Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis
Abstract
:1. Introduction
2. Results
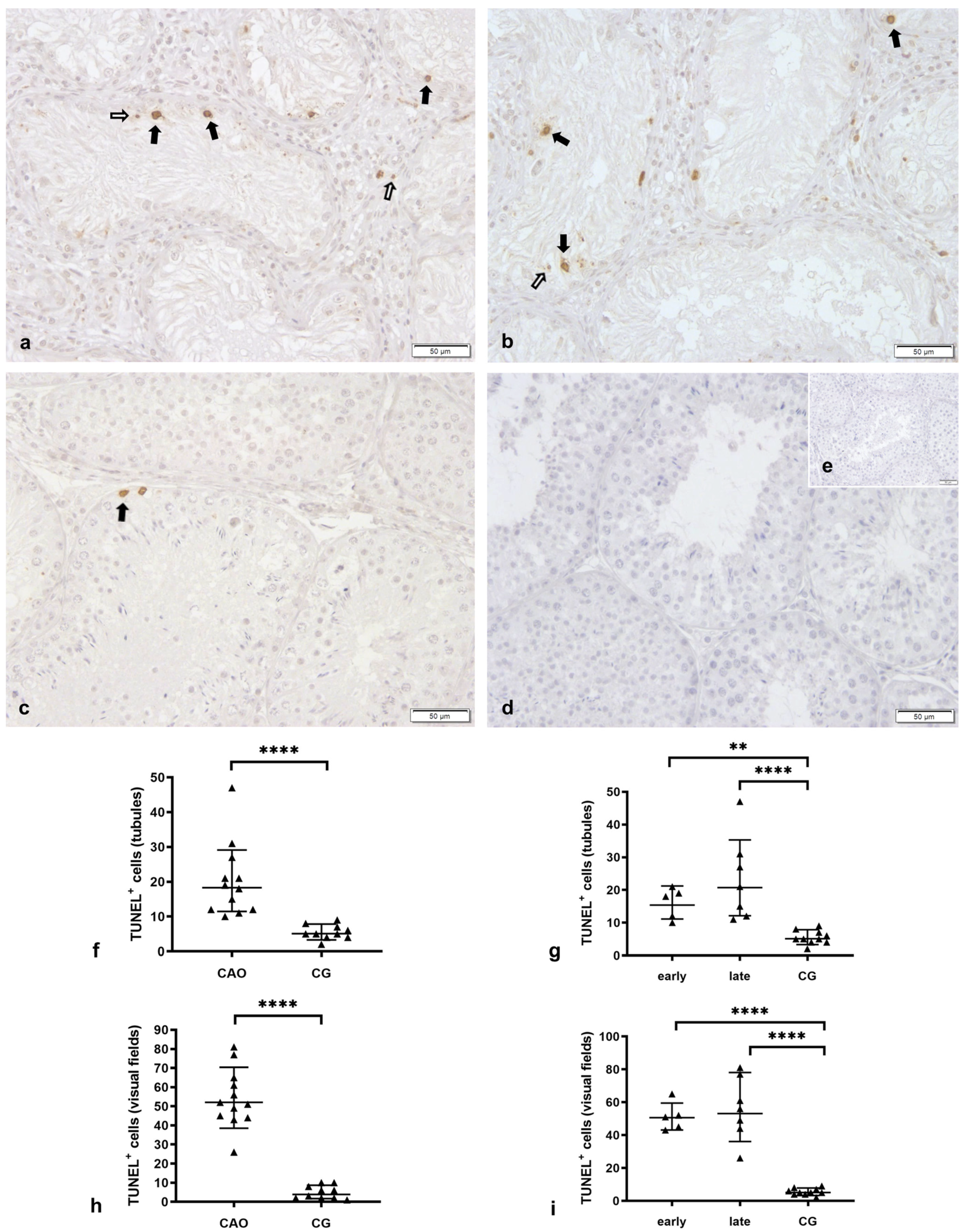
2.1. Number of Apoptotic Cells by TUNEL Method
Apoptosis Correlates with the Degree of Spermatogenesis Disturbance
2.2. Intrinsic Pathway of Apoptosis
2.2.1. Bcl-2 Is Upregulated in CAO
2.2.2. Bcl-2 Upregulation in CAO Is Associated with CD20-Expressing B Lymphocytes
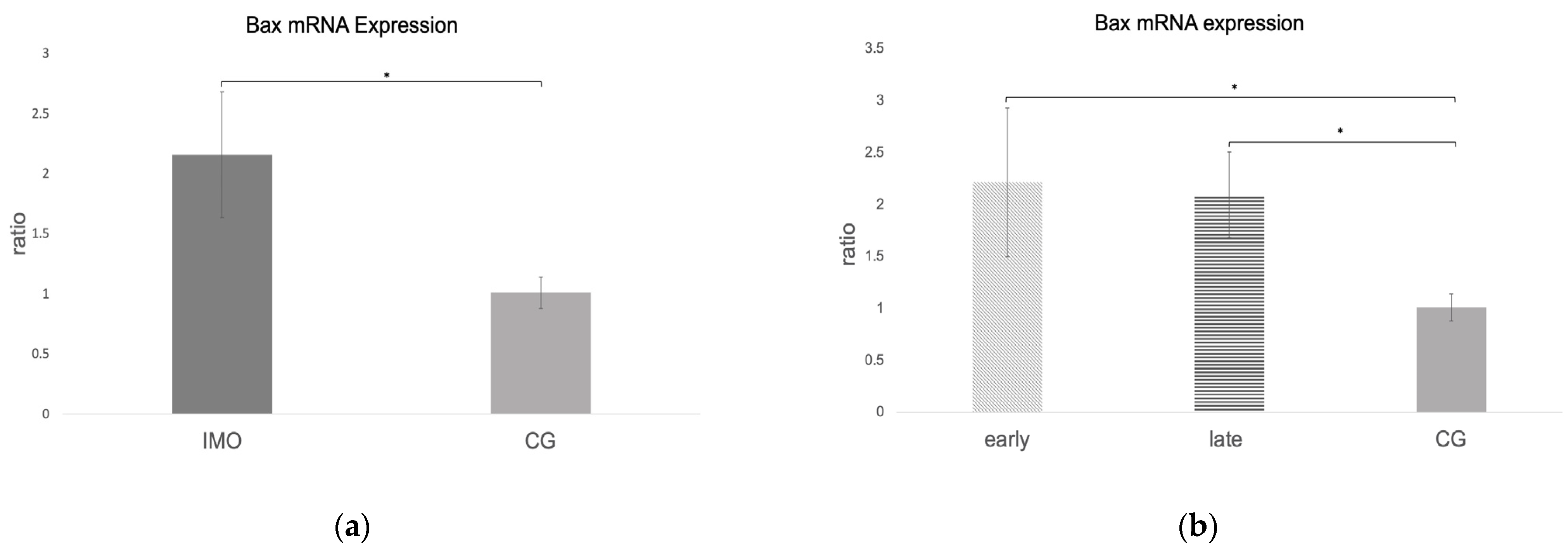
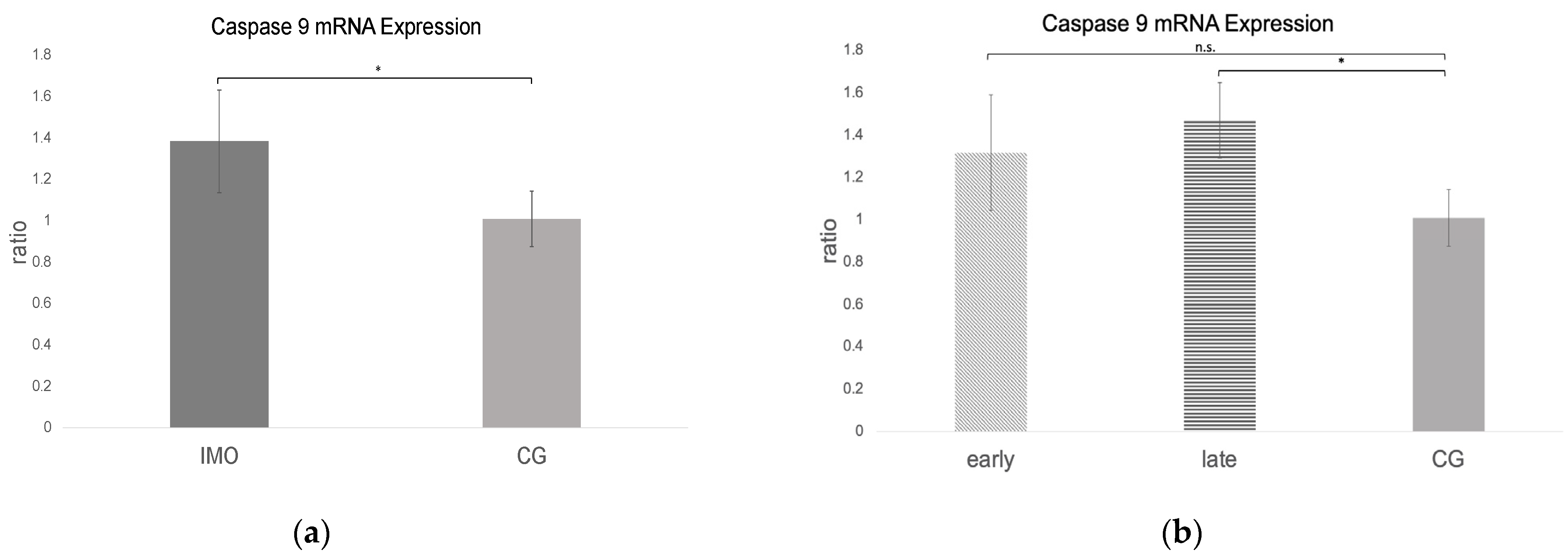
2.2.3. Bax and Caspase 9 mRNA Expression Is Increased in CAO
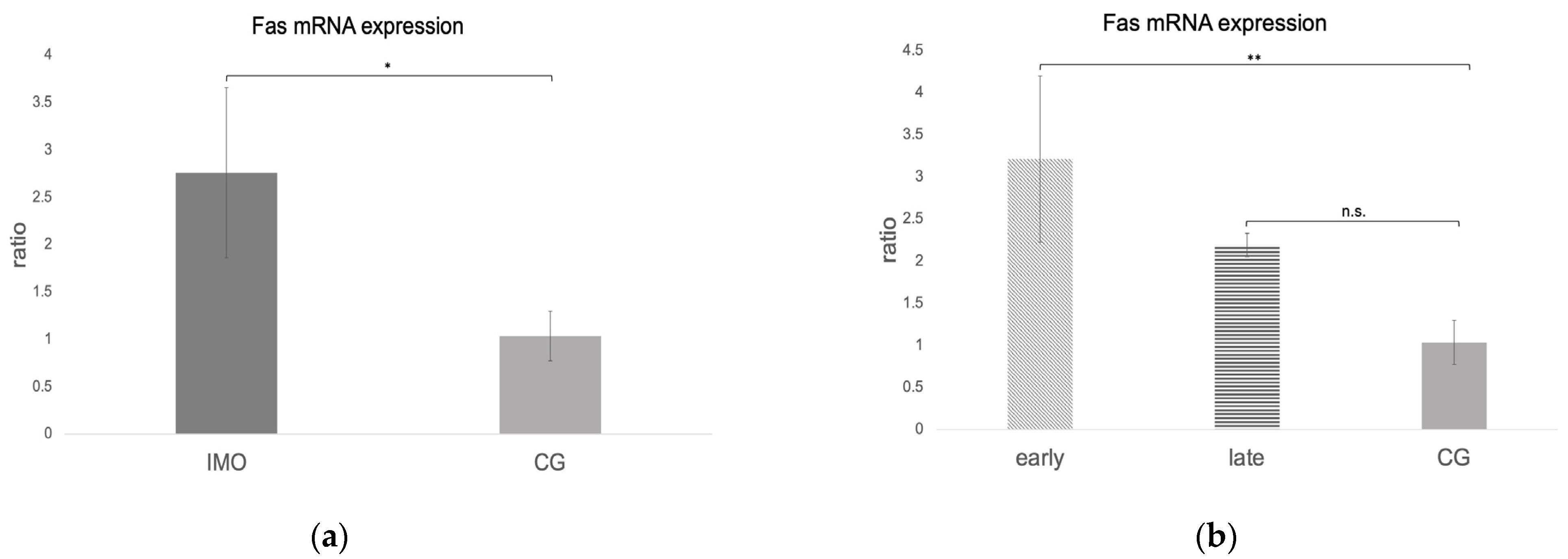
2.3. Extrinsic Pathway
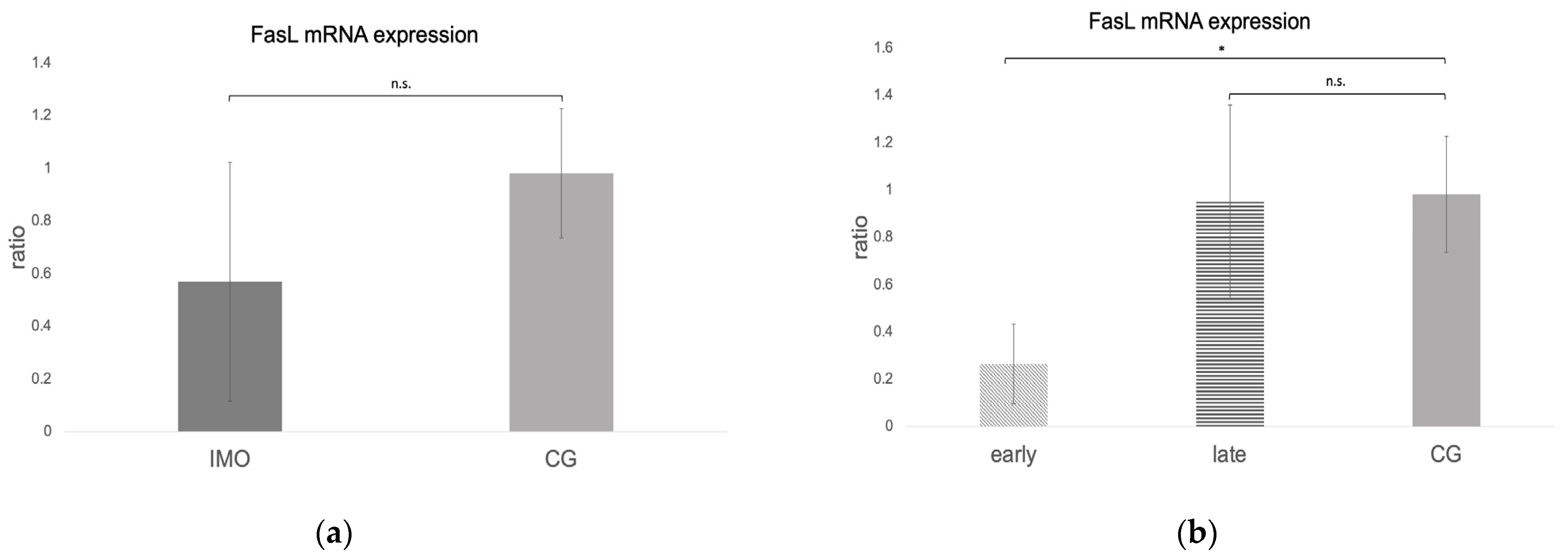
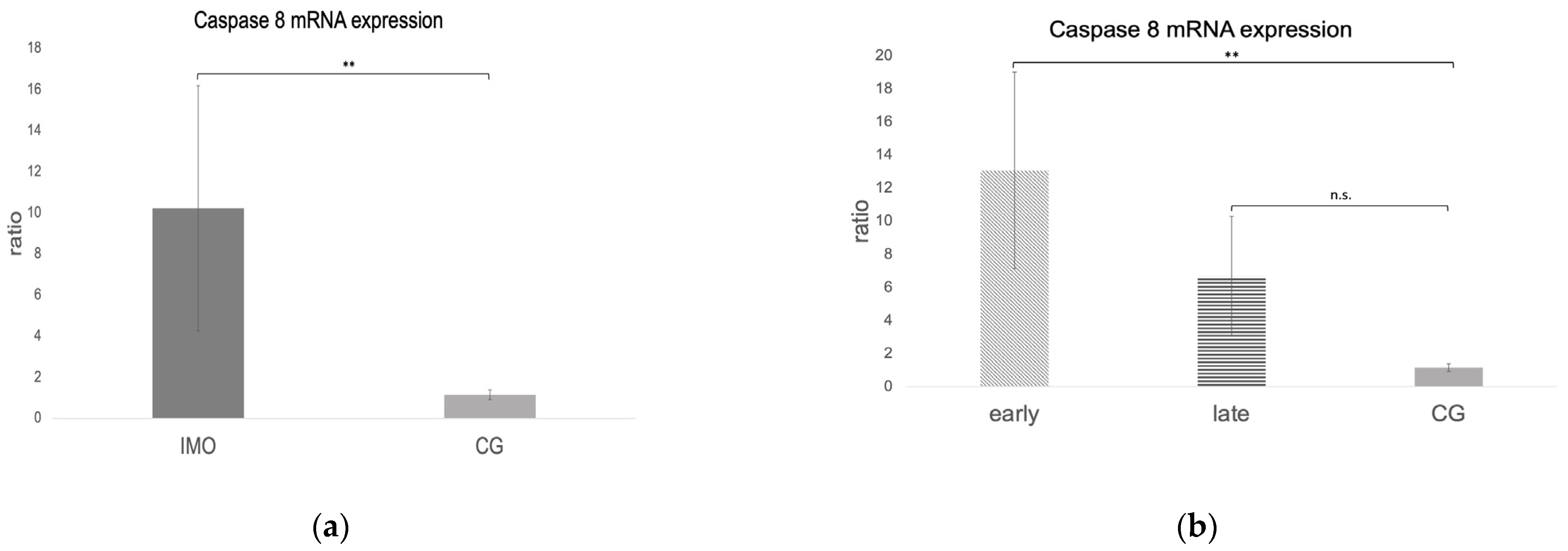
Fas, FasLigand, and Caspase 8 mRNA Expression
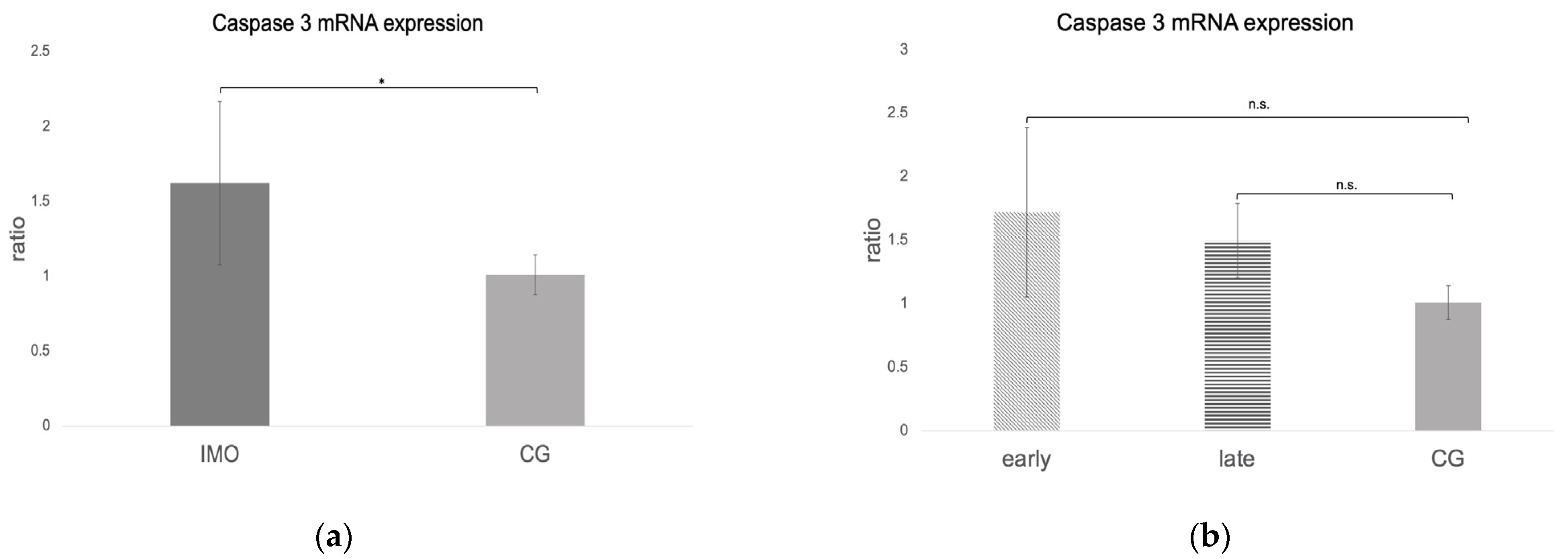
2.4. Caspase 3 mRNA and Protein Expression
3. Discussion
4. Materials and Methods
4.1. Study Design, Animals, and Grouping
4.2. Study Design, Sample Collection, and Processing
4.3. Quantitative Real-Time PCR (RT-qPCR)
4.4. Protein Extraction and Western Blot Analyses
4.5. TUNEL Method, Procedure, and Evaluation
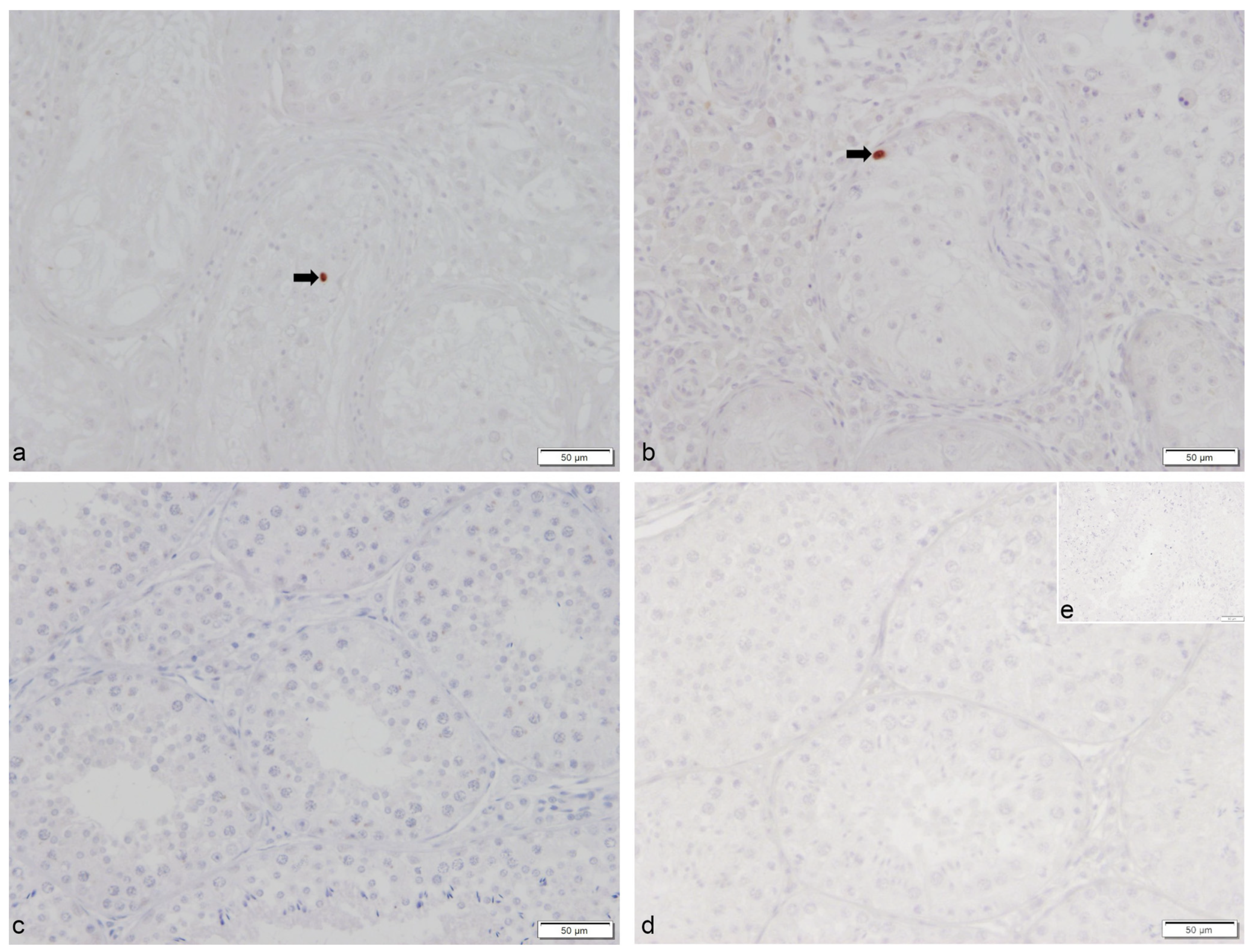
4.6. Immunohistochemistry and Evaluation of Bcl-2, CD20, and Caspase 3 Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romagnoli, S. Two Common Causes of Infertility in the Male Dog. Proc. World Small Anim. Vet. Assoc. 2006, 687–690. [Google Scholar]
- Behrens Mathiesen, C.; Körber, H.; Goericke-Pesch, S. Inflammatory Changes in Dogs with Azoospermia—A Normal Finding? Reprod. Dom. Anim. 2017, 52, 8–9. [Google Scholar]
- Goericke-Pesch, S.; Wehrend, A. Determination of the Alkaline Phosphatase in Canine Seminalplasma to Differentiate Azoospermia and Incomplete Ejaculation (German). Tierärztl. Prax. 2008, 36, A24. [Google Scholar]
- Romagnoli, S.; Bonaccini, P.; Stelletta, C.; Garolla, A.; Menegazzo, M.; Foresta, C.; Mollo, A.; Milani, C.; Gelli, D. Clinical Use of Testicular Fine Needle Aspiration Cytology in Oligozoospermic and Azoospermic Dogs. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 329–333. [Google Scholar] [CrossRef] [PubMed]
- Goericke-Pesch, S.; Reifarth, L.; Behrens Mathiesen, C.; Schuler, G.; Umbach, A.K.; Korber, H. Chronic Immune-Mediated Orchitis Is the Major Cause of Acquired Non-Obstructive Azoospermia in Dogs. Front. Vet. Sci. 2022, 9, 865967. [Google Scholar] [CrossRef] [PubMed]
- Fritz, T.E.; Lombard, S.A.; Tyler, S.A.; Norris, W.P. Pathology and Familial Incidence of Orchitis and Its Relation to Thyroiditis in a Closed Beagle Colony. Exp. Mol. Pathol. 1976, 24, 142–158. [Google Scholar] [CrossRef]
- Pröbstl, C.; Umbach, A.; Beineke, A.; Korber, H.; Goericke-Pesch, S. Immune Cell Characterization in Spontaneous Autoimmune Orchitis in Dogs. Theriogenology 2022, 187, 219–226. [Google Scholar] [CrossRef]
- Allen, W.E.; Longstaffe, J.A. Spermatogenic Arrest Associated with Focal Degenerative Orchitis in Related Dogs. J. Small Anim. Pract. 1982, 23, 337–343. [Google Scholar] [CrossRef]
- Allen, W.E.; Patel, J.R. Autoimmune Orchitis in Two Related Dogs. J. Small Anim. Pract. 1982, 23, 713–718. [Google Scholar] [CrossRef]
- Casal, M.L. Canine Autoimmune Orchitis. Clin. Theriogenol. 2012, 4, 251–254. [Google Scholar]
- Davidson, A.P.; von Dehn, B.J.; Schlafer, D.H. Adult-Onset Lymphoplasmacytic Orchitis in a Labrador Retriever Stud Dog. Top. Companion Anim. Med. 2015, 30, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Matschurat, C.; Rode, K.; Hollenbach, J.; Wolf, K.; Urhausen, C.; Beineke, A.; Gunzel-Apel, A.R.; Brehm, R. Impaired Spermatogenesis, Tubular Wall Disruption, Altered Blood-Testis Barrier Composition and Intratubular Lymphocytes in an Infertile Beagle Dog—A putative Case of Autoimmune Orchitis. Histol. Histopathol. 2019, 34, 525–535. [Google Scholar] [CrossRef]
- Metcalfe, S.S.; Gunn, I.M.; Champness, K.A. Azoospermia in Two Labrador Retrievers. Aust. Vet. J. 1999, 77, 570–573. [Google Scholar] [CrossRef]
- Tung, K.S.; Ellis, L.E.; Childs, G.V.; Dufau, M. The Dark Mink: A Model of Male Infertility. Endocrinology 1984, 114, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.M.; Yoon, S.R.; Akpovi, C.D.; Silvas, E.; Vitale, M.L. Defects in the Regulatory Clearance Mechanisms Favor the Breakdown of Self-Tolerance during Spontaneous Autoimmune Orchitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R743–R762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furbeth, C.; Hubner, G.; Thoenes, G.H. Spontaneous Immune Complex Orchitis in Brown Norway Rats. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 57, 37–45. [Google Scholar] [CrossRef]
- Tung, K.S.; Teuscher, C. Mechanisms of Autoimmune Disease in the Testis and Ovary. Hum. Reprod. Update 1995, 1, 35–50. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Pilatz, A.; Hossain, H.; Meinhardt, A.; Bergmann, M.; Haidl, G.; Weidner, W. Orchitis and Male Infertility. Urologe A 2010, 49, 629–635. [Google Scholar] [CrossRef]
- Pilatz, A.; Fijak, M.; Wagenlehner, F.; Schuppe, H.C. Orchitis. Urologe A 2019, 58, 697–710. [Google Scholar] [CrossRef]
- Silva, C.A.; Cocuzza, M.; Carvalho, J.F.; Bonfa, E. Diagnosis and Classification of Autoimmune Orchitis. Autoimmun. Rev. 2014, 13, 431–434. [Google Scholar] [CrossRef]
- Fijak, M.; Meinhardt, A. The Testis in Immune Privilege. Immunol. Rev. 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, Inflammatory and ‘Autoimmune’ Male Factor Infertility: How Do Rodent Models Inform Clinical Practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef] [Green Version]
- Jacobo, P.; Guazzone, V.A.; Theas, M.S.; Lustig, L. Testicular Autoimmunity. Autoimmun. Rev. 2011, 10, 201–204. [Google Scholar] [CrossRef]
- Lustig, L.; Guazzone, V.A.; Theas, M.S.; Pleuger, C.; Jacobo, P.; Perez, C.V.; Meinhardt, A.; Fijak, M. Pathomechanisms of Autoimmune Based Testicular Inflammation. Front. Immunol. 2020, 11, 583135. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodriguez, J.; Martinez-Garcia, C. Spontaneous Germ Cell Death in the Testis of the Adult Rat Takes the Form of Apoptosis: Re-Evaluation of Cell Types That Exhibit the Ability to Die during Spermatogenesis. Cell Prolif. 1996, 29, 13–31. [Google Scholar] [CrossRef]
- Billig, H.; Furuta, I.; Rivier, C.; Tapanainen, J.; Parvinen, M.; Hsueh, A.J. Apoptosis in Testis Germ Cells: Developmental Changes in Gonadotropin Dependence and Localization to Selective Tubule Stages. Endocrinology 1995, 136, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Huckins, C. The Morphology and Kinetics of Spermatogonial Degeneration in Normal Adult Rats: An Analysis Using a Simplified Classification of the Germinal Epithelium. Anat. Rec. 1978, 190, 905–926. [Google Scholar] [CrossRef]
- Oakberg, E.F. A Description of Spermiogenesis in the Mouse and Its Use in Analysis of the Cycle of the Seminiferous Epithelium and Germ Cell Renewal. Am. J. Anat. 1956, 99, 391–413. [Google Scholar] [CrossRef]
- Lee, J.; Richburg, J.H.; Younkin, S.C.; Boekelheide, K. The Fas System Is a Key Regulator of Germ Cell Apoptosis in the Testis. Endocrinology 1997, 138, 2081–2088. [Google Scholar] [CrossRef]
- Rodriguez, I.; Ody, C.; Araki, K.; Garcia, I.; Vassalli, P. An Early and Massive Wave of Germinal Cell Apoptosis Is Required for the Development of Functional Spermatogenesis. EMBO J. 1997, 16, 2262–2270. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Rodriguez, J. A matter of death and life: The Significance of Germ Cell Death during Spermatogenesis. Int. J. Androl. 1998, 21, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Koji, T.; Hishikawa, Y. Germ Cell Apoptosis and Its Molecular Trigger in Mouse Testes. Arch. Histol. Cytol. 2003, 66, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theas, M.S.; Rival, C.; Lustig, L. Germ Cell Apoptosis in Autoimmune Orchitis: Involvement of the Fas-FasL System. Am. J. Reprod. Immunol. 2003, 50, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S.; Rival, C.; Dietrich, S.J.; Guazzone, V.A.; Lustig, L. Death Receptor and Mitochondrial Pathways Are Involved in Germ Cell Apoptosis in an Experimental Model of Autoimmune Orchitis. Hum. Reprod. 2006, 21, 1734–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobo, P.V.; Fass, M.; Perez, C.V.; Jarazo-Dietrich, S.; Lustig, L.; Theas, M.S. Involvement of Soluble Fas Ligand in Germ Cell Apoptosis in Testis of Rats Undergoing Autoimmune Orchitis. Cytokine 2012, 60, 385–392. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The Executioners of Apoptosis. Biochem. J. 1997, 326 Pt 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ashe, P.C.; Berry, M.D. Apoptotic Signaling Cascades. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 199–214. [Google Scholar] [CrossRef]
- Zimmermann, K.C.; Bonzon, C.; Green, D.R. The Machinery of Programmed Cell Death. Pharmacol. Ther. 2001, 92, 57–70. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death Receptors: Signaling and Modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [Green Version]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The Polypeptide Encoded by the Cdna for Human Cell Surface Antigen Fas Can Mediate Apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.C.; Hahne, M.; Schroeter, M.; Frei, K.; Fontana, A.; Villunger, A.; Newton, K.; Tschopp, J.; Strasser, A. Activation of Fas by Fasl Induces Apoptosis by a Mechanism That Cannot Be Blocked by Bcl-2 or Bcl-x(L). Proc. Natl. Acad. Sci. USA 1999, 96, 14871–14876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewski, S.; Tanaka, S.; Takayama, S.; Schibler, M.J.; Fenton, W.; Reed, J.C. Investigation of the Subcellular Distribution of the Bcl-2 Oncoprotein: Residence in the Nuclear Envelope, Endoplasmic Reticulum, and Outer Mitochondrial Membranes. Cancer Res. 1993, 53, 4701–4714. [Google Scholar] [PubMed]
- Hockenbery, D.; Nunez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an Inner Mitochondrial Membrane Protein That Blocks Programmed Cell Death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of Apoptosis by Bcl-2: Release of Cytochrome C from Mitochondria Blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Orrenius, S. Mitochondrial Regulation of Apoptotic Cell Death. Toxicol. Lett. 2004, 149, 19–23. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome C and Datp-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Kuerban, M.; Naito, M.; Hirai, S.; Terayama, H.; Qu, N.; Musha, M.; Ikeda, A.; Koji, T.; Itoh, M. Involvement of Fas/Fas-L and Bax/Bcl-2 Systems in Germ Cell Death Following Immunization with Syngeneic Testicular Germ Cells in Mice. J. Androl. 2012, 33, 824–831. [Google Scholar] [CrossRef]
- Tedder, T.F.; Klejman, G.; Schlossman, S.F.; Saito, H. Structure of the Gene Encoding the Human B Lymphocyte Differentiation Antigen Cd20 (B1). J. Immunol. 1989, 142, 2560–2568. [Google Scholar] [CrossRef]
- Stashenko, P.; Nadler, L.M.; Hardy, R.; Schlossman, S.F. Characterization of a Human B Lymphocyte-Specific Antigen. J. Immunol. 1980, 125, 1678–1685. [Google Scholar] [CrossRef]
- Pentikainen, V.; Erkkila, K.; Dunkel, L. Fas Regulates Germ Cell Apoptosis in the Human Testis In Vitro. Am. J. Physiol. 1999, 276, E310–E316. [Google Scholar] [CrossRef]
- Henning, H.; Masal, C.; Herr, A.; Wolf, K.; Urhausen, C.; Beineke, A.; Beyerbach, M.; Kramer, S.; Gunzel-Apel, A.R. Effect of Short-Term Scrotal Hyperthermia on Spermatological Parameters, Testicular Blood Flow and Gonadal Tissue in Dogs. Reprod. Domest. Anim. 2014, 49, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, E.; Hori, T.; Tsutsui, T. Azoospermia of Dogs with Apoptotic Germ Cells and Leydig Cells. J. Vet. Med. Sci. 2000, 62, 529–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, M.; Dettwiler, M.; Vaughan, L.; Guscetti, F. Immunohistochemical Expression of Bax and Bak in Canine Non-Neoplastic Tissues. Vet. J. 2013, 198, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Saraste, A.; Pulkki, K. Morphologic and Biochemical Hallmarks of Apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of Programmed Cell Death In Situ via Specific Labeling of Nuclear DNA Fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Majtnerova, P.; Rousar, T. An Overview of Apoptosis Assays Detecting DNA Fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X.; Chen, Z.; Huang, X. Long-Term Reproductive Consequences of No-Scalpel Vasectomy in Beagles. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 899–905. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Do Tunel and Other Apoptosis Assays Detect Cell Death in Preclinical Studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S.; Rival, C.; Jarazo-Dietrich, S.; Jacobo, P.; Guazzone, V.A.; Lustig, L. Tumour Necrosis Factor-Alpha Released by Testicular Macrophages Induces Apoptosis of Germ Cells in Autoimmune Orchitis. Hum. Reprod. 2008, 23, 1865–1872. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Yuan, L.; Lin, F.; Yi, J.; Li, J.; Yuan, H.; Shi, J.; Yuan, T.; Zhang, S.; et al. Lead Induces Apoptosis in Mouse Tm3 Leydig Cells through the Fas/Fasl Death Receptor Pathway. Environ. Toxicol. Pharmacol. 2017, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, X.; Hu, X.; Zhou, L.; Zhang, C.; Zhang, M. Cadmium-Induced Apoptosis through Reactive Oxygen Species-Mediated Mitochondrial Oxidative Stress and the Jnk Signaling Pathway in Tm3 Cells, a Model of Mouse Leydig Cells. Toxicol. Appl. Pharmacol. 2019, 368, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Aoyama-Ishikawa, M.; Uemura, M.; Yamashita, H.; Koga, Y.; Terashima, M.; Usami, M.; Kotani, J.; Hirata, J. Interleukin-18 Levels and Mouse Leydig Cell Apoptosis during Lipopolysaccharide-Induced Acute Inflammatory Conditions. J. Reprod. Immunol. 2020, 141, 103167. [Google Scholar] [CrossRef]
- Beumer, T.L.; Roepers-Gajadien, H.L.; Gademan, I.S.; Lock, T.M.; Kal, H.B.; De Rooij, D.G. Apoptosis Regulation in the Testis: Involvement of Bcl-2 Family Members. Mol. Reprod. Dev. 2000, 56, 353–359. [Google Scholar] [CrossRef]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Heterodimerizes In Vivo with a Conserved Homolog, Bax, That Accelerates Programmed Cell Death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A Rheostat That Regulates an Anti-Oxidant Pathway and Cell Death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar] [PubMed]
- Russell, L.D.; Chiarini-Garcia, H.; Korsmeyer, S.J.; Knudson, C.M. Bax-Dependent Spermatogonia Apoptosis Is Required for Testicular Development and Spermatogenesis. Biol. Reprod. 2002, 66, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G.; Si, M.; Li, X.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone Induces Apoptosis of Rat Sertoli Cells through Fas-Fas Ligand and Mitochondrial Pathway. Environ. Toxicol. 2019, 34, 424–433. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yoon, H.J.; Oh, J.H.; Park, H.J.; Lee, E.H.; Song, C.W.; Yoon, S. 1,3-Dinitrobenzene Induces Apoptosis in Tm4 Mouse Sertoli Cells: Involvement of the C-Jun N-Terminal Kinase (Jnk) Mapk Pathway. Toxicol. Lett. 2009, 189, 145–151. [Google Scholar] [CrossRef]
- Aslani, F.; Sebastian, T.; Keidel, M.; Frohlich, S.; Elsasser, H.P.; Schuppe, H.C.; Klug, J.; Mahavadi, P.; Fijak, M.; Bergmann, M.; et al. Resistance to Apoptosis and Autophagy Leads to Enhanced Survival in Sertoli Cells. Mol. Hum. Reprod. 2017, 23, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Reifarth, L.; Körber, H.; Goericke-Pesch, S. Analysis of Putative Stem Cell Markers in Dogs with Spontaneous Immune Mediated Orchitis. Reprod. Domest. Anim. 2022, 57, 12. [Google Scholar]
- Hockenbery, D.M.; Zutter, M.; Hickey, W.; Nahm, M.; Korsmeyer, S.J. Bcl2 Protein Is Topographically Restricted in Tissues Characterized by Apoptotic Cell Death. Proc. Natl. Acad. Sci. USA 1991, 88, 6961–6965. [Google Scholar] [CrossRef] [Green Version]
- Nunez, G.; Hockenbery, D.; McDonnell, T.J.; Sorensen, C.M.; Korsmeyer, S.J. Bcl-2 Maintains B Cell Memory. Nature 1991, 353, 71–73. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Deane, N.; Platt, F.M.; Nunez, G.; Jaeger, U.; McKearn, J.P.; Korsmeyer, S.J. Bcl-2-Immunoglobulin Transgenic Mice Demonstrate Extended B Cell Survival and Follicular Lymphoproliferation. Cell 1989, 57, 79–88. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Nunez, G.; Platt, F.M.; Hockenberry, D.; London, L.; McKearn, J.P.; Korsmeyer, S.J. Deregulated Bcl-2-Immunoglobulin Transgene Expands a Resting but Responsive Immunoglobulin M and D-Expressing B-Cell Population. Mol. Cell Biol. 1990, 10, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Cory, S.; Adams, J.M. Bcl-2 Gene Promotes Haemopoietic Cell Survival and Cooperates with C-Myc to Immortalize Pre-B Cells. Nature 1988, 335, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Apoptosis and the Pathogenesis of Lymphoma. Acta Oncol. 2000, 39, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Sen, R. Control of B Lymphocyte Apoptosis by the Transcription Factor Nf-Kappab. Immunity 2006, 25, 871–883. [Google Scholar] [CrossRef] [Green Version]
- Bruun Jensen, H.; Engelhard Holm, J.; Körber, H.; Goericke-Pesch, S. Expression of Connexin 43 and Androgen Receptor in Testes of Azoospermic Dogs. Reprod. Domest. Anim. 2018, 53, 5. [Google Scholar]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B Cells Responses and Cytokine Production Are Regulated by Their Immune Microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.V.; Sobarzo, C.M.; Jacobo, P.V.; Pellizzari, E.H.; Cigorraga, S.B.; Denduchis, B.; Lustig, L. Loss of Occludin Expression and Impairment of Blood-Testis Barrier Permeability in Rats with Autoimmune Orchitis: Effect of Interleukin 6 on Sertoli Cell Tight Junctions. Biol. Reprod. 2012, 87, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 Disrupts Blood-Testis Barrier through Inhibiting Protein Degradation or Activating Phosphorylated Erk in Sertoli Cells. Sci. Rep. 2014, 4, 4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Xia, W.; Mruk, D.D.; Wang, C.Q.; Yan, H.H.; Siu, M.K.; Lui, W.Y.; Lee, W.M.; Cheng, C.Y. Tumor Necrosis Factor {Alpha} Reversibly Disrupts the Blood-Testis Barrier and Impairs Sertoli-Germ Cell Adhesion in the Seminiferous Epithelium of Adult Rat Testes. J. Endocrinol. 2006, 190, 313–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pröbstl, C.; Körber, H.; Goericke-Pesch, S. Germ Cell Apoptosis Induced by Tumor Necrosis Factor (Tnf) Alpha in Spontaneous Autoimmune Orchitis in Dogs Is Mainly Triggered by Interaction with Tnf Receptor 2 on Spermatogonia. Reprod. Domest. Anim. 2022, 57, 18. [Google Scholar]
- Kim, S.K.; Yoon, Y.D.; Park, Y.S.; Seo, J.T.; Kim, J.H. Involvement of the Fas-Fas Ligand System and Active Caspase-3 in Abnormal Apoptosis in Human Testes with Maturation Arrest and Sertoli Cell-Only Syndrome. Fertil. Steril. 2007, 87, 547–553. [Google Scholar] [CrossRef]
- Jhun, H.; Park, H.J.; Lee, R.; Song, H.; Hur, T.Y.; Lee, S.; Park, J.K.; Lee, W.Y. Germ Cell-Specific Apoptosis by Extracellular Clusterin in Cryptorchid Dog Testes. Anim. Reprod. Sci. 2018, 193, 158–164. [Google Scholar] [CrossRef]
- Almeida, C.; Correia, S.; Rocha, E.; Alves, A.; Ferraz, L.; Silva, J.; Sousa, M.; Barros, A. Caspase Signalling Pathways in Human Spermatogenesis. J. Assist. Reprod. Genet. 2013, 30, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Bellgrau, D.; Gold, D.; Selawry, H.; Moore, J.; Franzusoff, A.; Duke, R.C. A Role for Cd95 Ligand in Preventing Graft Rejection. Nature 1995, 377, 630–632. [Google Scholar] [CrossRef]
- Griffith, T.S.; Brunner, T.; Fletcher, S.M.; Green, D.R.; Ferguson, T.A. Fas Ligand-Induced Apoptosis as a Mechanism of Immune Privilege. Science 1995, 270, 1189–1192. [Google Scholar] [CrossRef]
- Head, J.R.; Neaves, W.B.; Billingham, R.E. Immune Privilege in the Testis. I. Basic Parameters of Allograft Survival. Transplantation 1983, 36, 423–431. [Google Scholar] [CrossRef]
- Jarazo Dietrich, S.; Fass, M.I.; Jacobo, P.V.; Sobarzo, C.M.; Lustig, L.; Theas, M.S. Inhibition of Nos-No System Prevents Autoimmune Orchitis Development in Rats: Relevance of No Released by Testicular Macrophages in Germ Cell Apoptosis and Testosterone Secretion. PLoS ONE 2015, 10, e0128709. [Google Scholar] [CrossRef]
- Yazdani, I.; Ghazi-Khansari, M.; Saeedi Saravi, S.S.; Nobakht, M.; Majdani, R.; Rezayat, S.M.; Mousavi, S.E.; Yari, A.; Dehpour, A.R. Nortriptyline Protects Testes against Germ Cell Apoptosis and Oxidative Stress Induced by Testicular Ischaemia/Reperfusion. Andrologia 2017, 49, e12605. [Google Scholar] [CrossRef] [PubMed]
- Lysiak, J.J.; Zheng, S.; Woodson, R.; Turner, T.T. Caspase-9-Dependent Pathway to Murine Germ Cell Apoptosis: Mediation by Oxidative Stress, Bax, and Caspase 2. Cell Tissue Res. 2007, 328, 411–419. [Google Scholar] [CrossRef]
- Riesenbeck, A.; Völger, D.; Hoffmann, B. Praxisnahe Beurteilung von Rüdensperma zur Bestimmung von VitalitäTsparametern. Tierärztliche Praxis 2001, 29, 116–120. [Google Scholar]
- Goericke-Pesch, S.; Spang, A.; Schulz, M.; Ozalp, G.; Bergmann, M.; Ludwig, C.; Hoffmann, B. Recrudescence of Spermatogenesis in the Dog Following Downregulation Using a Slow Release Gnrh Agonist Implant. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time Rt-Pcr. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Urhausen, C.; Beineke, A.; Piechotta, M.; Karre, I.; Beyerbach, M.; Gunzel-Apel, A.R. Apoptosis in the Uterotubal Junction and Oviductal Isthmus during the Estrous Cycle of the Bitch. Anat. Rec. 2011, 294, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Rempel, L.M.; Korber, H.; Reichler, I.M.; Balogh, O.; Goericke-Pesch, S. Investigations on the Potential Role of Prostaglandin E2 in Canine Uterine Inertia. Theriogenology 2021, 175, 134–147. [Google Scholar] [CrossRef]
- Pillai-Kastoori, L.; Heaton, S.; Shiflett, S.D.; Roberts, A.C.; Solache, A.; Schutz-Geschwender, A.R. Antibody Validation for Western Blot: By the User, for the User. J. Biol. Chem. 2020, 295, 926–939. [Google Scholar] [CrossRef]
- Körber, H.; Goericke-Pesch, S. Expression of Ptgs2, Pgfs and Ptgfr during Downregulation and Restart of Spermatogenesis Following Gnrh Agonist Treatment in the Dog. Cell Tissue Res. 2019, 375, 531–541. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Goldschmidt, M.H.; Shofer, F.S.; Goldkamp, C.; Ferracone, J. Evaluation of Cyclooxygenase-1 and Cyclooxygenase-2 Expression and the Effect of Cyclooxygenase Inhibitors in Canine Prostatic Carcinoma. Vet. Comp. Oncol. 2004, 2, 13–23. [Google Scholar] [CrossRef] [PubMed]




| Primer | Accession Nr. | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) | Amplicon Length (bp) | Efficiency |
|---|---|---|---|---|---|
| Bcl-2 | NM_ 001002949.1 | ATGTGTGTGGAGAGCGTCAAC | GCCAGGAGAAGTCAAACAGAGG | 175 | 1.98 |
| Bax | NM_ 001003011.1 | TCAAGCGCATCGGAGATGAAC | TCGAAGGAAGTCCAGTGTCCAG | 248 | 1.98 |
| Fas | XM_ 022410445.1 | AGACCCGGAATACCAAGTGCAG | GGATGAGGACGCAAAACCACAG | 180 | 1.91 |
| FasL | NM_ 001287153.1 | CAGCGAAAGGCATGTAGCACC | CACCCCAGAGACAAGGGCAAT | 169 | 2.05 |
| Caspase 3 | NM_ 001003042.1 | TCCAGTCACTTTGTGCGATGC | CCAAACCAAACCAAACCAACCC | 218 | 2.02 |
| Caspase 8 | NM_ 001048029.1 | GCTTCAGATACCAGGCAGAGC | CTCCCGGCTCAAGAGAAACTTA | 115 | 2.19 |
| Caspase 9 | NM_ 001031633.1 | TGTCTAGTTTGCCCACTCCCAG | TGCGAAACAGCATTAGCGACC | 184 | 1.98 |
| GAPDH | NM_001003142 | GGCCAAGAGGGTCATCATCTC | GGGGCCGTCCACGGTCTTC | 229 | 1.93 |
| ß-actin | AF484115.1 | GCTGTGCTGTCCCTGTATG | GCGTACCCCTCATAGATGG | 98 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawietz, J.; Körber, H.; Packeiser, E.-M.; Beineke, A.; Goericke-Pesch, S. Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis. Int. J. Mol. Sci. 2023, 24, 6083. https://doi.org/10.3390/ijms24076083
Morawietz J, Körber H, Packeiser E-M, Beineke A, Goericke-Pesch S. Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis. International Journal of Molecular Sciences. 2023; 24(7):6083. https://doi.org/10.3390/ijms24076083
Chicago/Turabian StyleMorawietz, Judith, Hanna Körber, Eva-Maria Packeiser, Andreas Beineke, and Sandra Goericke-Pesch. 2023. "Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis" International Journal of Molecular Sciences 24, no. 7: 6083. https://doi.org/10.3390/ijms24076083
APA StyleMorawietz, J., Körber, H., Packeiser, E.-M., Beineke, A., & Goericke-Pesch, S. (2023). Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis. International Journal of Molecular Sciences, 24(7), 6083. https://doi.org/10.3390/ijms24076083





