rhipicephalus (Boophilus) microplus: Acaricidal activity and acetylcholinesterase inhibition. Vet. Parasitol. 2020, 280, 109090. [Google Scholar] [CrossRef]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef]
- Lee, B.H.; Nam, T.G.; Park, W.J.; Kang, H.; Heo, H.J.; Chung, D.K.; Kim, G.H.; Kim, D.O. Antioxidative and neuroprotective effects of volatile components in essential oils from Chrysanthemum indicum Linné flowers. Food Sci. Biotechnol. 2015, 24, 717–723. [Google Scholar] [CrossRef]
- Diken, M.E.; Yılmaz Kardas, B. Inhibitory effect on acetylcholinesterase and toxicity analysis of some medicinal plants. Int. J. Second. Metab. 2022, 9, 27–42. [Google Scholar] [CrossRef]
- Zengin Kurt, B.; Durdagi, S.; Celebi, G.; Ekhteiari Salmas, R.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modeling studies of novel carvacrol-substituted amide derivatives. J. Biomol. Struct. Dyn. 2020, 38, 841–859. [Google Scholar] [CrossRef]
- Georgiev, B.; Nikolova, M.; Aneva, I.; Dzhurmanski, A.; Sidjimova, B.; Berkov, S. Plant products with acetylcholinesterase inhibitory activity for insect control. BioRisk 2022, 17, 309–315. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef]
- Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From ancient flavoring to neuromodulatory agent. Molecules 2013, 18, 6161–6172. [Google Scholar] [CrossRef] [PubMed]
- Siahbalaei, R.; Kavoosi, G.; Shakeri, R. In vitro antioxidant and antidiabetic activity of essential oils encapsulated in gelatin-pectin particles against sugar, lipid and protein oxidation and amylase and glucosidase activity. Food Sci. Nutr. 2020, 8, 6457–6466. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Nahid, S.; Manik, A.S.; Rahman, M.S.; Rumpa, F.T.; Majumder, M.; Paul, A. In silico molecular docking analysis of isolated compounds of Ocimum sanctum against two related targets to diabetes. Pharma Innov. Int. J. 2017, 6, 148–151. [Google Scholar]
- Stojanović, N.M.; Stevanović, M.; Randjelović, P.; Mitić, K.; Petrović, V.; Sokolović, D.; Mladenović, B.; Lalić, J.; Radulović, N.S. Low dose of carvacrol prevents rat pancreas tissue damage after l-arginine application, while higher doses cause pancreatic tissue impairment. Food Chem. Toxicol. 2019, 128, 280–285. [Google Scholar] [CrossRef]
- Kiliç, Y.; Geyikoglu, F.; Çolak, S.; Turkez, H.; Bakır, M.; Hsseinigouzdagani, M. Carvacrol modulates oxidative stress and decreases cell injury in pancreas of rats with acute pancreatitis. Cytotechnology 2016, 68, 1243–1256. [Google Scholar] [CrossRef]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–189. [Google Scholar] [CrossRef]
- Kabdal, T.; Kumar, R.; Prakash, O.; Nagarkoti, K.; Rawat, D.S.; Srivastava, R.M.; Kumar, S.; Dubey, S.K. Seasonal variation in the essential oil composition and biological activities of Thymus linearis Benth. Collected from the Kumaun region of Uttarakhand, India. Biochem. Syst. Ecol. 2022, 103, 104449. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Haile, T.; Cardoso, S.M.; de Oliveira Raphaelli, C.; Pereira, O.R.; Pereira, E.D.S.; Vizzotto, M.; Nora, L.; Asfaw, A.A.; Periasamy, G.; Karim, A. Chemical composition, antioxidant potential, and blood glucose lowering effect of aqueous extract and essential oil of Thymus Serrulatus Hochst. Ex Benth. Front. Pharmacol. 2021, 12, 858. [Google Scholar] [CrossRef]
- Aristatile, B.; Al-Numair, K.S.; Veeramani, C.; Pugalendi, K.V. Effect of carvacrol onhepatic marker enzymes and antioxidant statusin d-galactosamine-induced hepatotoxicity in rats. Fundam. Clin. Pharmacol. 2009, 23, 757–765. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Seifi-Nahavandi, B.; Yaghmaei, P.; Ahmadian, S.; Ghobeh, M.; Ebrahim-Habibi, A. Cymene consumption and physical activity effect in Alzheimer’s disease model: An in vivo and in vitro study. J. Diabetes Metab. Disord. 2020, 19, 1381–1389. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kiziltan, H.S.; Kocyigit, A.; Güler, E.M.; Karataş, E.; Toprak, A. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anticancer Drugs 2017, 28, 522–530. [Google Scholar] [CrossRef]
- Krstic, M.; Sovilj, S.P.; Grguri, Ć.; Šipka, S.; Evans, I.R.; Borozan, S.; Santibanez, J.F. Synthesis, structural and spectroscopic characterization, in vitro cytotoxicity and in vivo activity as free radical scavengers of chloride (p-cymene) complexes of ruthenium (II) containing N-alkylphenothiazines. Eur. J. Med. Chem. 2011, 46, 4168–4177. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Lu, R.; Qian, Q.; Lei, X.; Xiao, Q.; Huang, S.; Liu, L.; Huang, C.; Su, W. Synthesis, X-ray diffraction study, and cytotoxicity of a cationic p-cymene ruthenium chloro complex containing a chelating semicarbazone ligand. J. Inorg. Gen. Chem. 2013, 639, 943–946. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef]
- Quintans, J.D.S.S.; Menezes, P.P.; Santos, M.R.; Bonjardim, L.R.; Almeida, J.R.; Gelain, D.P.; Quintans-Júnior, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef]
- Hu, X.; Chu, Y.; Ma, G.; Li, W.; Wang, X.; Mo, H.; Yin, Q.; Guo, J.; Ma, X.; Zhou, S. Simultaneous determination of ascaridole, p-cymene and α-terpinene in rat plasma after oral administration of Chenopodium ambrosioides L. by GC-MS. Biomed. Chromatogr. 2015, 29, 1682–1686. [Google Scholar] [CrossRef]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Topkara, K.C.; Kilinc, E.; Cetinkaya, A.; Saylan, A.; Demir, S. Therapeutic effects of carvacrol on beta-amyloid-induced impairments in in vitro and in vivo models of Alzheimer’s disease. Eur. J. Neurosci. 2022, 56, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.W.; Xie, Z.X.; Wang, B.F.; Zhong, Z.H.; Chen, X.Y.; Sun, Y.H.; Sun, Q.F.; Yang, G.Y.; Bian, L.G. Carvacrol protects neuroblastoma SH-SY5Y cells against Fe(2+)-induced apoptosis by suppressing activation of MAPK/JNK-NF-κB signaling pathway. Acta Pharmacol. Sin. 2015, 36, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Akter, M.; Corpus Bondad, S.E.; Saito, T.; Hosokawa, T.; Kurasaki, M. Carvacrol inhibits cadmium toxicity through combating against caspase dependent/independent apoptosis in PC12 cells. Food Chem. Toxicol. 2019, 134, 110835. [Google Scholar] [CrossRef] [PubMed]
- Dati, L.M.; Ulrich, H.; Real, C.C.; Feng, Z.P.; Sun, H.S.; Britto, L.R. Carvacrol promotes neuroprotection in the mouse hemiparkinsonian model. Neuroscience 2017, 356, 176–181. [Google Scholar] [CrossRef]
- Chenet, A.L.; Duarte, A.R.; de Almeida, F.J.S.; Andrade, C.M.B.; de Oliveira, M.R. Carvacrol Depends on Heme Oxygenase-1 (HO-1) to Exert Antioxidant, Anti-inflammatory, and Mitochondria-Related Protection in the Human Neuroblastoma SH-SY5Y Cells Line Exposed to Hydrogen Peroxide. Neurochem. Res. 2019, 44, 884–896. [Google Scholar] [CrossRef]
- Delgado-Marín, L.; Sánchez-Borzone, M.; García, D.A. Neuroprotective effects of gabaergic phenols correlated with their pharmacological and antioxidant properties. Life Sci. 2017, 175, 11–15. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Sun, H.; Wang, H.; Peng, W.; Zhou, Z.; Hongwei, W.; Chenchen, P.; Yingai, S.; He, X. Metabolism: A novel shared link between diabetes mellitus and Alzheimer’s disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef]
- Chornenkyy, Y.; Wang, W.X.; Wei, A.; Nelson, P.T. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. 2019, 29, 3–17. [Google Scholar] [CrossRef]
- De la Monte, S.M. Relationships between diabetes and cognitive impairment. Endocrinol. Metab. Clin. N. Am. 2014, 43, 245–267. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Van Giau, V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.E.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B.E. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 15, 332. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Mitra, A.; Manjunatha, M. A comparative study of alpha amylase inhibitory activities of common anti-diabetic plants at Kharagpur 1 block. Int. J. Green Pharm. 2010, 4, 115–121. Available online: https://www.ijp-online.com/text.asp?2010/42/5/280/70107 (accessed on 22 October 2022).
- Mukhopadhyay, D.; Dasgupta, P.; Roy, D.S.; Palchoudhuri, S.; Chatterjee, I.; Ali, S.; Dastidar, S.G. A sensitive in vitro spectrophotometric hydrogen peroxide scavenging assay using 1,10-phenanthroline. Free Radic Antioxid. 2016, 6, 124–132. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Festa, M.; Del Valle, L.; Khalili, K.; Franco, R.; Scognamiglio, G.; Graziano, V.; Rosati, A. BAG3 protein is overexpressed in human glioblastoma and is a potential target for therapy. Am. J. Pathol. 2011, 178, 2504–2512. [Google Scholar] [CrossRef]
| Compound | IC50 a (μg/mL) | ||
|---|---|---|---|
| AChE | BChE | α-Amylase | |
| Carvacrol | 3.8 ± 1.3 **,#### | 32.7 ± 5.5 #### | 171.2 ± 10.8 |
| p-Cymene | 15.2 ± 3.6 **** | 1456.0 ± 56.9 **** | 215.2 ± 12.6 |
| Galantamine | 0.6 ± 0.3 | 4.5 ± 1.2 | - |
| Acarbose | - | - | 34.5 ± 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. https://doi.org/10.3390/ijms24076073
Caputo L, Amato G, De Martino L, De Feo V, Nazzaro F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. International Journal of Molecular Sciences. 2023; 24(7):6073. https://doi.org/10.3390/ijms24076073
Chicago/Turabian StyleCaputo, Lucia, Giuseppe Amato, Laura De Martino, Vincenzo De Feo, and Filomena Nazzaro. 2023. "Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells" International Journal of Molecular Sciences 24, no. 7: 6073. https://doi.org/10.3390/ijms24076073
APA StyleCaputo, L., Amato, G., De Martino, L., De Feo, V., & Nazzaro, F. (2023). Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. International Journal of Molecular Sciences, 24(7), 6073. https://doi.org/10.3390/ijms24076073










