Function of Innate Lymphoid Cells in Periodontal Tissue Homeostasis: A Narrative Review
Abstract
:1. Introduction
2. Innate Lymphoid Cells
| ILC Group | Subsets | Stimuli | Mediators | Phenotype | Transcription Factors | References | |
|---|---|---|---|---|---|---|---|
| Mouse | Human | ||||||
| Group 1 | ILC1s | tumors, intracellular microbe (bacteria, virus, parasites) | IFN-r, granzymes, perforin | CD49a, NK1.1, NKp46, CD122, CD127, CD200R, TRAIL | NKp46, CD122, CD127, CD200R, TRAIL | T-bet EOMEShig | [17,18,19,20,21] |
| NK cells | CD49b, NK1.1, NKp46, CD122, KLRG1 | NKp46, NKp30, NKp80, CD56, CD16, CD122, CD127, KLRG1 | T-bet EOMES | [20,22,23,24] | |||
| Group 2 | ILC2s | extracellular parasites and allergens | IL-4, IL-5, IL-9, IL-13, AREG | CD127, CD25, ST2, KLRG1 | NKp46, CD127 | GATA3 RORα | [25,26,27] |
| Group 3 | NCR+ILC3s | extracellular microbes (bacteria, fungi) | IL-22, IL-17, GM-CSF, Lymphotoxin | NKp46, CD127 | NKp46, NKp44, CD127, CCR6, CD56 | RORγt AhR | [9,28,29] |
| NCR−ILC3s | NKp46, NKp44, CD127, CD56, CCR6 | CD127, CCR6 | [30,31] | ||||
| Lti | mesenchymal organizer cells (CXCL13, RANKL) | RANK, Lymphotoxin, TNF, IL-17, IL-22 | CD127, CCR6 | CD127, CCR6, CD7 | [31,32] | ||
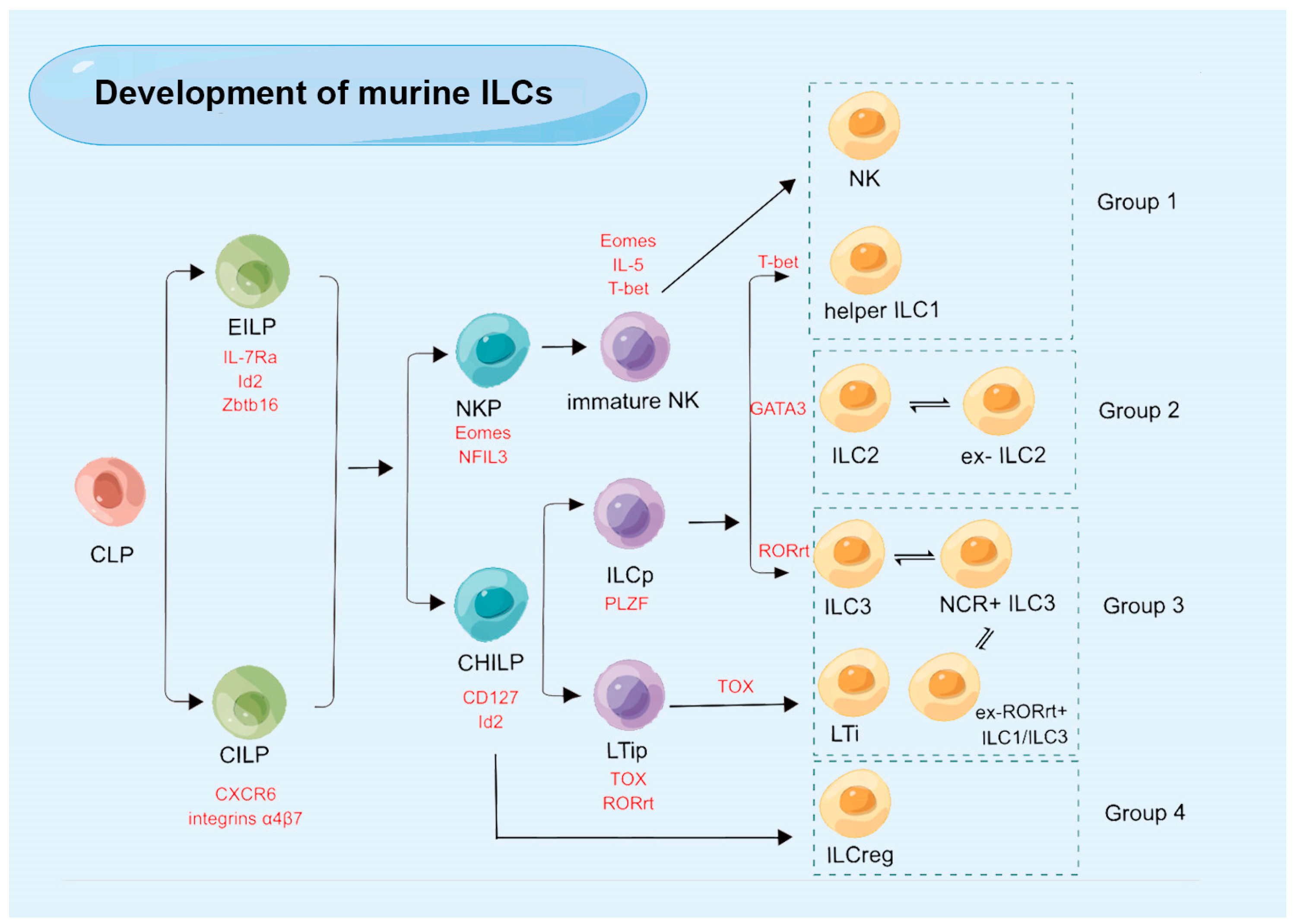
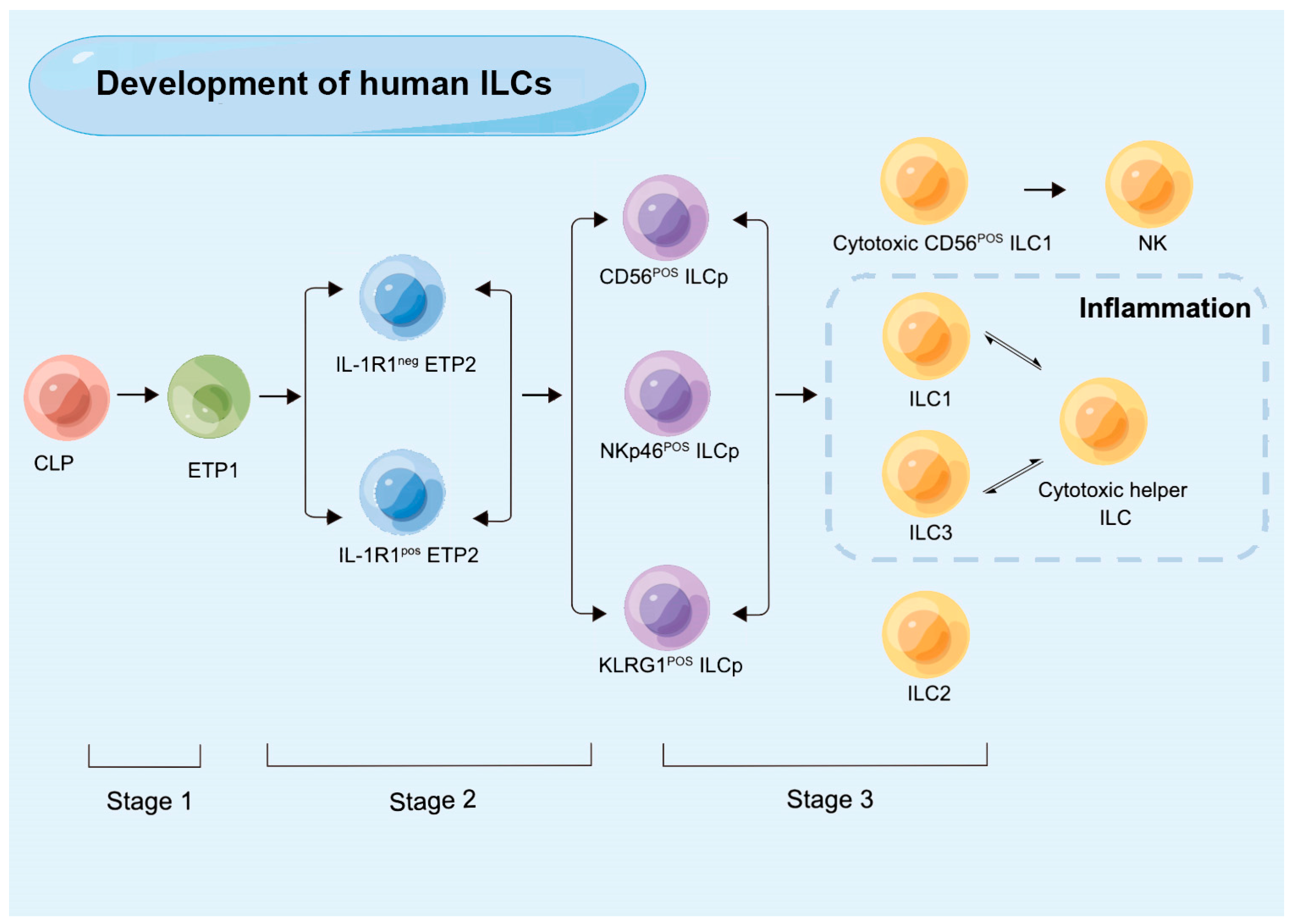
3. The Development of ILCs
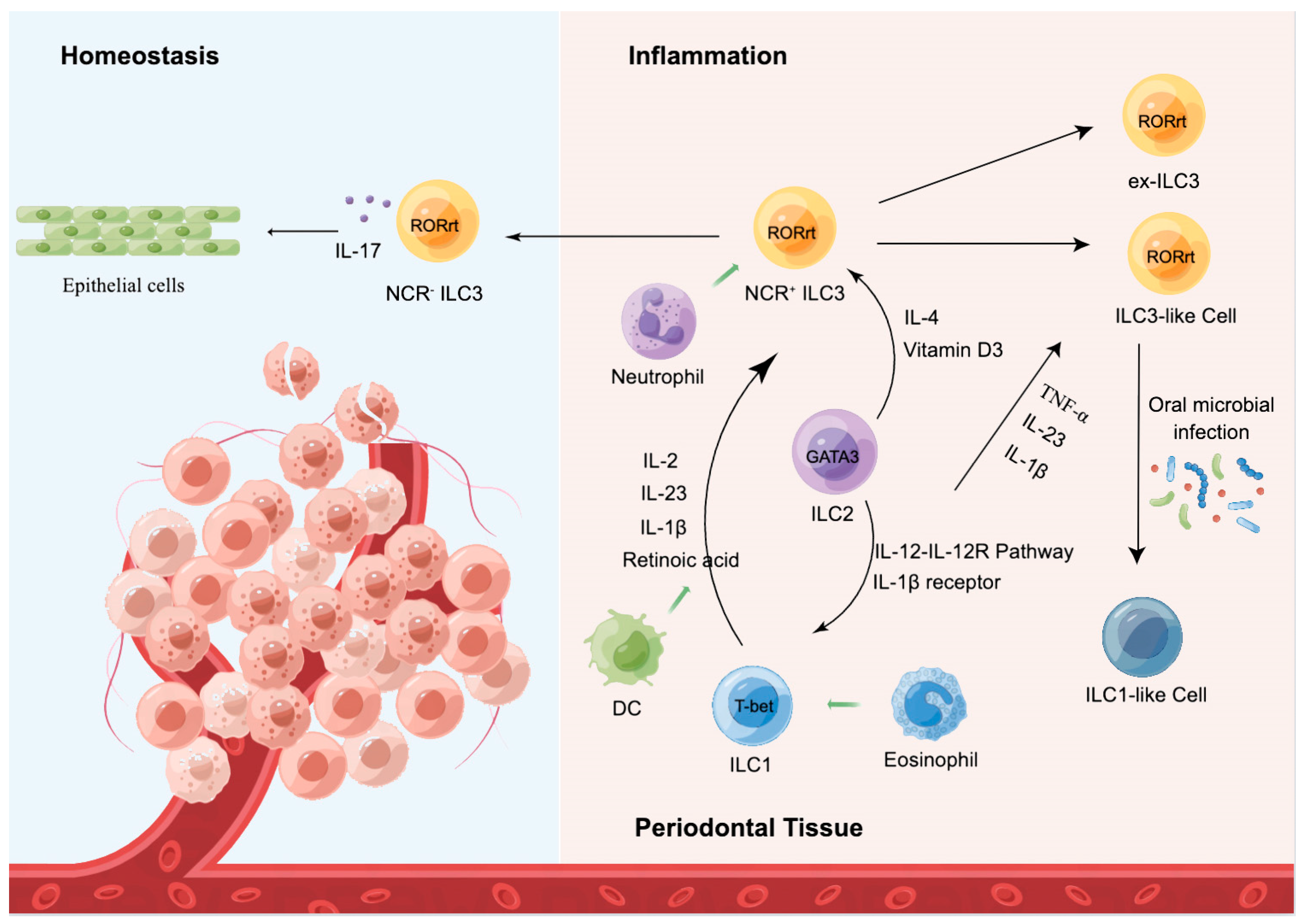
4. Plasticity of ILC Subsets
5. ILCs in Periodontal Homeostasis
5.1. ILC1
5.2. ILC2
5.3. ILC3
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 2018, 48, 1104–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goc, J.; Lv, M.; Bessman, N.J.; Flamar, A.L.; Sahota, S.; Suzuki, H.; Teng, F.; Putzel, G.G.; Eberl, G.; Withers, D.R.; et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 2021, 184, 5015–5030.e5016. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015, 21, 698–708. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.C.; Chang, C.; Boschetti, G.; Ungaro, R.; Giri, M.; Grout, J.A.; Gettler, K.; Chuang, L.S.; Nayar, S.; Greenstein, A.J.; et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019, 178, 1493–1508.e1420. [Google Scholar] [CrossRef]
- Kansler, E.R.; Li, M.O. Innate lymphocytes-lineage, localization and timing of differentiation. Cell. Mol. Immunol. 2019, 16, 627–633. [Google Scholar] [CrossRef]
- Pesce, S.; Trabanelli, S.; Di Vito, C.; Greppi, M.; Obino, V.; Guolo, F.; Minetto, P.; Bozzo, M.; Calvi, M.; Zaghi, E.; et al. Cancer Immunotherapy by Blocking Immune Checkpoints on Innate Lymphocytes. Cancers 2020, 12, 3504. [Google Scholar] [CrossRef]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.; Eberl, G.; Koyasu, S.; Locksley, R.; McKenzie, A.; Mebius, R.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Chen, Y.; Qu, Y.; Xiong, Z.; Ye, B.; Du, Y.; Tian, Y.; Yin, Z.; Xu, Z.; et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell 2017, 171, 201–216.e218. [Google Scholar] [CrossRef] [Green Version]
- Guia, S.; Fenis, A.; Vivier, E.; Narni-Mancinelli, E. Activating and inhibitory receptors expressed on innate lymphoid cells. Semin. Immunopathol. 2018, 40, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bennstein, S.B.; Scherenschlich, N.; Weinhold, S.; Manser, A.R.; Noll, A.; Raba, K.; Kögler, G.; Walter, L.; Uhrberg, M. Transcriptional and functional characterization of neonatal circulating Innate Lymphoid Cells. Stem. Cells Transl. Med. 2021, 10, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.; Rudensky, A. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Hashimoto-Hill, S.; Kim, M. Migration and Tissue Tropism of Innate Lymphoid Cells. Trends Immunol. 2016, 37, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Campbell, L.; Malcolm, J.; Adrados Planell, A.; Butcher, J.; Culshaw, S. Enrichment of Innate Lymphoid Cell Populations in Gingival Tissue. J. Dent. Res. 2018, 97, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Cortez, V.S.; Fuchs, A.; Cella, M.; Gilfillan, S.; Colonna, M. Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. J. Immunol. 2014, 192, 4487–4491. [Google Scholar] [CrossRef] [Green Version]
- Schuster, I.S.; Wikstrom, M.E.; Brizard, G.; Coudert, J.D.; Estcourt, M.J.; Manzur, M.; O’Reilly, L.A.; Smyth, M.J.; Trapani, J.A.; Hill, G.R.; et al. TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity 2014, 41, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Tessmer, M.S.; Reilly, E.C.; Brossay, L. Salivary gland NK cells are phenotypically and functionally unique. PLoS Pathog. 2011, 7, e1001254. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.M.; Chaix, J.; Rupp, L.J.; Wu, J.; Madera, S.; Sun, J.C.; Lindsten, T.; Reiner, S.L. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012, 36, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Diefenbach, A.; Colonna, M.; Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 2014, 41, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Bal, S.M.; Bernink, J.H.; Nagasawa, M.; Groot, J.; Shikhagaie, M.M.; Golebski, K.; van Drunen, C.M.; Lutter, R.; Jonkers, R.E.; Hombrink, P.; et al. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 2016, 17, 636–645. [Google Scholar] [CrossRef]
- Schulz-Kuhnt, A.; Wirtz, S.; Neurath, M.F.; Atreya, I. Regulation of Human Innate Lymphoid Cells in the Context of Mucosal Inflammation. Front. Immunol. 2020, 11, 1062. [Google Scholar] [CrossRef]
- Ishizuka, I.E.; Chea, S.; Gudjonson, H.; Constantinides, M.G.; Dinner, A.R.; Bendelac, A.; Golub, R. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat. Immunol. 2016, 17, 269–276. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Flores-Borja, F.; Irshad, S.; Deng, J.; Ng, T. Pleiotropic Role and Bidirectional Immunomodulation of Innate Lymphoid Cells in Cancer. Front. Immunol. 2019, 10, 3111. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Zheng, M.; Cui, K.; Martins, A.J.; Hu, G.; Li, D.; Tessarollo, L.; Kozlov, S.; Keller, J.R.; Tsang, J.S.; et al. Differential Expression of the Transcription Factor GATA3 Specifies Lineage and Functions of Innate Lymphoid Cells. Immunity 2020, 52, 83–95.e84. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaldo, E.; Juelke, K.; Romagnani, C. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice. Eur. J. Immunol. 2015, 45, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Scoville, S.D.; Freud, A.G.; Caligiuri, M.A. Cellular pathways in the development of human and murine innate lymphoid cells. Curr. Opin. Immunol. 2019, 56, 100–106. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.E.; Geary, C.D.; Weizman, O.E.; Geiger, T.L.; Rapp, M.; Dorn, G.W., 2nd; Overholtzer, M.; Sun, J.C. Atg5 Is Essential for the Development and Survival of Innate Lymphocytes. Cell Rep. 2016, 15, 1910–1919. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Wang, Y.; Deng, M.; Li, Y.; Ruhn, K.; Zhang, C.; Hooper, L. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. eLife 2014, 3, e04406. [Google Scholar] [CrossRef]
- Yu, Y.; Tsang, J.C.; Wang, C.; Clare, S.; Wang, J.; Chen, X.; Brandt, C.; Kane, L.; Campos, L.S.; Lu, L.; et al. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature 2016, 539, 102–106. [Google Scholar] [CrossRef]
- Seillet, C.; Mielke, L.A.; Amann-Zalcenstein, D.B.; Su, S.; Gao, J.; Almeida, F.F.; Shi, W.; Ritchie, M.E.; Naik, S.H.; Huntington, N.D.; et al. Deciphering the Innate Lymphoid Cell Transcriptional Program. Cell Rep. 2016, 17, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.I.; Verrier, T.; Vosshenrich, C.A.; Di Santo, J.P. Developmental options and functional plasticity of innate lymphoid cells. Curr. Opin. Immunol. 2017, 44, 61–68. [Google Scholar] [CrossRef]
- Curio, S.; Belz, G.T. The unique role of innate lymphoid cells in cancer and the hepatic microenvironment. Cell. Mol. Immunol. 2022, 19, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, M.G.; McDonald, B.D.; Verhoef, P.A.; Bendelac, A. A committed precursor to innate lymphoid cells. Nature 2014, 508, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Scoville, S.D.; Mundy-Bosse, B.L.; Zhang, M.H.; Chen, L.; Zhang, X.; Keller, K.A.; Hughes, T.; Chen, L.; Cheng, S.; Bergin, S.M.; et al. A Progenitor Cell Expressing Transcription Factor RORγt Generates All Human Innate Lymphoid Cell Subsets. Immunity 2016, 44, 1140–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Ephraim, Y.E.; Koning, J.J.; Burniol Ruiz, E.; Konijn, T.; Mourits, V.P.; Lakeman, K.A.; Boon, L.; Bögels, M.; van Maanen, J.P.; Den Haan, J.M.M.; et al. CD62L Is a Functional and Phenotypic Marker for Circulating Innate Lymphoid Cell Precursors. J. Immunol. 2019, 202, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Youssef, Y.; Robinson, C.; Ernst, G.F.; Carson, M.Y.; Young, K.A.; Scoville, S.D.; Zhang, X.; Harris, R.; Sekhri, P.; et al. CD56 Expression Marks Human Group 2 Innate Lymphoid Cell Divergence from a Shared NK Cell and Group 3 Innate Lymphoid Cell Developmental Pathway. Immunity 2018, 49, 464–476.e464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzurana, L.; Czarnewski, P.; Jonsson, V.; Wigge, L.; Ringnér, M.; Williams, T.C.; Ravindran, A.; Björklund, Å.K.; Säfholm, J.; Nilsson, G.; et al. Tissue-specific transcriptional imprinting and heterogeneity in human innate lymphoid cells revealed by full-length single-cell RNA-sequencing. Cell Res. 2021, 31, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Otero, K.; Colonna, M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc. Natl. Acad. Sci. USA 2010, 107, 10961–10966. [Google Scholar] [CrossRef] [Green Version]
- Crellin, N.K.; Trifari, S.; Kaplan, C.D.; Satoh-Takayama, N.; Di Santo, J.P.; Spits, H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 2010, 33, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Vonarbourg, C.; Mortha, A.; Bui, V.L.; Hernandez, P.P.; Kiss, E.A.; Hoyler, T.; Flach, M.; Bengsch, B.; Thimme, R.; Hölscher, C.; et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 2010, 33, 736–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, H.Y.; Sciumè, G.; Mikami, Y.; Guo, L.; Sun, H.W.; Brooks, S.R.; Urban, J.F., Jr.; Davis, F.P.; Kanno, Y.; O’Shea, J.J. Developmental Acquisition of Regulomes Underlies Innate Lymphoid Cell Functionality. Cell 2016, 165, 1120–1133. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, A.; Stockmann, C. The Metabolic Basis of ILC Plasticity. Front. Immunol. 2022, 13, 858051. [Google Scholar] [CrossRef]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J.; et al. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Cella, M.; Gamini, R.; Sécca, C.; Collins, P.L.; Zhao, S.; Peng, V.; Robinette, M.L.; Schettini, J.; Zaitsev, K.; Gordon, W.; et al. Subsets of ILC3-ILC1-like cells generate a diversity spectrum of innate lymphoid cells in human mucosal tissues. Nat. Immunol. 2019, 20, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Menegatti, S.; Bustamante, J.; Le Bourhis, L.; Allez, M.; Rogge, L.; Casanova, J.L.; Yssel, H.; Di Santo, J.P. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med. 2016, 213, 569–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohne, Y.; Silver, J.S.; Thompson-Snipes, L.; Collet, M.A.; Blanck, J.P.; Cantarel, B.L.; Copenhaver, A.M.; Humbles, A.A.; Liu, Y.J. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol. 2016, 17, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.S.; Kearley, J.; Copenhaver, A.M.; Sanden, C.; Mori, M.; Yu, L.; Pritchard, G.H.; Berlin, A.A.; Hunter, C.A.; Bowler, R.; et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 2016, 17, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Guo, L.; Qiu, J.; Chen, X.; Hu-Li, J.; Siebenlist, U.; Williamson, P.R.; Urban, J.F., Jr.; Paul, W.E. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 2015, 16, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Wallrapp, A.; Burkett, P.R.; Riesenfeld, S.J.; Kim, S.J.; Christian, E.; Abdulnour, R.E.; Thakore, P.I.; Schnell, A.; Lambden, C.; Herbst, R.H.; et al. Calcitonin Gene-Related Peptide Negatively Regulates Alarmin-Driven Type 2 Innate Lymphoid Cell Responses. Immunity 2019, 51, 709–723.e706. [Google Scholar] [CrossRef]
- Bernink, J.H.; Ohne, Y.; Teunissen, M.B.M.; Wang, J.; Wu, J.; Krabbendam, L.; Guntermann, C.; Volckmann, R.; Koster, J.; van Tol, S.; et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 2019, 20, 992–1003. [Google Scholar] [CrossRef]
- Golebski, K.; Ros, X.R.; Nagasawa, M.; van Tol, S.; Heesters, B.A.; Aglmous, H.; Kradolfer, C.M.A.; Shikhagaie, M.M.; Seys, S.; Hellings, P.W.; et al. IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat. Commun. 2019, 10, 2162. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Qu, Y.; Xia, P.; Chen, Y.; Zhu, X.; Zhang, J.; Wang, G.; Tian, Y.; Ying, J.; Fan, Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020, 30, 610–622. [Google Scholar] [CrossRef]
- Cortez, V.S.; Ulland, T.K.; Cervantes-Barragan, L.; Bando, J.K.; Robinette, M.L.; Wang, Q.; White, A.J.; Gilfillan, S.; Cella, M.; Colonna, M. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-β signaling. Nat. Immunol. 2017, 18, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.; Kim, H.Y.; Lee, Y.; Park, I.K.; Kang, C.H.; Kim, Y.T.; Kim, J.E.; Choi, M.; Lee, W.W.; Jeon, Y.K.; et al. IL23-Producing Human Lung Cancer Cells Promote Tumor Growth via Conversion of Innate Lymphoid Cell 1 (ILC1) into ILC3. Clin. Cancer Res. 2019, 25, 4026–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Hoda, M.N.; Susin, C.; Wheeler, J.N.; Marshall, B.; Perry, L.; Saad, N.; Yin, L.; Elsayed, R.; Elsalanty, M.; et al. Increased Innate Lymphoid Cells in Periodontal Tissue of the Murine Model of Periodontitis: The Role of AMP-Activated Protein Kinase and Relevance for the Human Condition. Front. Immunol. 2017, 8, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; Wyss, T.; Salomé, B.; Romero, P.; Trabanelli, S.; Jandus, C. Distinct and shared gene expression for human innate versus adaptive helper lymphoid cells. J. Leukoc. Biol. 2020, 108, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Kindstedt, E.; Koskinen Holm, C.; Palmqvist, P.; Sjostrom, M.; Lejon, K.; Lundberg, P. Innate lymphoid cells are present in gingivitis and periodontitis. J. Periodontol. 2019, 90, 200–207. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Pan, J.; Wang, Y.; Shen, L.; Xu, Y. ILC1s and ILC3s Exhibit Inflammatory Phenotype in Periodontal Ligament of Periodontitis Patients. Front. Immunol. 2021, 12, 708678. [Google Scholar] [CrossRef] [PubMed]
- Seillet, C.; Brossay, L.; Vivier, E. Natural killers or ILC1s? That is the question. Curr. Opin. Immunol. 2021, 68, 48–53. [Google Scholar] [CrossRef]
- Wilensky, A.; Chaushu, S.; Shapira, L. The role of natural killer cells in periodontitis. Periodontol 2000 2015, 69, 128–141. [Google Scholar] [CrossRef]
- Lujan, R.A.; Vrba, S.M.; Hickman, H.D. Antiviral Activities of Group I Innate Lymphoid Cells. J. Mol. Biol. 2022, 434, 167266. [Google Scholar] [CrossRef]
- Li, H.; Reeves, R.K. Functional perturbation of classical natural killer and innate lymphoid cells in the oral mucosa during SIV infection. Front. Immunol. 2012, 3, 417. [Google Scholar] [CrossRef] [Green Version]
- Nabekura, T.; Shibuya, A. ILC1: Guardians of the oral mucosa against enemy viruses. Immunity 2021, 54, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.P.; Vrba, S.M.; Reynoso, G.V.; Wynne-Jones, E.; Kamenyeva, O.; Malo, C.S.; Cherry, C.R.; McManus, D.T.; Hickman, H.D. Group 1 innate lymphoid-cell-derived interferon-gamma maintains anti-viral vigilance in the mucosal epithelium. Immunity 2021, 54, 276–290.e275. [Google Scholar] [CrossRef] [PubMed]
- Weizman, O.E.; Adams, N.M.; Schuster, I.S.; Krishna, C.; Pritykin, Y.; Lau, C.; Degli-Esposti, M.A.; Leslie, C.S.; Sun, J.C.; O’Sullivan, T.E. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171, 795–808 e712. [Google Scholar] [CrossRef] [PubMed]
- Björklund, Å.K.; Forkel, M.; Picelli, S.; Konya, V.; Theorell, J.; Friberg, D.; Sandberg, R.; Mjösberg, J. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol. 2016, 17, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Califano, D.; Cho, J.J.; Uddin, M.N.; Lorentsen, K.J.; Yang, Q.; Bhandoola, A.; Li, H.; Avram, D. Transcription Factor Bcl11b Controls Identity and Function of Mature Type 2 Innate Lymphoid Cells. Immunity 2015, 43, 354–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, M.; Heesters, B.A.; Kradolfer, C.M.A.; Krabbendam, L.; Martinez-Gonzalez, I.; de Bruijn, M.J.W.; Golebski, K.; Hendriks, R.W.; Stadhouders, R.; Spits, H.; et al. Correction: KLRG1 and NKp46 discriminate subpopulations of human CD117(+)CRTH2(-) ILCs biased toward ILC2 or ILC3. J. Exp. Med. 2019, 216, 2221–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, K.; Kabata, H.; Tanabe, M.; Koga, S.; Takeno, N.; Mochizuki, M.; Fukunaga, K.; Asano, K.; Betsuyaku, T.; Koyasu, S. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat. Immunol. 2016, 17, 76–86. [Google Scholar] [CrossRef]
- Karagiannis, F.; Masouleh, S.K.; Wunderling, K.; Surendar, J.; Schmitt, V.; Kazakov, A.; Michla, M.; Holzel, M.; Thiele, C.; Wilhelm, C. Lipid-Droplet Formation Drives Pathogenic Group 2 Innate Lymphoid Cells in Airway Inflammation. Immunity 2020, 52, 620–634 e626. [Google Scholar] [CrossRef]
- Fu, L.; Zhao, J.; Huang, J.; Li, N.; Dong, X.; He, Y.; Wang, W.; Wang, Y.; Qiu, J.; Guo, X. A mitochondrial STAT3-methionine metabolism axis promotes ILC2-driven allergic lung inflammation. J. Allergy Clin. Immunol. 2022, 149, 2091–2104. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef]
- Konya, V.; Czarnewski, P.; Forkel, M.; Rao, A.; Kokkinou, E.; Villablanca, E.J.; Almer, S.; Lindforss, U.; Friberg, D.; Hoog, C.; et al. Vitamin D downregulates the IL-23 receptor pathway in human mucosal group 3 innate lymphoid cells. J. Allergy Clin. Immunol. 2018, 141, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.I.; Li, Y.; Lopez-Lastra, S.; Stadhouders, R.; Paul, F.; Casrouge, A.; Serafini, N.; Puel, A.; Bustamante, J.; Surace, L.; et al. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell 2017, 168, 1086–1100.e1010. [Google Scholar] [CrossRef] [Green Version]
- Mebius, R.E.; Rennert, P.; Weissman, I.L. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997, 7, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnenberg, G.F.; Monticelli, L.A.; Elloso, M.M.; Fouser, L.A.; Artis, D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011, 34, 122–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Pavert, S.A. Lymphoid Tissue inducer (LTi) cell ontogeny and functioning in embryo and adult. Biomed. J. 2021, 44, 123–132. [Google Scholar] [CrossRef]
- Shikhagaie, M.M.; Björklund, Å.K.; Mjösberg, J.; Erjefält, J.S.; Cornelissen, A.S.; Ros, X.R.; Bal, S.M.; Koning, J.J.; Mebius, R.E.; Mori, M.; et al. Neuropilin-1 Is Expressed on Lymphoid Tissue Residing LTi-like Group 3 Innate Lymphoid Cells and Associated with Ectopic Lymphoid Aggregates. Cell Rep. 2017, 18, 1761–1773. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wang, J.; Hu, L.; Wang, S. Function of Innate Lymphoid Cells in Periodontal Tissue Homeostasis: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 6099. https://doi.org/10.3390/ijms24076099
Ma Z, Wang J, Hu L, Wang S. Function of Innate Lymphoid Cells in Periodontal Tissue Homeostasis: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(7):6099. https://doi.org/10.3390/ijms24076099
Chicago/Turabian StyleMa, Zhiyu, Jinsong Wang, Lei Hu, and Songlin Wang. 2023. "Function of Innate Lymphoid Cells in Periodontal Tissue Homeostasis: A Narrative Review" International Journal of Molecular Sciences 24, no. 7: 6099. https://doi.org/10.3390/ijms24076099
APA StyleMa, Z., Wang, J., Hu, L., & Wang, S. (2023). Function of Innate Lymphoid Cells in Periodontal Tissue Homeostasis: A Narrative Review. International Journal of Molecular Sciences, 24(7), 6099. https://doi.org/10.3390/ijms24076099





