The Liver and Glycogen: In Sickness and in Health
Abstract
1. Introduction
2. Glycogen in the Normal Liver
3. Pathological Conditions with Excessive Hepatic Glycogen Accumulation
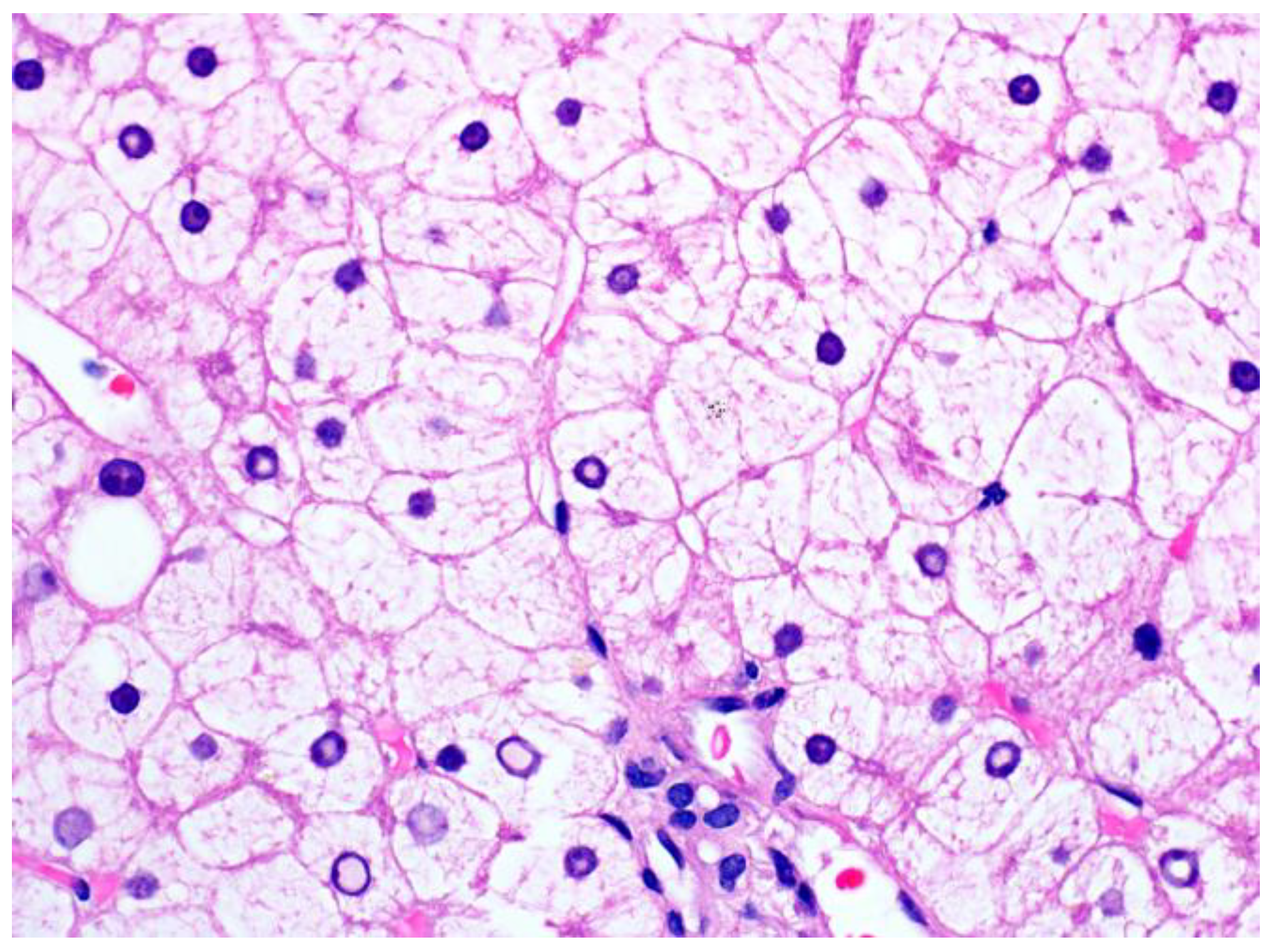
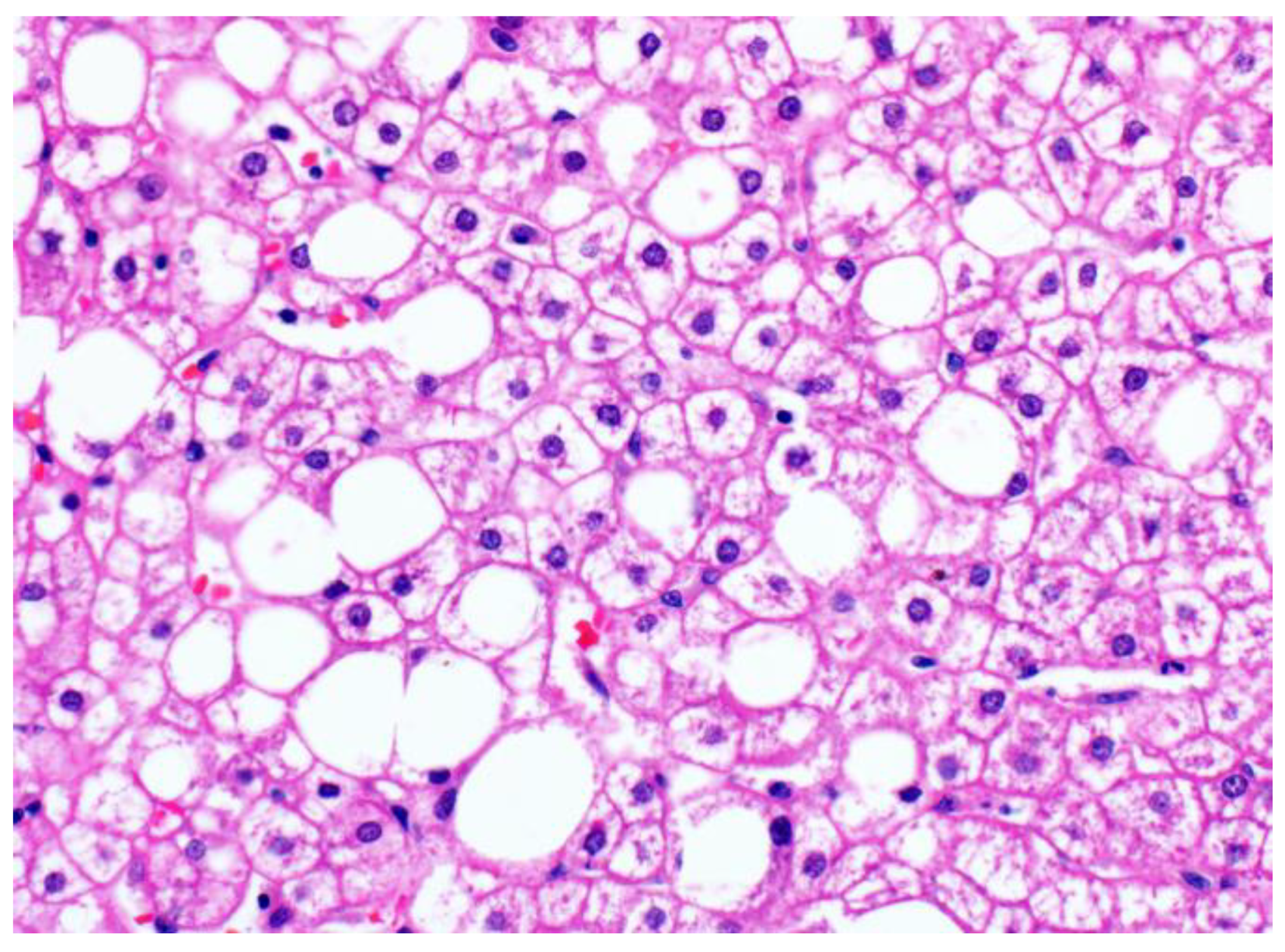
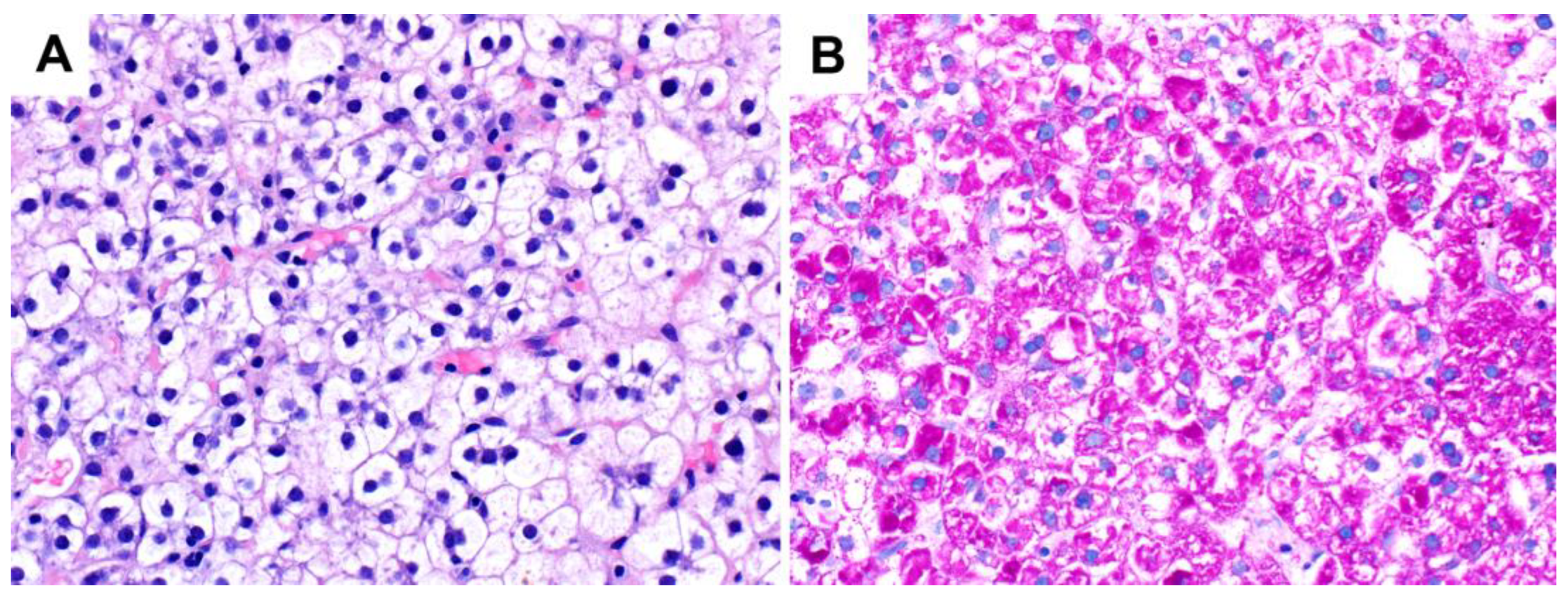
3.1. Glycogenic Hepatopathy
3.1.1. Clinical Findings and Pathogenesis
3.1.2. Pathological Findings
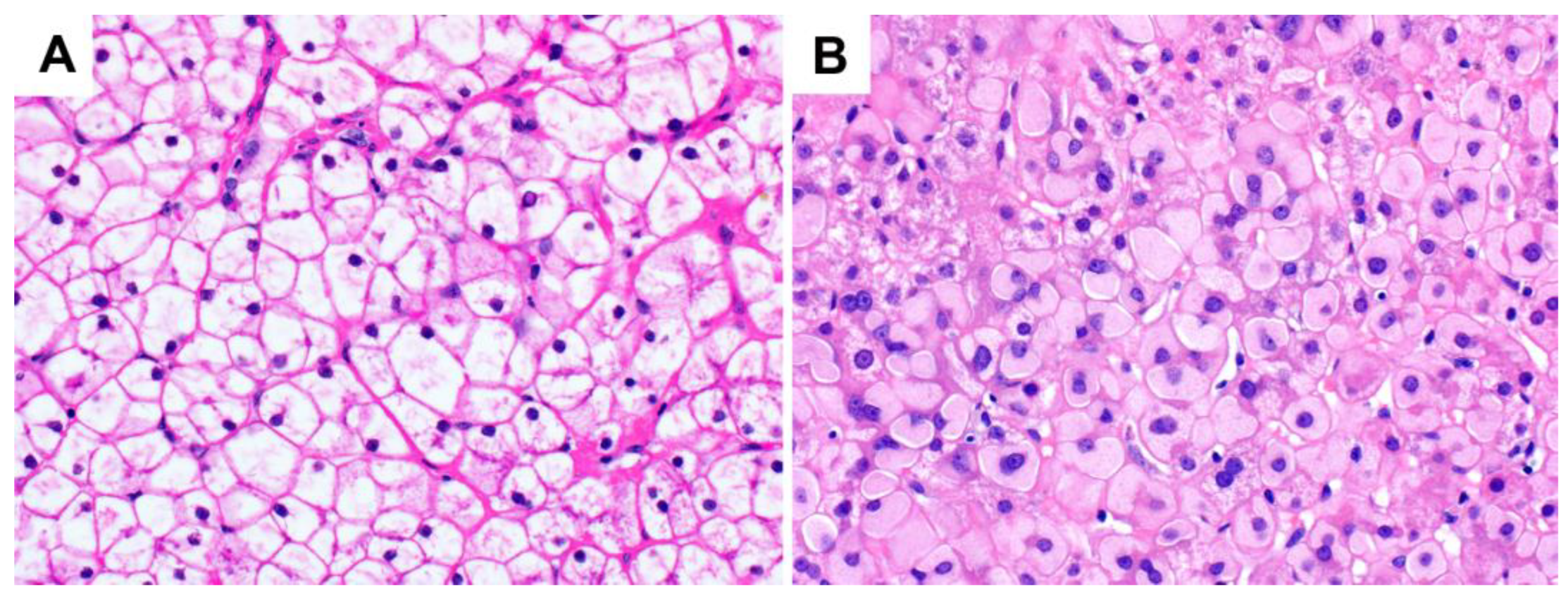
3.2. Glycogenosis in NAFLD
3.3. Glycogen Storage Disease
4. Abnormal Hepatic Glycogen Accumulation in Miscellaneous Conditions
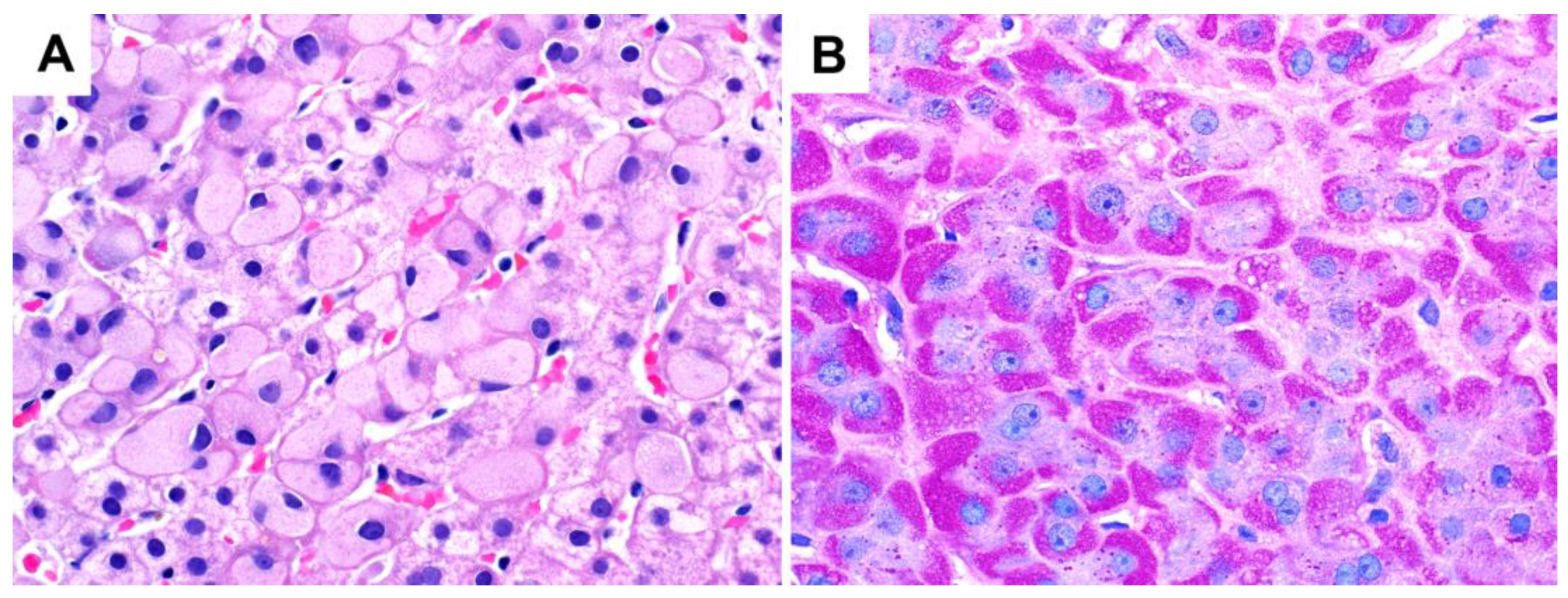
4.1. Urea Cycle Defects
4.2. Drug-Induced Pseudoground Glass Inclusions
5. Hepatic Masses with Abnormal Glycogen Accumulation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of Hepatic Glucose Metabolism in Health and Disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Metabolic Reprogramming: The Emerging Concept and Associated Therapeutic Strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in The Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Khan, T.; Sullivan, M.A.; Gunter, J.H.; Kryza, T.; Lyons, N.; He, Y.; Hooper, J.D. Revisiting Glycogen in Cancer: A Conspicuous and Targetable Enabler of Malignant Transformation. Front. Oncol. 2020, 10, 592455. [Google Scholar] [CrossRef]
- Roach, P.J. Glycogen and Its Metabolism. Curr. Mol. Med. 2002, 2, 101–120. [Google Scholar] [CrossRef]
- Marr, L.; Biswas, D.; Daly, L.A.; Browning, C.; Vial, S.C.M.; Maskell, D.P.; Hudson, C.; Bertrand, J.A.; Pollard, J.; Ranson, N.A.; et al. Mechanism of Glycogen Synthase Inactivation and Interaction with Glycogenin. Nat. Commun. 2022, 13, 3372. [Google Scholar] [CrossRef]
- Tan, X.; Sullivan, M.A.; Nada, S.S.; Deng, B.; Schulz, B.L.; Gilbert, R.G. Proteomic Investigation of the Binding Agent between Liver Glycogen β Particles. ACS Omega 2018, 3, 3640–3645. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, P.; Li, S.; Tan, X.; Hu, Z.; Deng, B.; Wang, K.; Li, C.; Sullivan, M.A.; Li, E.; et al. Molecular-Size Dependence of Glycogen Enzymatic Degradation and Its Importance for Diabetes. Eur. Polym. J. 2016, 82, 175–180. [Google Scholar] [CrossRef]
- Jungermann, K. Metabolic Zonation of Liver Parenchyma: Significance for the Regulation of Glycogen Metabolism, Gluconeogenesis, and Glycolysis. Diabetes Metab. Rev. 1987, 3, 269–293. [Google Scholar] [CrossRef]
- Layton, C.; Bancroft, J.D. Carbohydrates. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 176–197. [Google Scholar]
- Schwertheim, S.; Kälsch, J.; Jastrow, H.; Schaefer, C.M.; Theurer, S.; Ting, S.; Canbay, A.; Wedemeyer, H.; Schmid, K.W.; Baba, H.A. Characterization of Two Types of Intranuclear Hepatocellular Inclusions in NAFLD. Sci. Rep. 2020, 10, 16533. [Google Scholar] [CrossRef]
- Abraham, S.; Furth, E.E. Receiver Operating Characteristic Analysis of Glycogenated Nuclei in Liver Biopsy Specimens: Quantitative Evaluation of Their Relationship with Diabetes and Obesity. Hum. Pathol. 1994, 25, 1063–1068. [Google Scholar] [CrossRef]
- Mauriac, P. Gros Ventre, Hepatomegalie, Troubles de Las Croissance Chez Les Enfants Diabetiques Traits Depuis Plusieurs Annes Par l’insuline. Gax. Hebd. Med. Bordx. 1930, 26, 402–410. [Google Scholar]
- Carcione, L.; Lombardo, F.; Messina, M.F.; Rosano, M.; De Luca, F. Liver Glycogenosis as Early Manifestation in Type 1 Diabetes Mellitus. Diabetes Nutr. Metab. 2003, 16, 182–184. [Google Scholar]
- Chatila, R.; West, A.B. Hepatomegaly and Abnormal Liver Tests Due to Glycogenosis in Adults with Diabetes. Medicine 1996, 75, 327–333. [Google Scholar] [CrossRef]
- Evans, R.W.; Littler, T.R.; Pemberton, H.S. Glycogen Storage in the Liver in Diabetes Mellitus. J. Clin. Pathol. 1955, 8, 110–113. [Google Scholar] [CrossRef]
- Torres, M.; López, D. Liver Glycogen Storage Associated with Uncontrolled Type 1 Diabetes Mellitus. J. Hepatol. 2001, 35, 538. [Google Scholar] [CrossRef]
- Nakamuta, M.; Ohashi, M.; Goto, K.; Tanabe, Y.; Hiroshige, K.; Nawata, H. Diabetes Mellitus-Associated Glycogen Storage Hepatomegaly: Report of a Case and Review of the Japanese Literature. Fukuoka Igaku Zasshi 1993, 84, 354–358. [Google Scholar]
- Torbenson, M.; Chen, Y.-Y.; Brunt, E.; Cummings, O.W.; Gottfried, M.; Jakate, S.; Liu, Y.-C.; Yeh, M.M.; Ferrell, L. Glycogenic Hepatopathy: An Underrecognized Hepatic Complication of Diabetes Mellitus. Am. J. Surg. Pathol. 2006, 30, 508–513. [Google Scholar] [CrossRef]
- Haffar, S.; Izzy, M.; Habib, H.; Sugihara, T.; Li, D.K.; Sharma, A.; Wang, Z.; Murad, M.H.; Watt, K.D.; Bazerbachi, F. Liver Chemistries in Glycogenic Hepatopathy Associated with Type 1 Diabetes Mellitus: A Systematic Review and Pooled Analysis. Liver Int. 2021, 41, 1545–1555. [Google Scholar] [CrossRef]
- Bronstein, H.D.; Kantrowitz, P.A.; Schaffner, F. Marked Enlargement of the Liver and Transient Ascites Associated with the Treatment of Diabetic Acidosis. N. Engl. J. Med. 1959, 261, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.; Sharma, A.; Lackore, K.A.; Enders, F.T.; Torbenson, M.S.; Kamath, P.S.; Roberts, L.R.; Kudva, Y.C. Clinical, Biochemical, and Histopathology Features of Patients With Glycogenic Hepatopathy. Clin. Gastroenterol. Hepatol. 2017, 15, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E.; Cotoi, C.; Quaglia, A.; Sakellariou, S.; Ford-Adams, M.E.; Hadzic, N. Hepatopathy of Mauriac Syndrome: A Retrospective Review from a Tertiary Liver Centre. Arch. Dis. Child. 2014, 99, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; McCrossin, R.B.; Thomsett, M.J.; Batch, J. Hepatic Glycogenosis: Reversible Hepatomegaly in Type 1 Diabetes. J. Paediatr. Child Health 2000, 36, 449–452. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Takano, M.; Nishiofuku, M.; Yoshiji, H.; Matsumura, Y.; Kuriyama, S.; Uemura, M.; Okamoto, S.; Fukui, H. Rapid Onset of Glycogen Storage Hepatomegaly in a Type-2 Diabetic Patient after a Massive Dose of Long-Acting Insulin and Large Doses of Glucose. Intern. Med. 2006, 45, 469–473. [Google Scholar] [CrossRef]
- Umpaichitra, V. Unusual Glycogenic Hepatopathy Causing Abnormal Liver Enzymes in a Morbidly Obese Adolescent with Well-Controlled Type 2 Diabetes: Resolved after A1c Was Normalized by Metformin. Clin. Obes. 2016, 6, 281–284. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced Alterations in Hepatic Glucose and Lipid Metabolism: The Role of Type 1 and Type 2 Diabetes Mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Allende, D.S.; Gawrieh, S.; Cummings, O.W.; Belt, P.; Wilson, L.; Van Natta, M.; Behling, C.A.; Carpenter, D.; Gill, R.M.; Kleiner, D.E.; et al. Glycogenosis Is Common in Nonalcoholic Fatty Liver Disease and Is Independently Associated with Ballooning, but Lower Steatosis and Lower Fibrosis. Liver Int. 2021, 41, 996–1011. [Google Scholar] [CrossRef]
- Iancu, T.C.; Shiloh, H.; Dembo, L. Hepatomegaly Following Short-Term High-Dose Steroid Therapy. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 41–46. [Google Scholar] [CrossRef]
- Resnick, J.M.; Zador, I.; Fish, D.L. Dumping Syndrome, a Cause of Acquired Glycogenic Hepatopathy. Pediatr. Dev. Pathol. 2011, 14, 318–321. [Google Scholar] [CrossRef]
- Kransdorf, L.N.; Millstine, D.; Smith, M.L.; Aqel, B.A. Hepatic Glycogen Deposition in a Patient with Anorexia Nervosa and Persistently Abnormal Transaminase Levels. Clin. Res. Hepatol. Gastroenterol. 2016, 40, e15–e18. [Google Scholar] [CrossRef]
- Komuta, M.; Harada, M.; Ueno, T.; Uchimura, Y.; Inada, C.; Mitsuyama, K.; Sakisaka, S.; Sata, M.; Tanikawa, K. Unusual Accumulation of Glycogen in Liver Parenchymal Cells in a Patient with Anorexia Nervosa. Intern. Med. 1998, 37, 678–682. [Google Scholar] [CrossRef]
- Manderson, W.G.; McKiddie, M.T.; Manners, D.J.; Stark, J.R. Liver Glycogen Accumulation in Unstable Diabetes. Diabetes 1968, 17, 13–16. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Hasan, N.M.; Ansari, I.-U.H.; Longacre, M.J.; Kendrick, M.A.; Stoker, S.W. Discovery of a Genetic Metabolic Cause for Mauriac Syndrome in Type 1 Diabetes. Diabetes 2016, 65, 2051–2059. [Google Scholar] [CrossRef]
- Tomihira, M.; Kawasaki, E.; Nakajima, H.; Imamura, Y.; Sato, Y.; Sata, M.; Kage, M.; Sugie, H.; Nunoi, K. Intermittent and Recurrent Hepatomegaly Due to Glycogen Storage in a Patient with Type 1 Diabetes: Genetic Analysis of the Liver Glycogen Phosphorylase Gene (PYGL). Diabetes Res. Clin. Pract. 2004, 65, 175–182. [Google Scholar] [CrossRef]
- Sherigar, J.M.; Darouichi, Y.; Guss, D.; Mohanty, S.R. An Unusual Presentation of Glycogenic Hepatopathy with Bridging Fibrosis. ACG Case Rep. J 2018, 5, e31. [Google Scholar] [CrossRef]
- Harrison, S.A.; Brunt, E.M.; Goodman, Z.D.; Di Bisceglie, A.M. Diabetic Hepatosclerosis: Diabetic Microangiopathy of the Liver. Arch. Pathol. Lab. Med. 2006, 130, 27–32. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Garcia-Tsao, G.; Deng, Y.; Ciarleglio, M.; Jain, D. Hepatic Arteriolosclerosis: A Small-Vessel Complication of Diabetes and Hypertension. Am. J. Surg. Pathol. 2015, 39, 1000–1009. [Google Scholar] [CrossRef]
- Lackner, C.; Gogg-Kamerer, M.; Zatloukal, K.; Stumptner, C.; Brunt, E.M.; Denk, H. Ballooned Hepatocytes in Steatohepatitis: The Value of Keratin Immunohistochemistry for Diagnosis. J. Hepatol. 2008, 48, 821–828. [Google Scholar] [CrossRef]
- Gramlich, T.; Kleiner, D.E.; McCullough, A.J.; Matteoni, C.A.; Boparai, N.; Younossi, Z.M. Pathologic Features Associated with Fibrosis in Nonalcoholic Fatty Liver Disease. Hum. Pathol. 2004, 35, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Petersen, M.C.; Zhang, D.; Vatner, D.F.; Perry, R.J.; Abulizi, A.; Haedersdal, S.; Zhang, X.-M.; Butrico, G.M.; Samuel, V.T.; et al. Metabolic Control Analysis of Hepatic Glycogen Synthesis In Vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 8166–8176. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Dalla Man, C.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhäusl, W.; et al. Alterations in Postprandial Hepatic Glycogen Metabolism in Type 2 Diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Meroni, M.; Mancina, R.M.; Baselli, G.; Rametta, R.; Pelusi, S.; Männistö, V.; Fracanzani, A.L.; Badiali, S.; Miele, L.; et al. Protein Phosphatase 1 Regulatory Subunit 3B Gene Variation Protects against Hepatic Fat Accumulation and Fibrosis in Individuals at High Risk of Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2018, 2, 666–675. [Google Scholar] [CrossRef]
- Stender, S.; Smagris, E.; Lauridsen, B.K.; Kofoed, K.F.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Pennacchio, L.A.; Dickel, D.E.; Cohen, J.C.; Hobbs, H.H. Relationship between Genetic Variation at PPP1R3B and Levels of Liver Glycogen and Triglyceride. Hepatology 2018, 67, 2182–2195. [Google Scholar] [CrossRef]
- Ozen, H. Glycogen Storage Diseases: New Perspectives. World J. Gastroenterol. 2007, 13, 2541–2553. [Google Scholar] [CrossRef]
- Wright, T.L.F.; Umaña, L.A.; Ramirez, C.M. Update on Glycogen Storage Disease: Primary Hepatic Involvement. Curr. Opin. Pediatr. 2022, 34, 496–502. [Google Scholar] [CrossRef]
- Massese, M.; Tagliaferri, F.; Dionisi-Vici, C.; Maiorana, A. Glycogen Storage Diseases with Liver Involvement: A Literature Review of GSD Type 0, IV, VI, IX and XI. Orphanet. J. Rare Dis. 2022, 17, 241. [Google Scholar] [CrossRef]
- Torbenson, M.S. Genetic Diseases of the Liver. In Biopsy Interpretation of the Liver, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 546–552. [Google Scholar]
- Derks, T.G.J.; van Rijn, M. Lipids in Hepatic Glycogen Storage Diseases: Pathophysiology, Monitoring of Dietary Management and Future Directions. J. Inherit. Metab. Dis. 2015, 38, 537–543. [Google Scholar] [CrossRef]
- Badizadegan, K.; Perez-Atayde, A.R. Focal Glycogenosis of the Liver in Disorders of Ureagenesis: Its Occurrence and Diagnostic Significance. Hepatology 1997, 26, 365–373. [Google Scholar] [CrossRef]
- Yaplito-Lee, J.; Chow, C.-W.; Boneh, A. Histopathological Findings in Livers of Patients with Urea Cycle Disorders. Mol. Genet. Metab. 2013, 108, 161–165. [Google Scholar] [CrossRef]
- Miles, L.; Heubi, J.E.; Bove, K.E. Hepatocyte Glycogen Accumulation in Patients Undergoing Dietary Management of Urea Cycle Defects Mimics Storage Disease. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 471–476. [Google Scholar] [CrossRef]
- Burrage, L.C.; Madan, S.; Li, X.; Ali, S.; Mohammad, M.; Stroup, B.M.; Jiang, M.-M.; Cela, R.; Bertin, T.; Jin, Z.; et al. Chronic Liver Disease and Impaired Hepatic Glycogen Metabolism in Argininosuccinate Lyase Deficiency. JCI Insight 2020, 5, e132342. [Google Scholar] [CrossRef]
- Wisell, J.; Boitnott, J.; Haas, M.; Anders, R.A.; Hart, J.; Lewis, J.T.; Abraham, S.C.; Torbenson, M. Glycogen Pseudoground Glass Change in Hepatocytes. Am. J. Surg. Pathol. 2006, 30, 1085–1090. [Google Scholar] [CrossRef]
- Lefkowitch, J.H.; Lobritto, S.J.; Brown, R.S.; Emond, J.C.; Schilsky, M.L.; Rosenthal, L.A.; George, D.M.; Cairo, M.S. Ground-Glass, Polyglucosan-like Hepatocellular Inclusions: A “New” Diagnostic Entity. Gastroenterology 2006, 131, 713–718. [Google Scholar] [CrossRef]
- Lu, H.-C.; González, I.A.; Byrnes, K. Ground-Glass Hepatocellular Inclusions Are Associated with Polypharmacy. Ann. Diagn. Pathol. 2021, 52, 151740. [Google Scholar] [CrossRef]
- Callea, F.; Francalanci, P.; Grimaldi, C.; Camassei, F.D.; Devito, R.; Facchetti, F.; Alaggio, R.; Bellacchio, E. Pathomorphogenesis of Glycogen-Ground Glass Hepatocytic Inclusions (Polyglucosan Bodies) in Children after Liver Transplantation. Int. J. Mol. Sci. 2022, 23, 9996. [Google Scholar] [CrossRef]
- Torbenson, M.S. Drug-Induced Liver Injury. In Biopsy Interpretation of the Liver, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 245–246. [Google Scholar]
- Sala, M.; Gonzales, D.; Leste-Lasserre, T.; Dugot-Senant, N.; Paradis, V.; Di Tommaso, S.; Dupuy, J.-W.; Pitard, V.; Dourthe, C.; Sciarra, A.; et al. ASS1 Overexpression: A Hallmark of Sonic Hedgehog Hepatocellular Adenomas; Recommendations for Clinical Practice. Hepatol. Commun. 2020, 4, 809–824. [Google Scholar] [CrossRef]
- Callea, F.; Giovannoni, I.; Stefanelli, M.; Villanacci, V.; Lorini, G.; Francalanci, P. Glycogenotic Hepatocellular Carcinoma with Glycogen-Ground-Glass Hepatocytes: Histological, Histochemical and Microbiochemical Characterization of the Novel Variant. Histopathology 2012, 60, 1010–1012. [Google Scholar] [CrossRef]
- Haas, S.; Gütgemann, I.; Wolff, M.; Fischer, H.-P. Intrahepatic Clear Cell Cholangiocarcinoma: Immunohistochemical Aspects in a Very Rare Type of Cholangiocarcinoma. Am. J. Surg. Pathol. 2007, 31, 902–906. [Google Scholar] [CrossRef]
- Wu, W.W.; Gu, M.; Lu, D. Cytopathologic, Histopathologic, and Immunohistochemical Features of Intrahepatic Clear Cell Bile Duct Adenoma: A Case Report and Review of the Literature. Case Rep. Pathol. 2014, 2014, 874826. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Torbenson, M.S.; Park, Y.N.; Sakamoto, M.; Roncalli, M.; Ng, I. Hepatocellular Carcinoma. In WHO Classification of Tumours Editorial Board. Digestive System Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1. [Google Scholar]
- Lee, J.H.; Shin, D.H.; Park, W.Y.; Shin, N.; Kim, A.; Lee, H.J.; Kim, Y.K.; Choi, K.U.; Kim, J.Y.; Yang, Y.I.; et al. IDH1 R132C Mutation Is Detected in Clear Cell Hepatocellular Carcinoma by Pyrosequencing. World J. Surg. Oncol. 2017, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Hillis, D.M.; Patton, J.C.; Bradley, R.D.; Baker, R.J. Cytosolic Isocitrate Dehydrogenase in Humans, Mice, and Voles and Phylogenetic Analysis of the Enzyme Family. Mol. Biol. Evol. 1998, 15, 1674–1684. [Google Scholar] [CrossRef]
- Minard, K.I.; McAlister-Henn, L. Dependence of Peroxisomal Beta-Oxidation on Cytosolic Sources of NADPH. J. Biol. Chem. 1999, 274, 3402–3406. [Google Scholar] [CrossRef]
- Koh, H.-J.; Lee, S.-M.; Son, B.-G.; Lee, S.-H.; Ryoo, Z.Y.; Chang, K.-T.; Park, J.-W.; Park, D.-C.; Song, B.J.; Veech, R.L.; et al. Cytosolic NADP+-Dependent Isocitrate Dehydrogenase Plays a Key Role in Lipid Metabolism. J. Biol. Chem. 2004, 279, 39968–39974. [Google Scholar] [CrossRef]
- Ye, J.; Gu, Y.; Zhang, F.; Zhao, Y.; Yuan, Y.; Hao, Z.; Sheng, Y.; Li, W.Y.; Wakeham, A.; Cairns, R.A.; et al. IDH1 Deficiency Attenuates Gluconeogenesis in Mouse Liver by Impairing Amino Acid Utilization. Proc. Natl. Acad. Sci. USA 2017, 114, 292–297. [Google Scholar] [CrossRef]
- Ricoult, S.J.H.; Dibble, C.C.; Asara, J.M.; Manning, B.D. Sterol Regulatory Element Binding Protein Regulates the Expression and Metabolic Functions of Wild-Type and Oncogenic IDH1. Mol. Cell Biol. 2016, 36, 2384–2395. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Wu, P.C.; Lai, C.L.; Lam, K.C.; Lok, A.S.; Lin, H.J. Clear Cell Carcinoma of Liver. An Ultrastructural Study. Cancer 1983, 52, 504–507. [Google Scholar] [CrossRef]
- Yang, S.H.; Watanabe, J.; Nakashima, O.; Kojiro, M. Clinicopathologic Study on Clear Cell Hepatocellular Carcinoma. Pathol. Int. 1996, 46, 503–509. [Google Scholar] [CrossRef]
- Audisio, R.A.; Bombelli, L.; Lombardi, L.; Andreola, S. A Clinico-Pathologic Study of Clear-Cell Hepatocellular Carcinoma. Tumori 1987, 73, 389–395. [Google Scholar] [CrossRef]
- Kiyomatsu, K. Pathomorphologic study on hepatocellular carcinoma (HCC). A study of fatty change in HCC. Acta Hepatol. Jpn. 1989, 30, 974–979. [Google Scholar] [CrossRef][Green Version]
- Pelletier, J.; Bellot, G.; Gounon, P.; Lacas-Gervais, S.; Pouysségur, J.; Mazure, N.M. Glycogen Synthesis Is Induced in Hypoxia by the Hypoxia-Inducible Factor and Promotes Cancer Cell Survival. Front. Oncol. 2012, 2, 18. [Google Scholar] [CrossRef]
- Shen, C.; Kaelin, W.G. The VHL/HIF Axis in Clear Cell Renal Carcinoma. Semin. Cancer Biol. 2013, 23, 18–25. [Google Scholar] [CrossRef]
- Chen, S.-L.; Huang, Q.-S.; Huang, Y.-H.; Yang, X.; Yang, M.-M.; He, Y.-F.; Cao, Y.; Guan, X.-Y.; Yun, J.-P. GYS1 Induces Glycogen Accumulation and Promotes Tumor Progression via the NF-ΚB Pathway in Clear Cell Renal Carcinoma. Theranostics 2020, 10, 9186–9199. [Google Scholar] [CrossRef]
- Chen, S.-L.; Zhang, C.Z.; Liu, L.-L.; Lu, S.-X.; Pan, Y.-H.; Wang, C.-H.; He, Y.-F.; Lin, C.-S.; Yang, X.; Xie, D.; et al. A GYS2/P53 Negative Feedback Loop Restricts Tumor Growth in HBV-Related Hepatocellular Carcinoma. Cancer Res. 2019, 79, 534–545. [Google Scholar] [CrossRef]
- Tan, P.S.; Nakagawa, S.; Goossens, N.; Venkatesh, A.; Huang, T.; Ward, S.C.; Sun, X.; Song, W.-M.; Koh, A.; Canasto-Chibuque, C.; et al. Clinicopathological Indices to Predict Hepatocellular Carcinoma Molecular Classification. Liver Int. 2016, 36, 108–118. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological Subtypes of Hepatocellular Carcinoma Are Related to Gene Mutations and Molecular Tumour Classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhang, W.; Xiao, C.; Zhang, S.; Nian, C.; Li, J.; Su, D.; Chen, L.; Zhao, Q.; et al. Glycogen Accumulation and Phase Separation Drives Liver Tumor Initiation. Cell 2021, 184, 5559–5576. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Song, J.; Godfrey, J.; Riscal, R.; Skuli, N.; Nissim, I.; Simon, M.C. Glycogen Metabolism Is Dispensable for Tumour Progression in Clear Cell Renal Cell Carcinoma. Nat. Metab. 2021, 3, 327–336. [Google Scholar] [CrossRef] [PubMed]


| Types | Gene Mutation | Defective Protein | Clinical Manifestations | Main Liver Findings |
|---|---|---|---|---|
| 0 | GYS2 | Glycogen synthetase | Hypoglycemia | Steatosis |
| Ia | G6PC | Glucose-6-phosphatase | Short stature, hepatomegaly, hypoglycemia, and increased triglycerides, lactate, and uric acid | Steatosis Glycogenosis |
| Ib | SLC37A4 | Glucose-6-phosphate translocase | Similar to type Ia with additional neutropenia, infections, and poor wound healing | Steatosis Glycogenosis |
| III | AGL | Debranching enzyme and variants | Short stature, hepatomegaly, hypoglycemia, hyperlipidemia, and cardiomyopathy | Steatosis Glycogenosis |
| IV | GBE1 | 1,4-alpha-glucan branching enzyme 1 | Progressive form: short stature, hepatomegaly, splenomegaly, cirrhosis, and late-onset hypoglycemia Non-progressive form: hepatomegaly | Hepatocyte inclusions resembling ground glass changes |
| VI | PYGL | Liver phosphorylase | Short stature, hepatomegaly, and hypoglycemia | Steatosis Glycogenosis |
| IX | PHKA1, PHKA2, PHKB, PHKG2 | Phosphorylase kinase and variants | Short stature, hepatomegaly, hypoglycemia, hyperlipidemia, and myopathy | Glycogenosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soon, G.S.T.; Torbenson, M. The Liver and Glycogen: In Sickness and in Health. Int. J. Mol. Sci. 2023, 24, 6133. https://doi.org/10.3390/ijms24076133
Soon GST, Torbenson M. The Liver and Glycogen: In Sickness and in Health. International Journal of Molecular Sciences. 2023; 24(7):6133. https://doi.org/10.3390/ijms24076133
Chicago/Turabian StyleSoon, Gwyneth S. T., and Michael Torbenson. 2023. "The Liver and Glycogen: In Sickness and in Health" International Journal of Molecular Sciences 24, no. 7: 6133. https://doi.org/10.3390/ijms24076133
APA StyleSoon, G. S. T., & Torbenson, M. (2023). The Liver and Glycogen: In Sickness and in Health. International Journal of Molecular Sciences, 24(7), 6133. https://doi.org/10.3390/ijms24076133






