Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells
Abstract
1. Introduction
2. Results
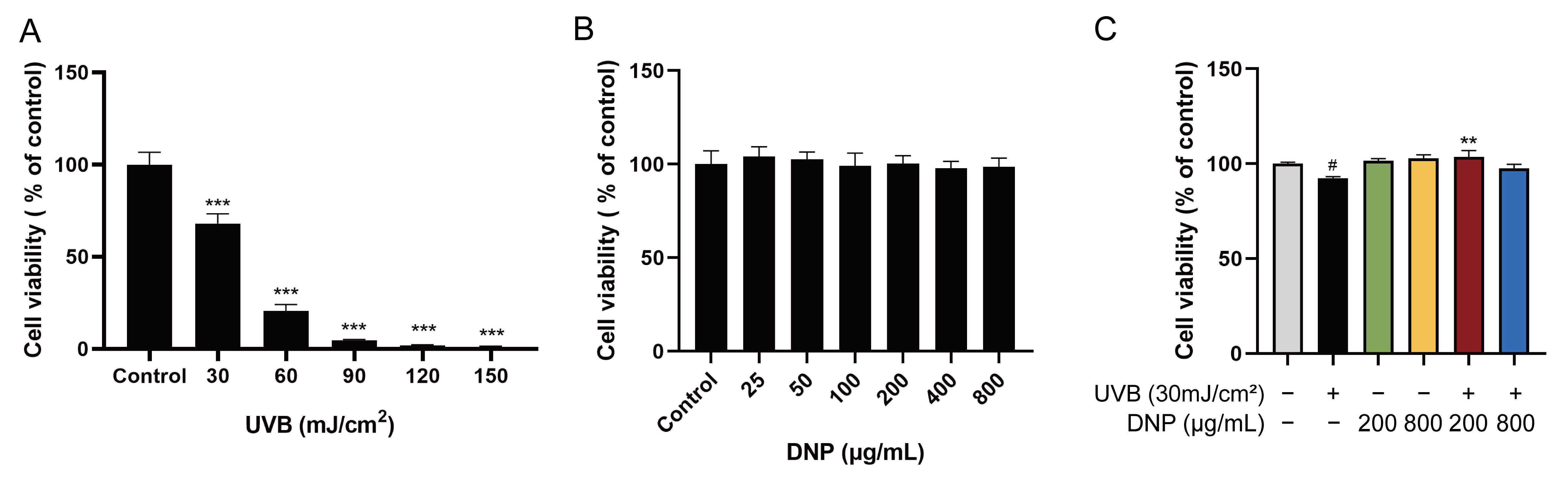
2.1. DNPs Attenuate UVB-Induced Damage in HaCaT Cells
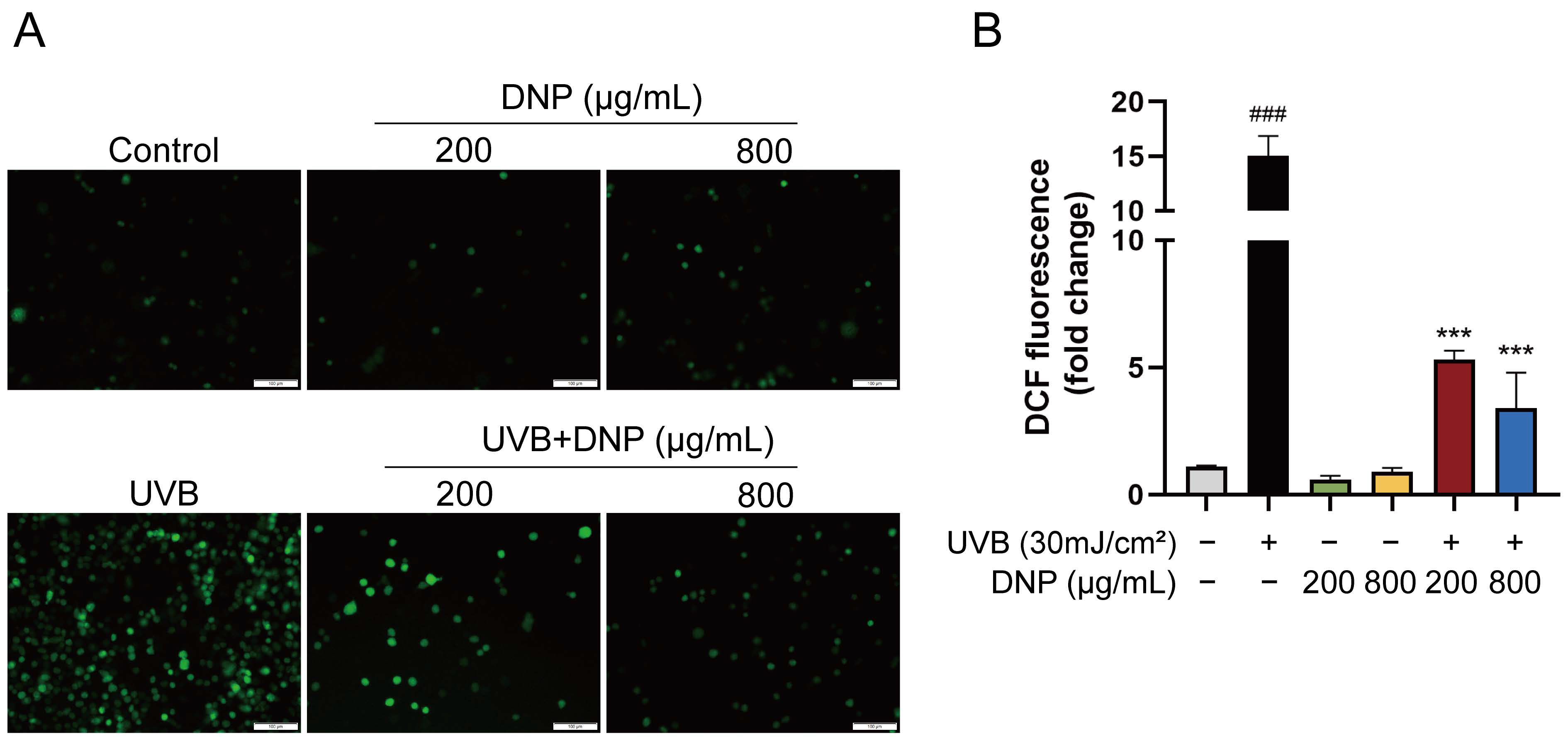
2.2. DNPs Scavenge Intracellular ROS
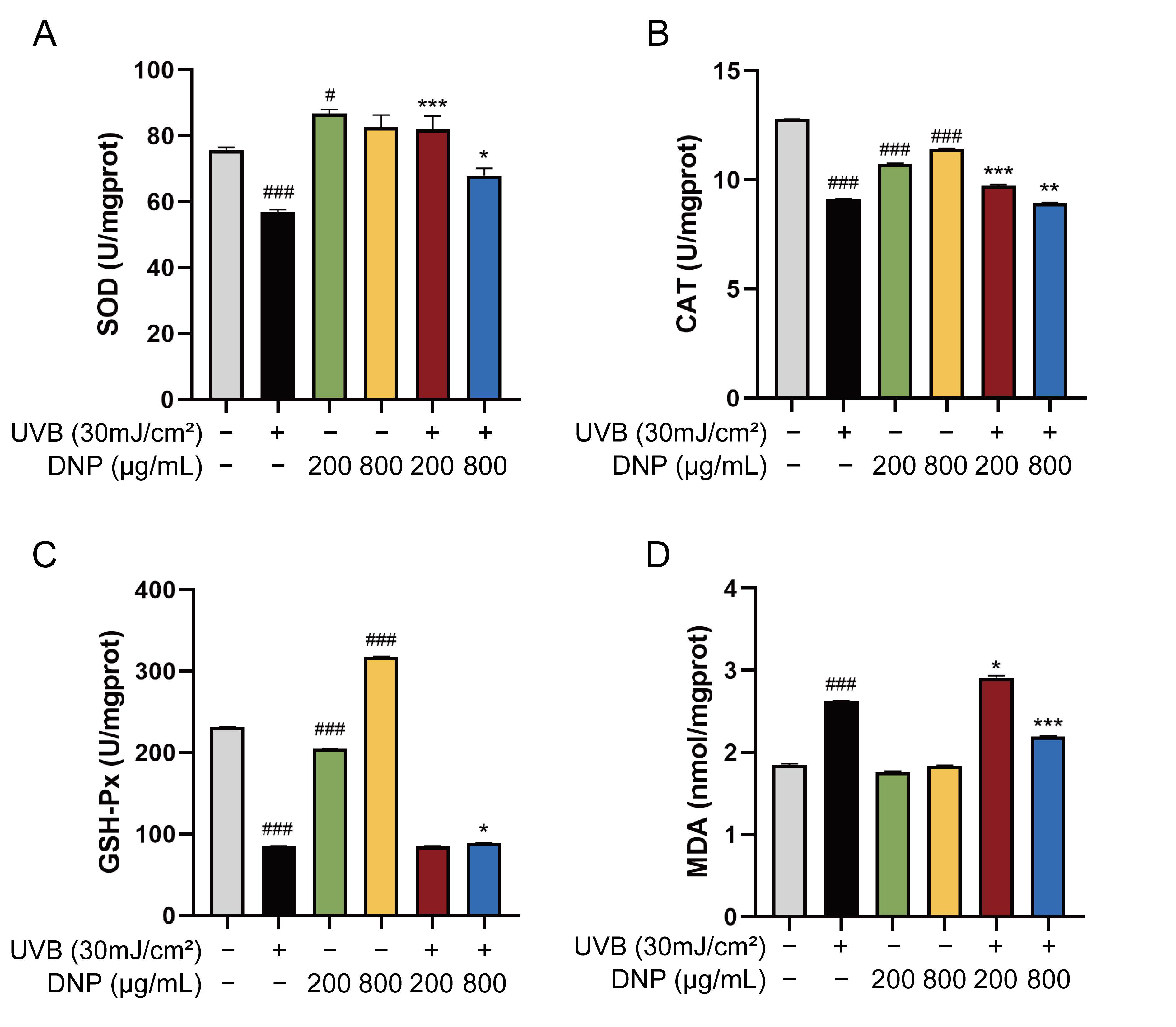
2.3. DNPs Alleviate UVB-Induced Oxidative Stress Damage
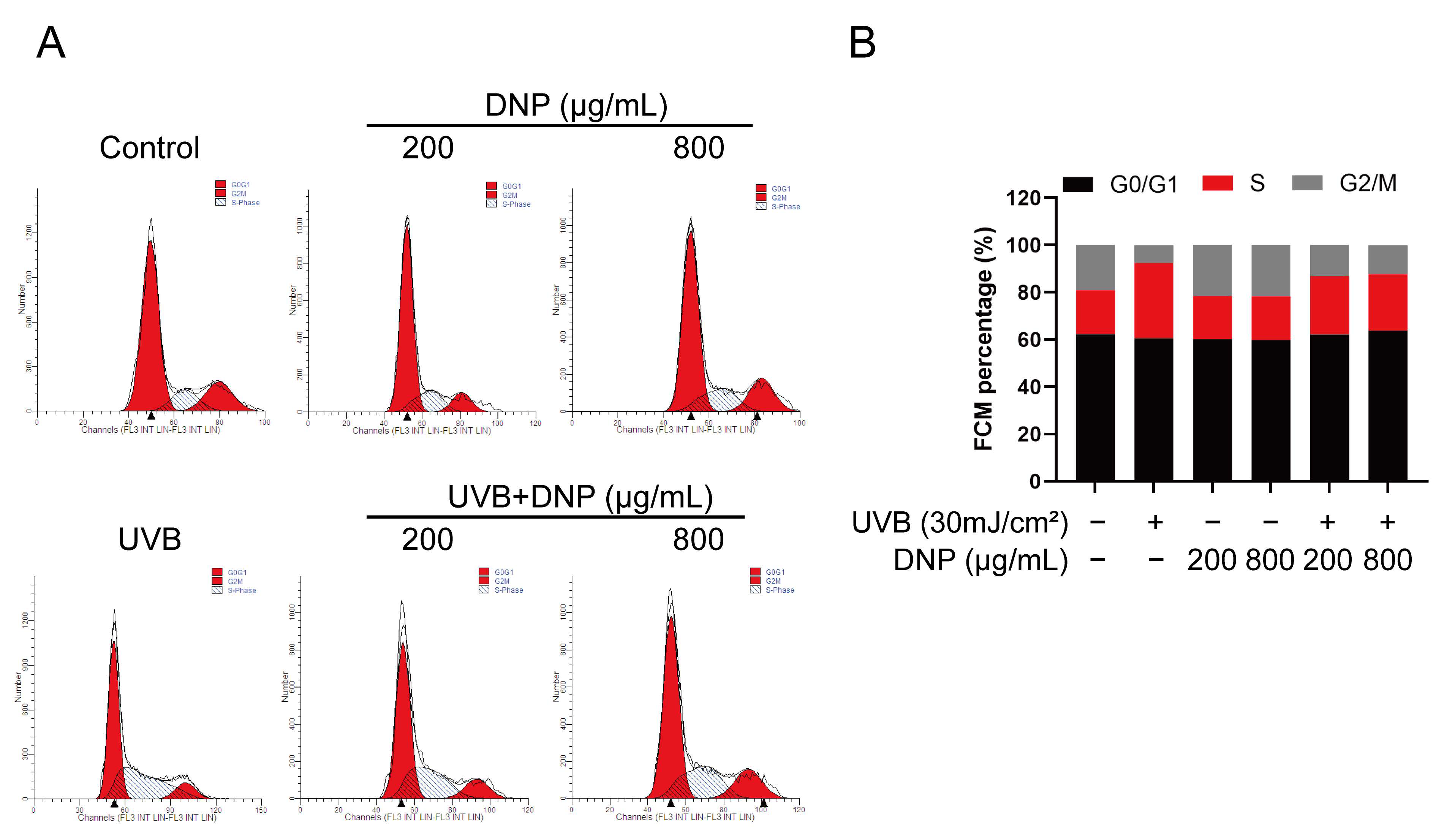
2.4. DNPs Rescue UVB-Induced Cell Cycle Arrest
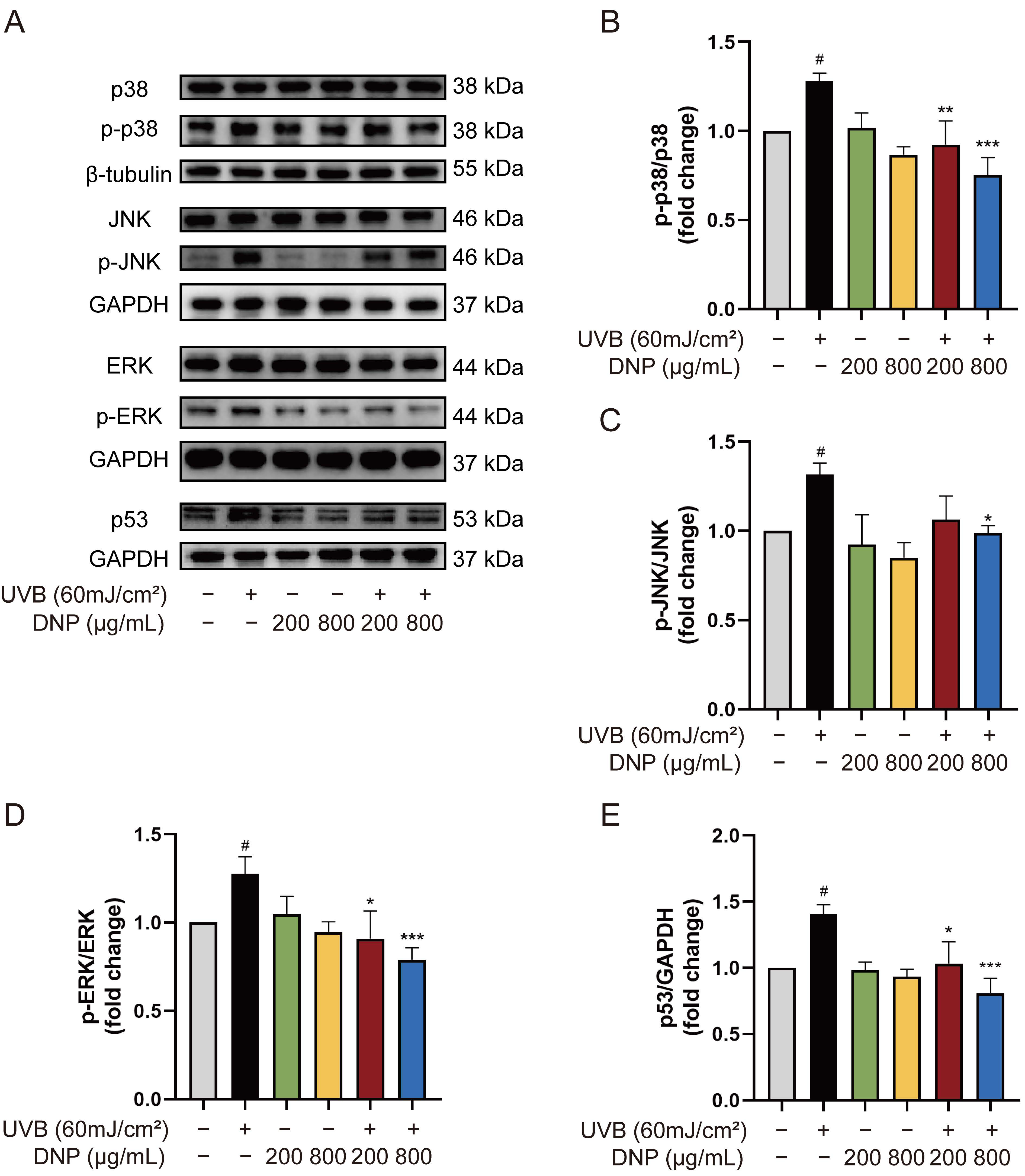
2.5. DNPs Inhibit UVB-Induced Activation of MAPKs
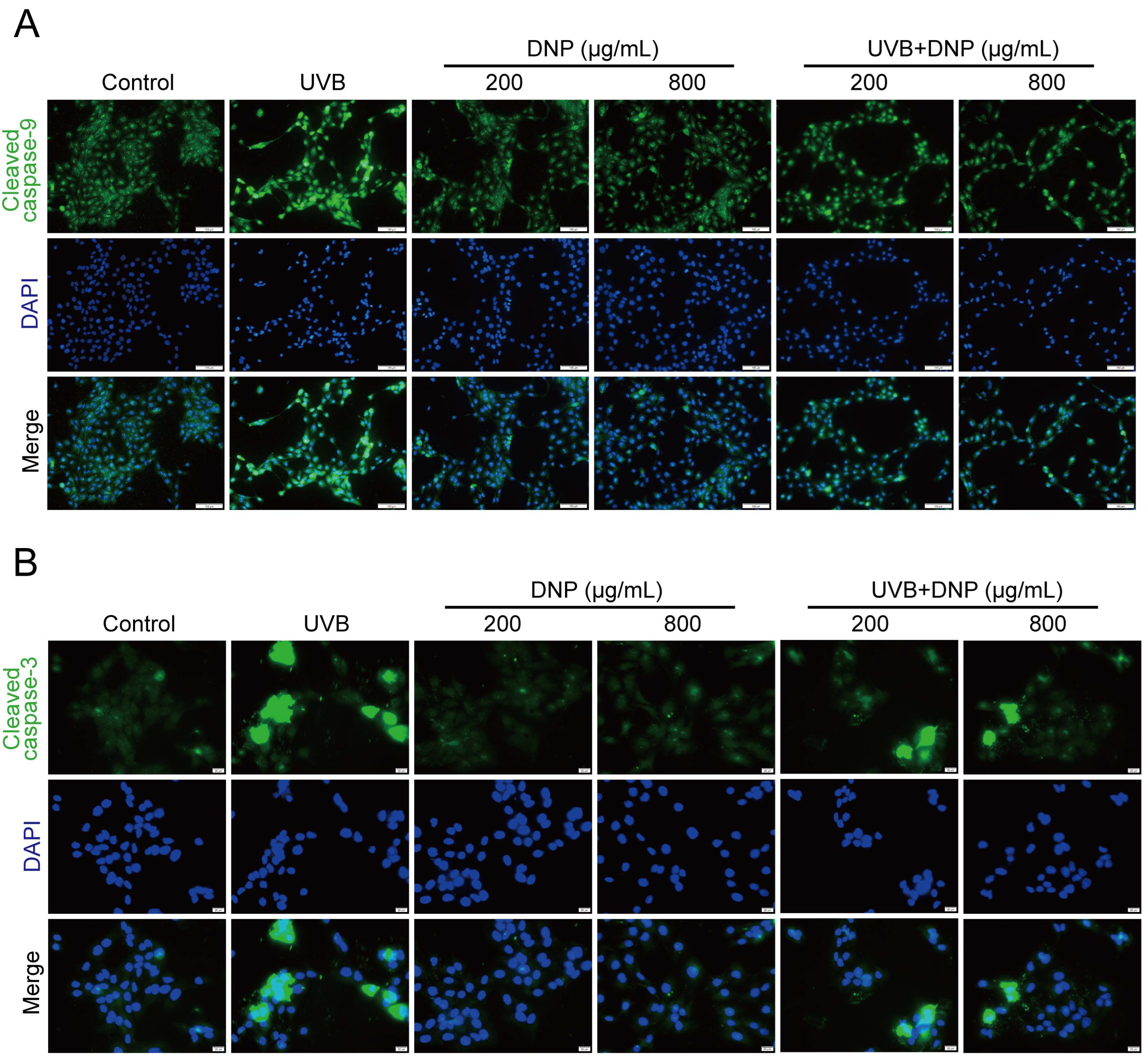
2.6. DNPs Inhibit UVB-Induced Apoptosis of HaCaT Cells
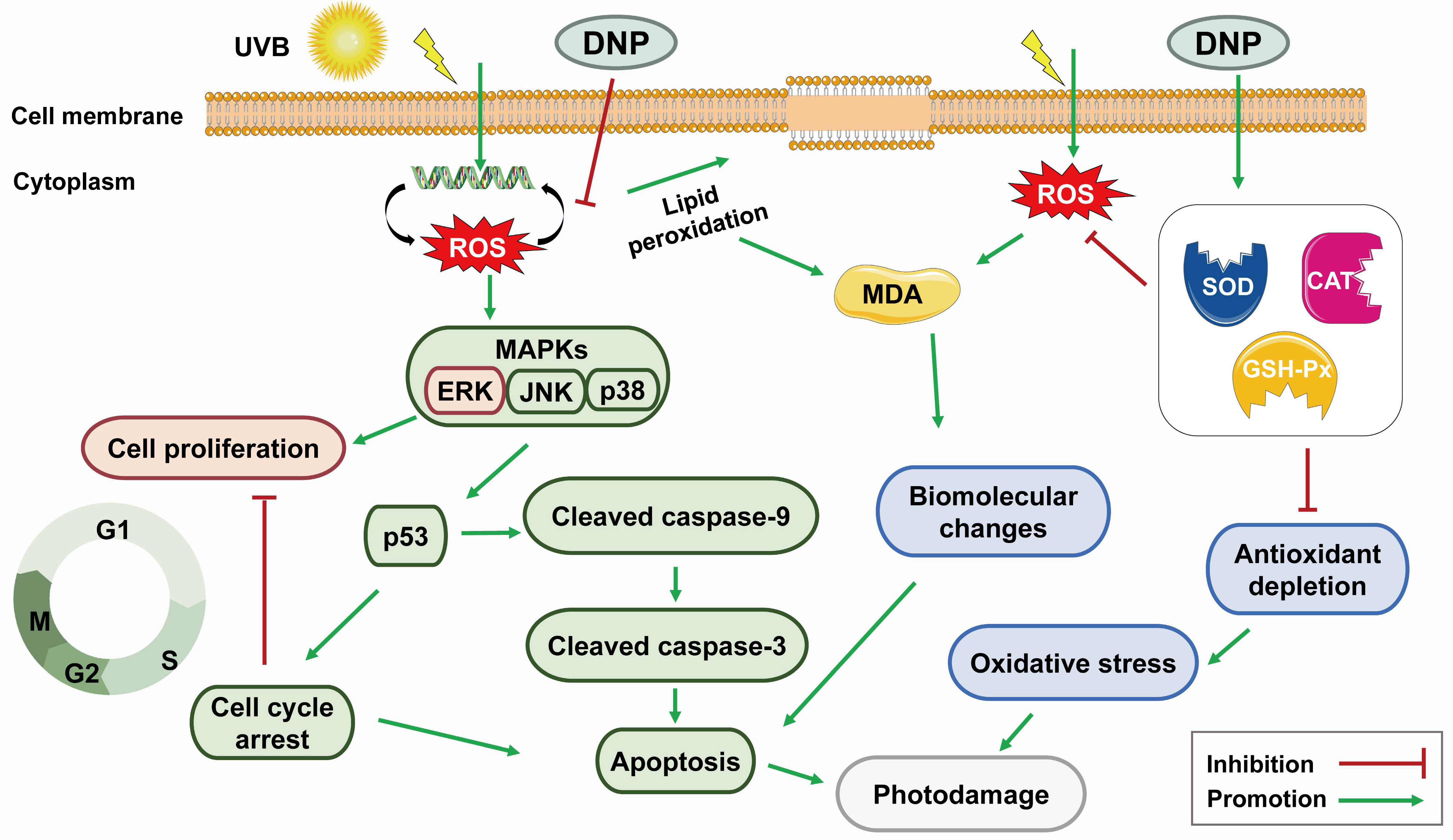
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. UVB Irradiation
4.4. Cell Viability Assay
4.5. Determination of Intracellular ROS Levels
4.6. Measurement of Oxidative Stress-Related Marker
4.7. Cell Cycle Analysis
4.8. Hoechst 33258 Staining
4.9. EdU Staining
4.10. Immunofluorescence
4.11. Western Blotting Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afnan, Q.; Kaiser, P.J.; Rafiq, R.A.; Nazir, L.A.; Bhushan, S.; Bhardwaj, S.C.; Sandhir, R.; Tasduq, S.A. Glycyrrhizic acid prevents ultraviolet-B-induced photodamage: A role for mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp. Dermatol. 2016, 25, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pawelek, J. Animals under the sun: Effects of ultraviolet radiation on mammalian skin. Clin. Dermatol. 1998, 16, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UVB-induced photo-aging by polyphenolic-rich Spatholobus Suberectus Stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef]
- Van Laethem, A.; Nys, K.; Van Kelst, S.; Claerhout, S.; Ichijo, H.; Vandenheede, J.R.; Garmyn, M.; Agostinis, P. Apoptosis signal regulating kinase-1 connects reactive oxygen species to p38 MAPK-induced mitochondrial apoptosis in UVB-irradiated human keratinocytes. Free Radic. Biol. Med. 2006, 41, 1361–1371. [Google Scholar] [CrossRef]
- López-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Pérez-Plasencia, C.; Alvarez-Sánchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef]
- Shimizu, H.; Banno, Y.; Sumi, N.; Naganawa, T.; Kitajima, Y.; Nozawa, Y. Activation of p38 mitogen-activated protein kinase and caspases in UVB-induced apoptosis of human keratinocyte HaCaT cells. J. Investig. Dermatol. 1999, 112, 769–774. [Google Scholar] [CrossRef]
- Finlay, C.A.; Hinds, P.W.; Levine, A.J. The p53 proto-oncogene can act as a suppressor of transformation. Cell 1989, 57, 1083–1093. [Google Scholar] [CrossRef]
- Baker, S.J.; Markowitz, S.; Fearon, E.R.; Willson, J.K.; Vogelstein, B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 1990, 249, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P.; Midgley, C.A.; Hupp, T.R.; Lu, X.; Vojtesek, B.; Picksley, S.M. On the regulation of the p53 tumour suppressor, and its role in the cellular response to DNA damage. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995, 347, 83–87. [Google Scholar] [PubMed]
- Chouinard, N.; Valerie, K.; Rouabhia, M.; Huot, J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J. 2002, 365, 133–145. [Google Scholar] [CrossRef]
- Ma, C.; Meng, C.W.; Zhou, Q.M.; Peng, C.; Liu, F.; Zhang, J.W.; Zhou, F.; Xiong, L. New sesquiterpenoids from the stems of Dendrobium nobile and their neuroprotective activities. Fitoterapia 2019, 138, 104351. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zou, S.; Lin, J.; Zhai, L.; Zhang, Y.; Fu, X.; Chen, S.; Niu, H.; Liu, F.; Wu, C.; et al. Antioxidant and anti-inflammatory activity of constituents isolated from Dendrobium nobile (Lindl.). Front. Chem. 2022, 10, 988459. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shi, X.; Zhang, L.; Yao, L.; Cen, L.; Li, L.; Lv, Y.; Wei, C. Ultrasonic extraction process of polysaccharides from Dendrobium nobile Lindl.: Optimization, physicochemical properties and anti-inflammatory activity. Foods 2022, 11, 2957. [Google Scholar] [CrossRef]
- Pi, T.; Lang, G.; Liu, B.; Shi, J. Protective effects of Dendrobium nobile Lindl. alkaloids on Alzheimer’s disease-like symptoms induced by high-methionine diet. Curr. Neuropharmacol. 2022, 20, 983–997. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef]
- Zhang, S.; Tu, H.; Zhu, J.; Liang, A.; Huo, P.; Shan, K.; He, J.; Zhao, M.; Chen, X.; Lei, X. Dendrobium nobile Lindl. polysaccharides improve follicular development in PCOS rats. Int. J. Biol. Macromol. 2020, 149, 826–834. [Google Scholar] [CrossRef]
- Lei, X.; Huo, P.; Xie, Y.J.; Wang, Y.; Liu, G.; Tu, H.; Shi, Q.; Mo, Z.C.; Zhang, S. Dendrobium nobile Lindl polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats. Mol. Reprod. Dev. 2022, 89, 202–213. [Google Scholar] [CrossRef]
- Li, W.; Mu, X.; Wu, X.; He, W.; Liu, Y.; Liu, Y.; Deng, J.; Nie, X. Dendrobium nobile Lindl. polysaccharides protect fibroblasts against UVA-induced photoaging via JNK/c-Jun/MMPs pathway. J. Ethnopharmacol. 2022, 298, 115590. [Google Scholar] [CrossRef]
- Long, Y.; Wang, W.; Zhang, Y.; Zhang, S.; Li, Z.; Deng, J.; Li, J. Dendrobium nobile Lindl polysaccharides attenuate UVB-induced photodamage by regulating oxidative stress, inflammation and MMPs expression in mice model. Photochem. Photobiol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, G.H.; Ahn, E.M.; Park, C.I.; Jang, J.H. Sargassum fulvellum protects HaCaT cells and BALB/c mice from UVB-induced proinflammatory responses. Evid. Based Complement. Alternat. Med. 2013, 2013, 747846. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.M.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.A.; De Benedetto, A. Epidermal tight junctions in health and disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef]
- Muthusamy, V.; Piva, T.J. A comparative study of UV-induced cell signalling pathways in human keratinocyte-derived cell lines. Arch. Dermatol. Res. 2013, 305, 817–833. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflam. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J. Investig. Dermatol. 2007, 127, 707–715. [Google Scholar] [CrossRef]
- Mishra, V.; Pathak, C. Human toll-like receptor 4 (hTLR4): Structural and functional dynamics in cancer. Int. J. Biol. Macromol. 2019, 122, 425–451. [Google Scholar] [CrossRef]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem. Photobiol. 2005, 81, 38–45. [Google Scholar] [CrossRef]
- Dai, J.; Ma, H.; Fan, J.; Li, Y.; Wang, J.; Ni, H.; Xia, G.; Chen, S. Crude polysaccharide from an anti-UVB cell clone of Bupleurum scorzonerifolium protect HaCaT cells against UVB-induced oxidative stress. Cytotechnology 2011, 63, 599–607. [Google Scholar] [CrossRef]
- Black, A.T.; Gray, J.P.; Shakarjian, M.P.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. Distinct effects of ultraviolet B light on antioxidant expression in undifferentiated and differentiated mouse keratinocytes. Carcinogenesis 2008, 29, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Mukhtar, H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B 2001, 63, 61–69. [Google Scholar] [CrossRef]
- Paz, M.L.; González Maglio, D.H.; Weill, F.S.; Bustamante, J.; Leoni, J. Mitochondrial dysfunction and cellular stress progression after ultraviolet B irradiation in human keratinocytes. Photodermatol. Photoimmunol. Photomed. 2008, 24, 115–122. [Google Scholar] [CrossRef]
- Soter, N.A. Acute effects of ultraviolet radiation on the skin. Semin. Dermatol. 1990, 9, 11–15. [Google Scholar] [PubMed]
- Shieh, S.Y.; Ikeda, M.; Taya, Y.; Prives, C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997, 91, 325–334. [Google Scholar] [CrossRef]
- Rezvani, H.R.; Mazurier, F.; Cario-André, M.; Pain, C.; Ged, C.; Taïeb, A.; de Verneuil, H. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J. Biol. Chem. 2006, 281, 17999–18007. [Google Scholar] [CrossRef]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Yu, S.M.; Kang, C.H.; Kim, G.Y. Protective effect of anthocyanin-enriched polyphenols from Hibiscus syriacus L. (Malvaceae) against ultraviolet B-induced damage. Antioxidants 2021, 10, 584. [Google Scholar] [CrossRef]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W.; et al. Fucodiphlorethol G Purified from Ecklonia cava suppresses ultraviolet B radiation-induced oxidative stress and cellular damage. Biomol. Ther. 2014, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective effects of a hyperoside-enriched fraction prepared from Houttuynia cordata Thunb. on ultraviolet B-induced skin aging in human fibroblasts through the MAPK signaling pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef]
- Tebbe, B.; Wu, S.; Geilen, C.C.; Eberle, J.; Kodelja, V.; Orfanos, C.E. L-ascorbic acid inhibits UVA-induced lipid peroxidation and secretion of IL-1alpha and IL-6 in cultured human keratinocytes in vitro. J. Investig. Dermatol. 1997, 108, 302–306. [Google Scholar] [CrossRef]
- Marionnet, C.; Pierrard, C.; Lejeune, F.; Sok, J.; Thomas, M.; Bernerd, F. Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS ONE 2010, 5, e12059. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Rhee, J.S.; Lee, K.W.; Kim, M.J.; Shin, K.H.; Lee, S.J.; Lee, Y.M.; Lee, J.S. UV-B radiation-induced oxidative stress and p38 signaling pathway involvement in the benthic copepod Tigriopus japonicus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 167, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.S.; Shapiro, P.S.; Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998, 74, 49–139. [Google Scholar]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef]
- Han, W.; Ming, M.; He, Y.Y. Caffeine promotes ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J. Biol. Chem. 2011, 286, 22825–22832. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 1999, 11, 732–736. [Google Scholar] [CrossRef] [PubMed]
- MacKeigan, J.P.; Collins, T.S.; Ting, J.P. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J. Biol. Chem. 2000, 275, 38953–38956. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef]
- Takasawa, R.; Nakamura, H.; Mori, T.; Tanuma, S. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis 2005, 10, 1121–1130. [Google Scholar] [CrossRef]
- Sitailo, L.A.; Tibudan, S.S.; Denning, M.F. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem. 2002, 277, 19346–19352. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Devin, A.; Lin, Y.; Liu, Z.G. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 2003, 4, 623–627. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Hall, C.K.; Ngo, L.; Jay, G. Extracellular deposition of beta-amyloid upon p53-dependent neuronal cell death in transgenic mice. J. Clin. Investig. 1996, 98, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.A.; Youle, R.J. The role of free radicals and p53 in neuron apoptosis in vivo. J. Neurosci. 1995, 15, 5851–5857. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.X.; Zhao, Y.; Brautigan, D.L.; Zhang, Z.Y. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J. Biol. Chem. 2002, 277, 31818–31825. [Google Scholar] [CrossRef]
- Tantini, B.; Pignatti, C.; Fattori, M.; Fiumana, E.; Facchini, A.; Stefanelli, C.; Caldarera, C.M.; Pegg, A.E.; Flamigni, F. Polyamine depletion inhibits etoposide-induced NF-kappaB activation in transformed mouse fibroblasts. Amino Acids 2004, 27, 207–214. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Mathov, I.; Aguirre, J.I.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 2005, 289, C633–C643. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Wang, W.; Zhang, Y.; Du, F.; Zhang, S.; Li, Z.; Deng, J.; Li, J. Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells. Int. J. Mol. Sci. 2023, 24, 6120. https://doi.org/10.3390/ijms24076120
Long Y, Wang W, Zhang Y, Du F, Zhang S, Li Z, Deng J, Li J. Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells. International Journal of Molecular Sciences. 2023; 24(7):6120. https://doi.org/10.3390/ijms24076120
Chicago/Turabian StyleLong, Yunluan, Wuji Wang, Yanyan Zhang, Fanpan Du, Shiqian Zhang, Zheng Li, Jiang Deng, and Jingjie Li. 2023. "Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells" International Journal of Molecular Sciences 24, no. 7: 6120. https://doi.org/10.3390/ijms24076120
APA StyleLong, Y., Wang, W., Zhang, Y., Du, F., Zhang, S., Li, Z., Deng, J., & Li, J. (2023). Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells. International Journal of Molecular Sciences, 24(7), 6120. https://doi.org/10.3390/ijms24076120





