Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust
Abstract
1. Introduction
2. Inflammation: An Overview
2.1. Recognition of Damaging Stimuli by Cell Surface Receptors: PRRs (Pattern Recognition Receptors)
2.2. Activation of Inflammatory Pathways
2.3. Activation and Release of Inflammatory Markers
2.4. Recruitment of Inflammatory Cells
2.5. Resolution of Inflammation
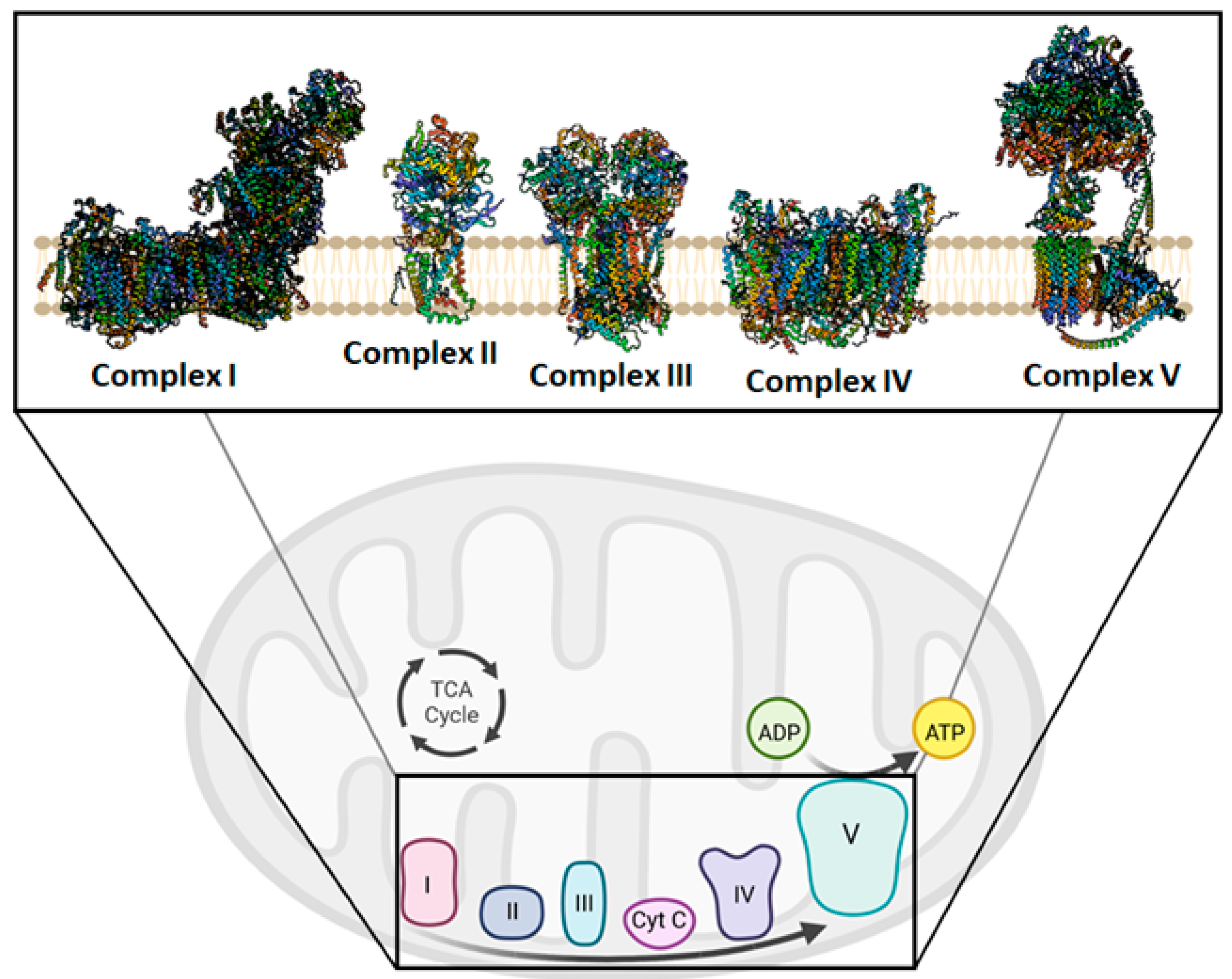
3. Mitochondrial Bioenergetics and Dysfunction
4. Mitochondria Are Potentially a New Target of Natural Compounds to Counteract Inflammation
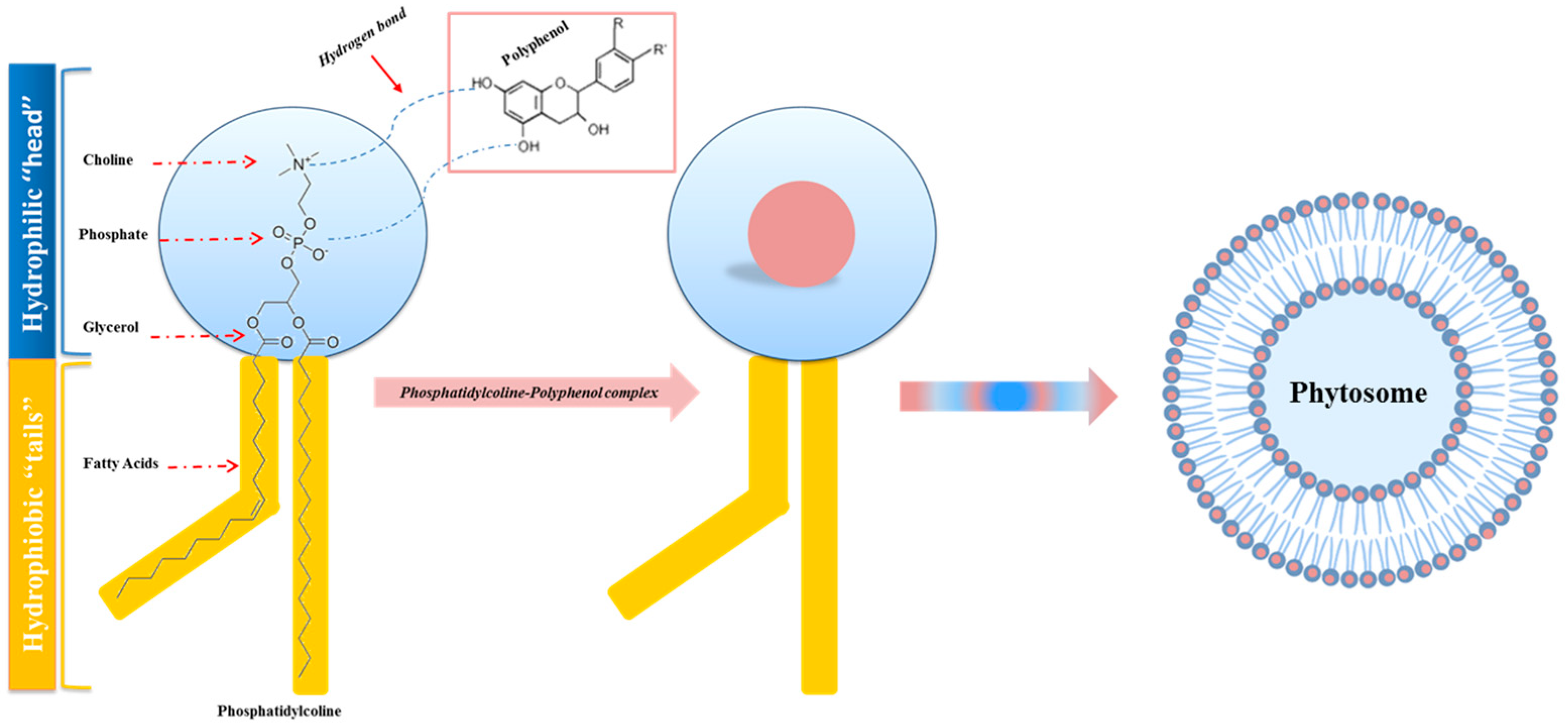
5. Phytochemicals and Phytosomes
- The phytochemical complexed in the phytosome is better protected from oxidation and degradation, improving its stability and increasing its long-term effects in pharmaceutical and cosmetic compositions [97];
- Compared with other nano-delivery systems, phytosomes have a simpler production method [98];
- The process of phytosome formation ensures efficient and meaningful drug/nutraceutical entrapment [99];
- Phosphatidylcholine acts both as a hepatoprotective agent, providing a synergistic effect, especially with hepatoprotective constituents found in the phytosomal complex, and as a supplement, with beneficial nutritional effects [102];
- Compared with liposomes, they are much smaller, and have different hydrogen bonds; the phytochemicals present in the phytosome are not dissolved in solvents or enveloped by the liposomal membrane, but are bound through chemical bonds with the polar head of phospholipids [103];
- Pharmacokinetic-type studies and pharmacodynamic tests conducted in experimental animals and human subjects have shown improved oral and percutaneous absorption of polar phytoactive compounds, increased bioavailability of the bioactive compound compared with non-complexed botanical derivatives, and enhanced therapeutic effects [104].
- The main disadvantages associated with phytosomes are:
- The pH sensitivity of phospholipids, due to their Zeta potential values, which must be considered when preparing phytosome formulations [105];
- Phytosome formation technology produces the rapid removal of phytoconstituents from the phytosome [106].
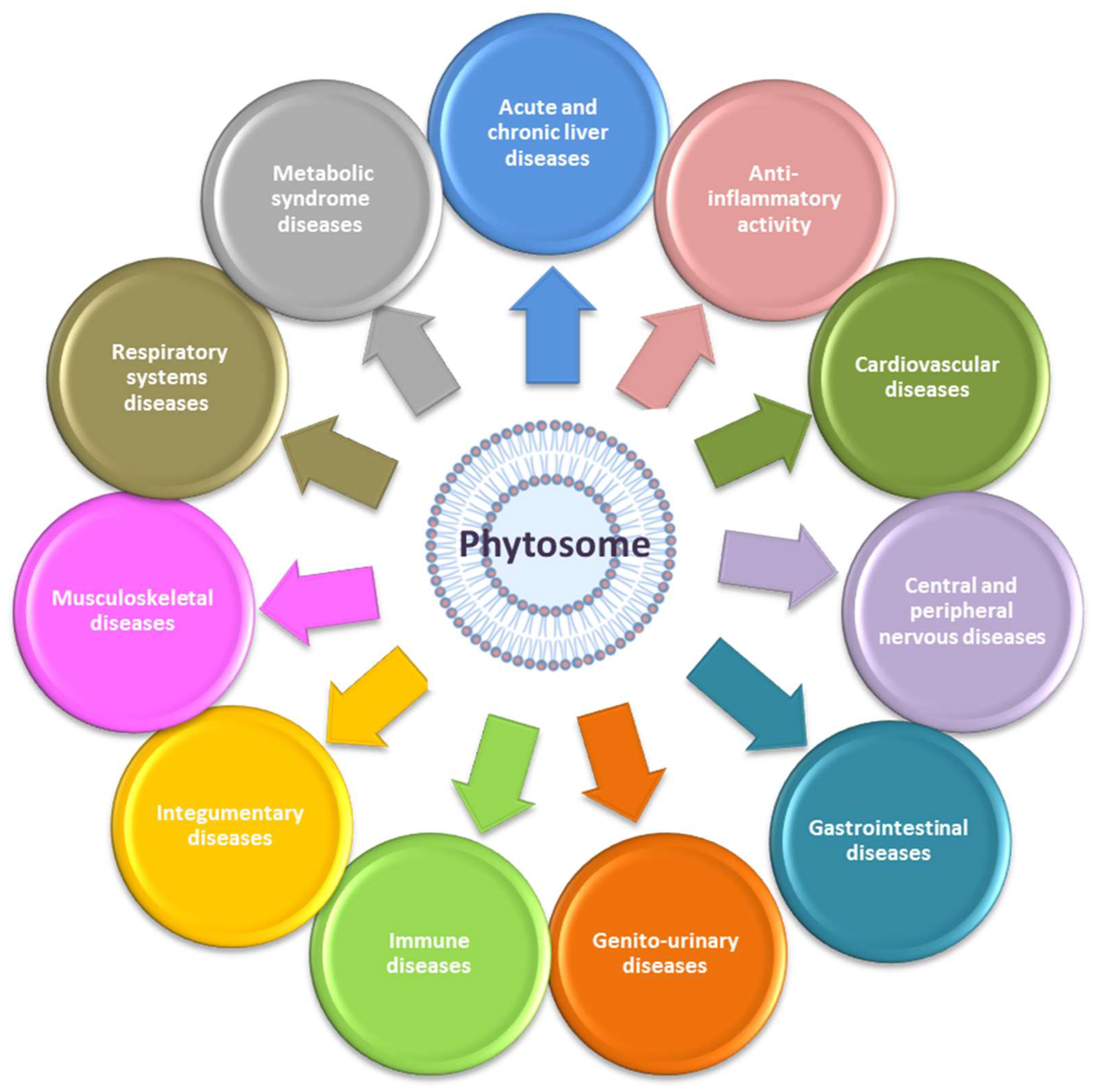
6. Phytosome and Pathologies
6.1. Curcumin Phytosome
6.2. Silybin Phytosome
6.3. Quercetin Phytosome
6.4. Berberine Phytosome
6.5. Mulberry and Ginger Phytosome
6.6. Eufortyn® Colesterolo Plus
6.7. Naringenin Phytosome
6.8. Centella Asiatica Phytosome
6.9. Leucoselect Phytosome
6.10. CoQ10 Phytosome
6.11. Phytosomes and Cytotoxicity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Lenaz, G. Impaired Mitochondrial Bioenergetics under Pathological Conditions. Life 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2022, 23, 159–173. [Google Scholar] [CrossRef]
- Picard, M.; Wallace, D.C.; Burelle, Y. The rise of mitochondria in medicine. Mitochondrion 2016, 30, 105–116. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Kulkarni, O.P.; Lichtnekert, J.; Anders, H.-J.; Mulay, S.R. The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is “Inflammation” Always Inflammation? Mediat. Inflamm. 2016, 2016, 2856213. [Google Scholar] [CrossRef]
- Shen, H.; Kreisel, D.; Goldstein, D.R. Processes of Sterile Inflammation. J. Immunol. 2013, 191, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Eletto, D.; Mentucci, F.; Voli, A.; Petrella, A.; Porta, A.; Tosco, A. Helicobacter pylori Pathogen-Associated Molecular Patterns: Friends or Foes? Int. J. Mol. Sci. 2022, 23, 3531. [Google Scholar] [CrossRef]
- Moriyama, K.; Nishida, O. Targeting Cytokines, Pathogen-Associated Molecular Patterns, and Damage-Associated Molecular Patterns in Sepsis via Blood Purification. Int. J. Mol. Sci. 2021, 22, 8882. [Google Scholar] [CrossRef]
- Bortolotti, P.; Faure, E.; Kipnis, E. Inflammasomes in Tissue Damages and Immune Disorders after Trauma. Front. Immunol. 2018, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- McKernan, D.P. Pattern Recognition Receptors as Potential Drug Targets in Inflammatory Disorders. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 119, pp. 65–109. ISBN 978-0-12-816844-8. [Google Scholar]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, M.J. Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol. Cells 2014, 37, 365–371. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (CoV-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Park, J.; Min, J.-S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Sandhir, R.; Halder, A.; Sunkaria, A. Mitochondria as a centrally positioned hub in the innate immune response. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1090–1097. [Google Scholar] [CrossRef]
- Vringer, E.; Tait, S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Stramer, B.M.; Mori, R.; Martin, P. The Inflammation–Fibrosis Link? A Jekyll and Hyde Role for Blood Cells during Wound Repair. J. Investig. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Mitchell, P. Keilin’s Respiratory Chain Concept and Its Chemiosmotic Consequences. Science 1979, 206, 1148–1159. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2021, 23, 141–161. [Google Scholar] [CrossRef]
- Saraste, M. Oxidative Phosphorylation at the Fin de Siècle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Pagliarani, A.; Algieri, C.; Trombetti, F. Mitochondrial F-Type ATP Synthase: Multiple Enzyme Functions Revealed by the Membrane-Embedded FO Structure. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 309–321. [Google Scholar] [CrossRef]
- Nesci, S.; Romeo, G. ‘Rotor free-wheeling’ in impaired F1FO-ATPase induces congenital hypermetabolism. Trends Endocrinol. Metab. 2022, 34, 63–65. [Google Scholar] [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial Cristae Shape Determines Respiratory Chain Supercomplexes Assembly and Respiratory Efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef]
- Strauss, M.; Hofhaus, G.; Schröder, R.R.; Kühlbrandt, W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008, 27, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Rampelt, H.; Wollweber, F.; Licheva, M.; de Boer, R.; Perschil, I.; Steidle, L.; Becker, T.; Bohnert, M.; van der Klei, I.; Kraft, C.; et al. Dual role of Mic10 in mitochondrial cristae organization and ATP synthase-linked metabolic adaptation and respiratory growth. Cell Rep. 2022, 38, 110290. [Google Scholar] [CrossRef] [PubMed]
- Blum, T.B.; Hahn, A.; Meier, T.; Davies, K.M.; Kühlbrandt, W. Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proc. Natl. Acad. Sci. USA 2019, 116, 4250–4255. [Google Scholar] [CrossRef]
- Daum, B.; Walter, A.; Horst, A.; Osiewacz, H.D.; Kühlbrandt, W. Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc. Natl. Acad. Sci. USA 2013, 110, 15301–15306. [Google Scholar] [CrossRef]
- Nesci, S. A Lethal Channel between the ATP Synthase Monomers. Trends Biochem. Sci. 2018, 43, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Algieri, C.; Tioli, G.; Lenaz, G. Molecular and Supramolecular Structure of the Mitochondrial Oxidative Phosphorylation System: Implications for Pathology. Life 2021, 11, 242. [Google Scholar] [CrossRef]
- Lenaz, G.; Strocchi, P. Reactive Oxygen Species in the Induction of Toxicity. In General, Applied and Systems Toxicology; American Cancer Society: Atlanta, GA, USA, 2011; ISBN 978-0-470-74430-7. [Google Scholar]
- Lenaz, G. Mitochondria and Reactive Oxygen Species. Which Role in Physiology and Pathology? Adv. Exp. Med. Biol. 2012, 942, 93–136. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Huang, L.S.; Hong, Z.; Wu, W.; Xiong, S.; Zhong, M.; Gao, X.; Rehman, J.; Malik, A.B. mtDNA Activates cGAS Signaling and Suppresses the YAP-Mediated Endothelial Cell Proliferation Program to Promote Inflammatory Injury. Immunity 2020, 52, 475–486.e5. [Google Scholar] [CrossRef]
- Wu, Z.; Sainz, A.G.; Shadel, G.S. Mitochondrial DNA: Cellular genotoxic stress sentinel. Trends Biochem. Sci. 2021, 46, 812–821. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Troisio, I.; et al. The Impairment of Cell Metabolism by Cardiovascular Toxicity of Doxorubicin Is Reversed by Bergamot Polyphenolic Fraction Treatment in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 8977. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Yi, J.; Olguín-Albuerne, M.; Morán, J.; Hornsveld, M.; Dansen, T.B.; Genova, M.L.; Lenaz, G.; Nunes, P.; et al. Redox Sensing by Proteins: Oxidative Modifications on Cysteines and the Consequent Events. Antioxid. Redox Signal. 2012, 16, 649–657. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Russo, M.T.; De Luca, G.; Degan, P.; Parlanti, E.; Dogliotti, E.; Barnes, D.E.; Lindahl, T.; Yang, H.; Miller, J.H.; Bignami, M. Accumulation of the Oxidative Base Lesion 8-Hydroxyguanine in DNA of Tumor-Prone Mice Defective in Both the Myh and Ogg1 DNA Glycosylases. Cancer Res. 2004, 64, 4411–4414. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Wang, Y.; Ning, X.; Gao, P.; Wu, S.; Sha, M.; Lv, M.; Zhou, X.; Gao, J.; Fang, R.; Meng, G.; et al. Inflammasome Activation Triggers Caspase-1-Mediated Cleavage of cGAS to Regulate Responses to DNA Virus Infection. Immunity 2017, 46, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Bleicken, S.; Assafa, T.E.; Stegmueller, C.; Wittig, A.; Garcia-Saez, A.J.; Bordignon, E. Topology of active, membrane-embedded Bax in the context of a toroidal pore. Cell Death Differ. 2018, 25, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San Chin, H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef]
- McArthur, K.; Kile, B.T. Apoptotic Caspases: Multiple or Mistaken Identities? Trends Cell Biol. 2018, 28, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Ventrella, V.; Pagliarani, A. From the Ca2+-Activated F1FO-ATPase to the Mitochondrial Permeability Transition Pore: An Overview. Biochimie 2018, 152, 85–93. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Morciano, G.; Naumova, N.; Koprowski, P.; Valente, S.; Sardão, V.A.; Potes, Y.; Rimessi, A.; Wieckowski, M.R.; Oliveira, P.J. The mitochondrial permeability transition pore: An evolving concept critical for cell life and death. Biol. Rev. 2021, 96, 2489–2521. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Chen, Z.J. cGAS in action: Expanding roles in immunity and inflammation. Science 2019, 363, eaat8657. [Google Scholar] [CrossRef]
- He, B.; Yu, H.; Liu, S.; Wan, H.; Fu, S.; Liu, S.; Yang, J.; Zhang, Z.; Huang, H.; Li, Q.; et al. Mitochondrial cristae architecture protects against mtDNA release and inflammation. Cell Rep. 2022, 41, 111774. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, W.; Zhang, B.; Li, W. NLRP3 Inflammasome: A Promising Therapeutic Target for Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 634607. [Google Scholar] [CrossRef]
- Nesci, S. The mitochondrial permeability transition pore in cell death: A promising drug binding bioarchitecture. Med. Res. Rev. 2020, 40, 811–817. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Nesci, S. Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction. Cells 2022, 11, 1401. [Google Scholar] [CrossRef]
- Bayir, A.G.; Kiziltan, H.S.; Kocyigit, A. Plant Family, Carvacrol, and Putative Protection in Gastric Cancer. In Dietary Interventions in Gastrointestinal Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–18. ISBN 978-0-12-814468-8. [Google Scholar]
- Sangiorgio, P.; Verardi, A.; Spagnoletta, A.; Balducchi, R.; Leone, G.P.; Pizzichini, D.; Raimondo, S.; Conigliaro, A.; Alessandro, R. Citrus as a Multifunctional Crop to Promote New Bio-Products and Valorize the Supply Chain. Environ. Eng. Manag. J. 2020, 19, 1869–1889. [Google Scholar] [CrossRef]
- Barreira, S.; Moutinho, C.; Silva, A.M.N.; Neves, J.; Seo, E.-J.; Hegazy, M.-E.F.; Efferth, T.; Gomes, L.R. Phytochemical characterization and biological activities of green tea (Camellia sinensis) produced in the Azores, Portugal. Phytomed. Plus 2021, 1, 100001. [Google Scholar] [CrossRef]
- Wu, P.; Li, F.; Zhang, J.; Yang, B.; Ji, Z.; Chen, W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complement. Altern. Med. 2017, 17, 151. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Magrone, T.; Salvatore, R.; Spagnoletta, A.; Magrone, M.; Russo, M.A.; Jirillo, E. In Vitro Effects of Nickel on Healthy Non-Allergic Peripheral Blood Mononuclear Cells. The Role of Red Grape Polyphenols. Endocr. Metab. Immune Disord.-Drug Targets 2017, 17, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E.; Spagnoletta, A.; Magrone, M.; Russo, M.A.; Fontana, S.; Laforgia, F.; Donvito, I.; Campanella, A.; Silvestris, F.; et al. Immune Profile of Obese People and In Vitro Effects of Red Grape Polyphenols on Peripheral Blood Mononuclear Cells. Oxidative Med. Cell. Longev. 2017, 2017, 9210862. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Complex Compounds of Bioflavonoids with Phospholipids, Their Preparation and Use, and Pharmaceutical and Cosmetic Compositions Containing Them—Patent US-5043323-A. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-5043323-A (accessed on 18 February 2023).
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Osipova, D.; Kokoreva, K.; Lazebnik, L.; Golovanova, E.; Pavlov, C.; Dukhanin, A.; Orlova, S.; Starostin, K. Regression of Liver Steatosis Following Phosphatidylcholine Administration: A Review of Molecular and Metabolic Pathways Involved. Front. Pharmacol. 2022, 13, 797923. [Google Scholar] [CrossRef]
- Bombardelli, E.; Curri, S.B.; Loggia, R.D.; Negro, P.D.; Tubaro, A.; Gariboldi, P. Complexes between Phospholipids and Vegetal Derivatives of Biological Interest. Fitoterapia 1989, 60, 1–9. [Google Scholar]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Habbu, P.; Madagundi, S.; Shastry, R.; Vanakudri, R.; Kulkarni, V. Preparation and Evaluation of Antidiabetic Activity of Allium cepa-Phospholipid Complex (Phytosome) in Streptozotocin Induced Diabetic Rats. RGUHS J. Pharm. Sci. 2016, 5, 132–141. [Google Scholar] [CrossRef]
- Dewan, N.; Dasgupta, D.; Pandit, S.; Ahmed, P. Review on- Herbosomes, A New Arena for Drug Delivery. J. Pharmacogn. Phytochem. 2016, 5, 104–108. [Google Scholar]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the “dark side of the force” in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar]
- Mastellone, I.; Polichetti, E.; Grès, S.; de la Maisonneuve, C.; Domingo, N.; Marin, V.; Lorec, A.-M.; Farnarier, C.; Portugal, H.; Kaplanski, G.; et al. Dietary soybean phosphatidylcholines lower lipidemia: Mechanisms at the levels of intestine, endothelial cell, and hepato-biliary axis. J. Nutr. Biochem. 2000, 11, 461–466. [Google Scholar] [CrossRef]
- Babazadeh, A.; Zeinali, M.; Hamishehkar, H. Nano-Phytosome: A Developing Platform for Herbal Anti-Cancer Agents in Cancer Therapy. Curr. Drug Targets 2018, 19, 170–180. [Google Scholar] [CrossRef]
- Ripoli, M.; Angelico, R.; Sacco, P.; Ceglie, A.; Mangia, A. Phytoliposome-Based Silibinin Delivery System as a Promising Strategy to Prevent Hepatitis C Virus Infection. J. Biomed. Nanotechnol. 2016, 12, 770–780. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-phytosome as a potential food-grade delivery system. Food Biosci. 2016, 15, 126–135. [Google Scholar] [CrossRef]
- Sanha, A.M.F.; Sharma, P.K.; Kumar, S. Phytosome as a Prominent Option in Drug Delivery for the Treatment of the Diseases: A Review. Indo Glob. J. Pharm. Sci. 2018, 8, 119–123. [Google Scholar] [CrossRef]
- Vivarelli, F.; Granata, S.; Rullo, L.; Mussoni, M.; Candeletti, S.; Romualdi, P.; Fimognari, C.; Cruz-Chamorro, I.; Carrillo-Vico, A.; Paolini, M.; et al. On the toxicity of e-cigarettes consumption: Focus on pathological cellular mechanisms. Pharmacol. Res. 2022, 182, 106315. [Google Scholar] [CrossRef]
- Al-Kahtani, M.; Abdel-Daim, M.M.; Sayed, A.A.; El-Kott, A.; Morsy, K. Curcumin phytosome modulates aluminum-induced hepatotoxicity via regulation of antioxidant, Bcl-2, and caspase-3 in rats. Environ. Sci. Pollut. Res. 2020, 27, 21977–21985. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, S.; Hajizadeh Moghaddam, A.; Khanjani Jelodar, S.; Moradi-Kor, N. Protective Effects of Curcumin and its Nano-Phytosome on Carrageenan-Induced Inflammation in Mice Model: Behavioral and Biochemical Responses. J. Inflamm. Res. 2020, 13, 45–51. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Chi, C.; Zhang, C.; Liu, Y.; Nie, H.; Zhou, J.; Ding, Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur. J. Pharm. Sci. 2020, 144, 105212. [Google Scholar] [CrossRef]
- Vecchione, G.; Grasselli, E.; Voci, A.; Baldini, F.; Grattagliano, I.; Wang, D.Q.; Portincasa, P.; Vergani, L. Silybin counteracts lipid excess and oxidative stress in cultured steatotic hepatic cells. World J. Gastroenterol. 2016, 22, 6016–6026. [Google Scholar] [CrossRef]
- Pasala, P.K.; Uppara, R.K.; Rudrapal, M.; Zothantluanga, J.H.; Umar, A.K. Silybin phytosome attenuates cerebral ischemia-reperfusion injury in rats by suppressing oxidative stress and reducing inflammatory response: In vivo and in silico approaches. J. Biochem. Mol. Toxicol. 2022, 36, e23073. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Bernardini, C.; Fabbri, M.; Forni, M.; Nesci, S. Mitochondrial Ca2+-activated F1FO-ATPase hydrolyzes ATP and promotes the permeability transition pore. Ann. N. Y. Acad. Sci. 2019, 1457, 142–157. [Google Scholar] [CrossRef]
- Di Pierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a Potential Candidate for Managing COVID-19. Minerva Gastroenterol. 2021, 67, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Mumtaz, S.U.; Chaudhry, M.M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.I.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.-R.A.; Mohamed, E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Interact. 2017, 271, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Giacosa, A.; Fazia, T.; Bernardinelli, L.; Gasparri, C.; Peroni, G.; Perna, S. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest–Post-Test Explanatory Study. Nutrients 2021, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W. Antimetabolic Syndrome Effect of Phytosome Containing the Combined Extracts of Mulberry and Ginger in an Animal Model of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 5972575. [Google Scholar] [CrossRef]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W. Phytosome Loading the Combined Extract of Mulberry Fruit and Ginger Protects against Cerebral Ischemia in Metabolic Syndrome Rats. Oxidative Med. Cell. Longev. 2020, 2020, 5972575. [Google Scholar] [CrossRef]
- Fogacci, F.; Rizzoli, E.; Giovannini, M.; Bove, M.; D’Addato, S.; Borghi, C.; Cicero, A.F.G. Effect of Dietary Supplementation with Eufortyn® Colesterolo Plus on Serum Lipids, Endothelial Reactivity, Indexes of Non-Alcoholic Fatty Liver Disease and Systemic Inflammation in Healthy Subjects with Polygenic Hypercholesterolemia: The ANEMONE Study. Nutrients 2022, 14, 2099. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Chen, H.; Zhu, L. Naringenin-Loaded Dipalmitoylphosphatidylcholine Phytosome Dry Powders for Inhaled Treatment of Acute Lung Injury. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 194–204. [Google Scholar] [CrossRef]
- Ho, P.J.; Sung, J.J.; Cheon, K.K.; Tae, H.J. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 2018, 43, 110–119. [Google Scholar] [CrossRef]
- Mao, J.T.; Xue, B.; Fan, S.; Neis, P.; Qualls, C.; Massie, L.; Fiehn, O. Leucoselect Phytosome Modulates Serum Eicosapentaenoic Acid, Docosahexaenoic Acid, and Prostaglandin E3 in a Phase I Lung Cancer Chemoprevention Study. Cancer Prev. Res. 2021, 14, 619–626. [Google Scholar] [CrossRef]
- Magrone, T.; Pugliese, V.; Fontana, S.; Jirillo, E. Human Use of Leucoselect® Phytosome® with Special Reference to Inflammatory-Allergic Pathologies in Frail Elderly Patients. Curr. Pharm. Des. 2014, 20, 1011–1019. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, A.; Veronesi, M.; Borghi, C. Noninvasive instrumental evaluation of coenzyme Q10 phytosome on endothelial reactivity in healthy nonsmoking young volunteers: A double-blind, randomized, placebo-controlled crossover clinical trial. Biofactors 2022, 48, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, N.; Liparulo, I.; Antonelli, G.; Orsini, F.; Riva, A.; Bergamini, C.; Fato, R. Coenzyme Q10 Phytosome Formulation Improves CoQ10 Bioavailability and Mitochondrial Functionality in Cultured Cells. Antioxidants 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; et al. Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics 2020, 12, 346. [Google Scholar] [CrossRef]
- Al-Rabia, M.W.; Alhakamy, N.A.; Rizg, W.Y.; Alghaith, A.F.; Ahmed, O.A.A.; Fahmy, U.A. Boosting curcumin activity against human prostatic cancer PC3 cells by utilizing scorpion venom conjugated phytosomes as promising functionalized nanovesicles. Drug Deliv. 2022, 29, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Neamatallah, T.; Malebari, A.M.; Alamoudi, A.J.; Nazreen, S.; Alam, M.M.; Bin-Melaih, H.H.; Abuzinadah, O.A.; Badr-Eldin, S.M.; Alhassani, G.; Makki, L.; et al. Andrographolide nanophytosomes exhibit enhanced cellular delivery and pro-apoptotic activities in HepG2 liver cancer cells. Drug Deliv. 2023, 30, 2174209. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. https://doi.org/10.3390/ijms24076106
Nesci S, Spagnoletta A, Oppedisano F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. International Journal of Molecular Sciences. 2023; 24(7):6106. https://doi.org/10.3390/ijms24076106
Chicago/Turabian StyleNesci, Salvatore, Anna Spagnoletta, and Francesca Oppedisano. 2023. "Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust" International Journal of Molecular Sciences 24, no. 7: 6106. https://doi.org/10.3390/ijms24076106
APA StyleNesci, S., Spagnoletta, A., & Oppedisano, F. (2023). Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. International Journal of Molecular Sciences, 24(7), 6106. https://doi.org/10.3390/ijms24076106







