Oestrogen and Vibration Improve Intervertebral Disc Cell Viability and Decrease Catabolism in Bovine Organ Cultures
Abstract
:1. Introduction
2. Results
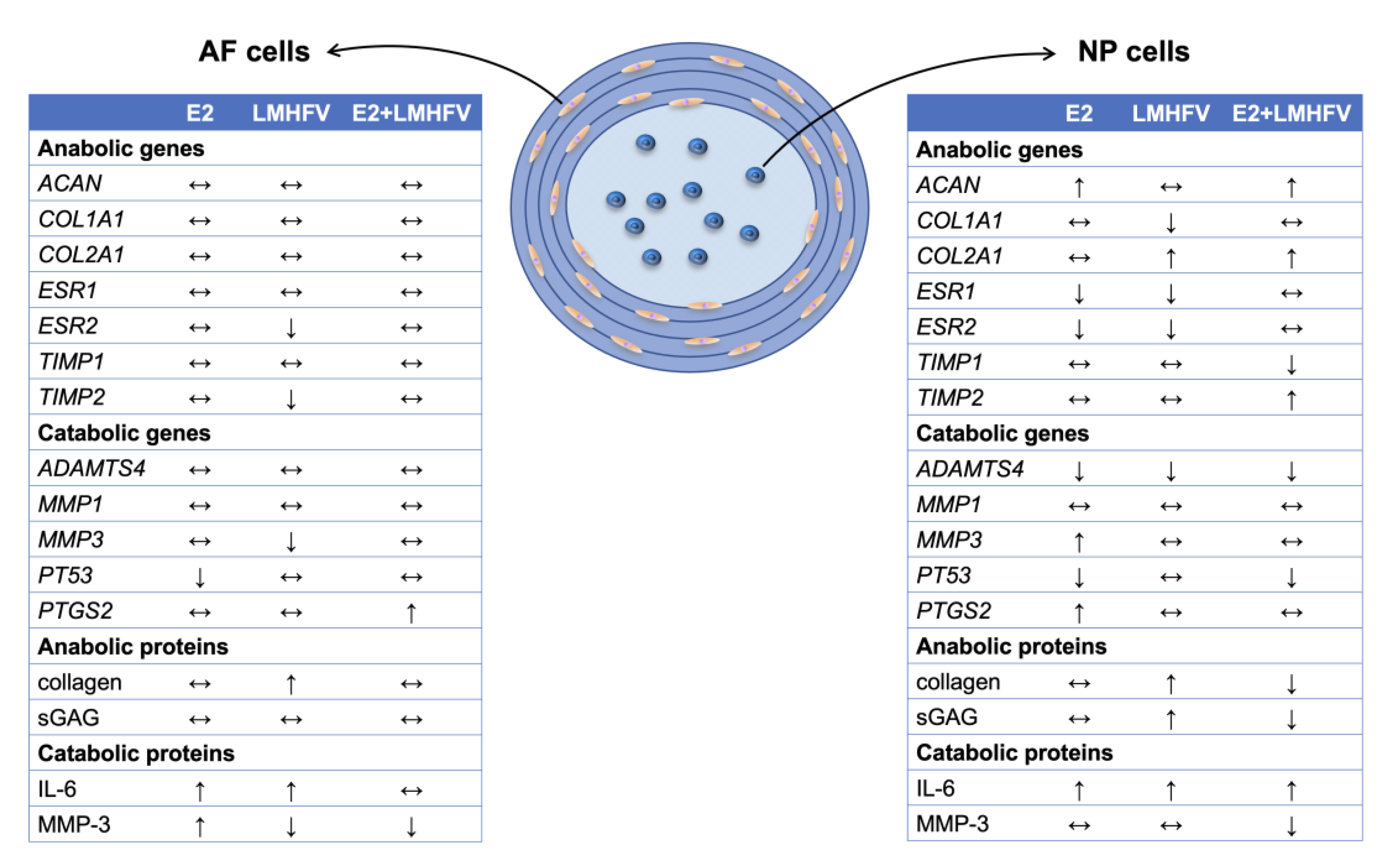
2.1. Effect of Oestrogen and Vibration on the Expression of Genes of the Extracellular Matrix and Its Metabolism
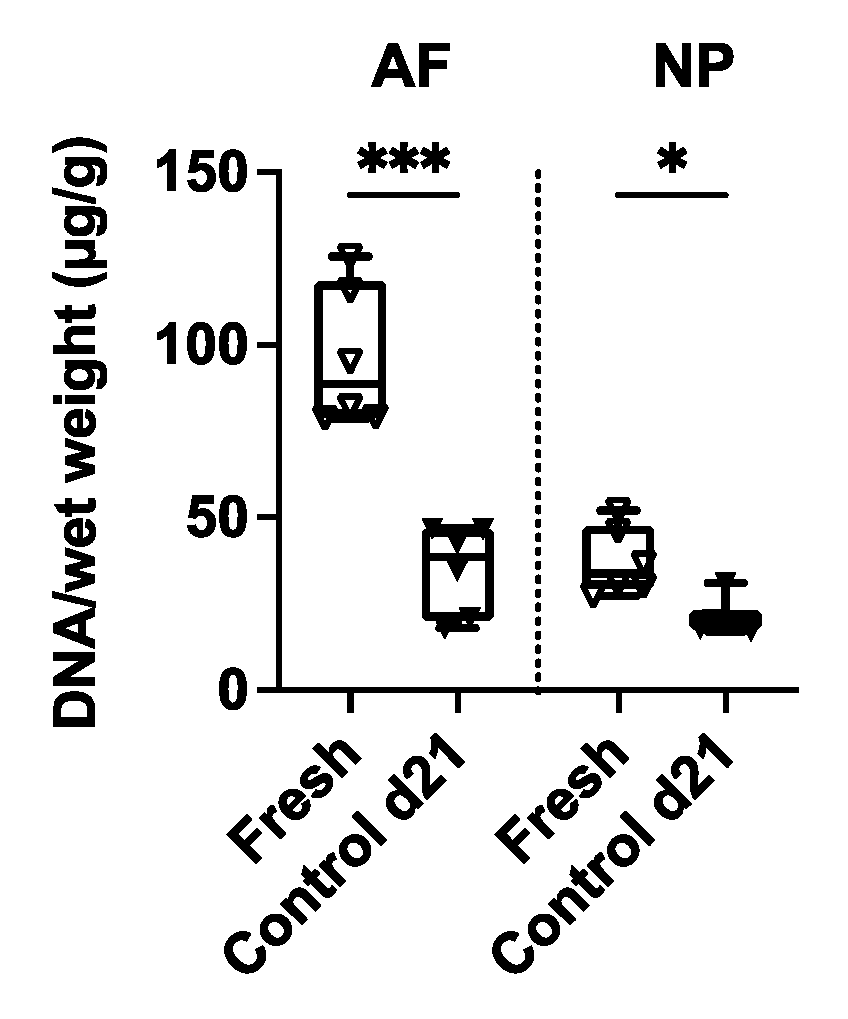
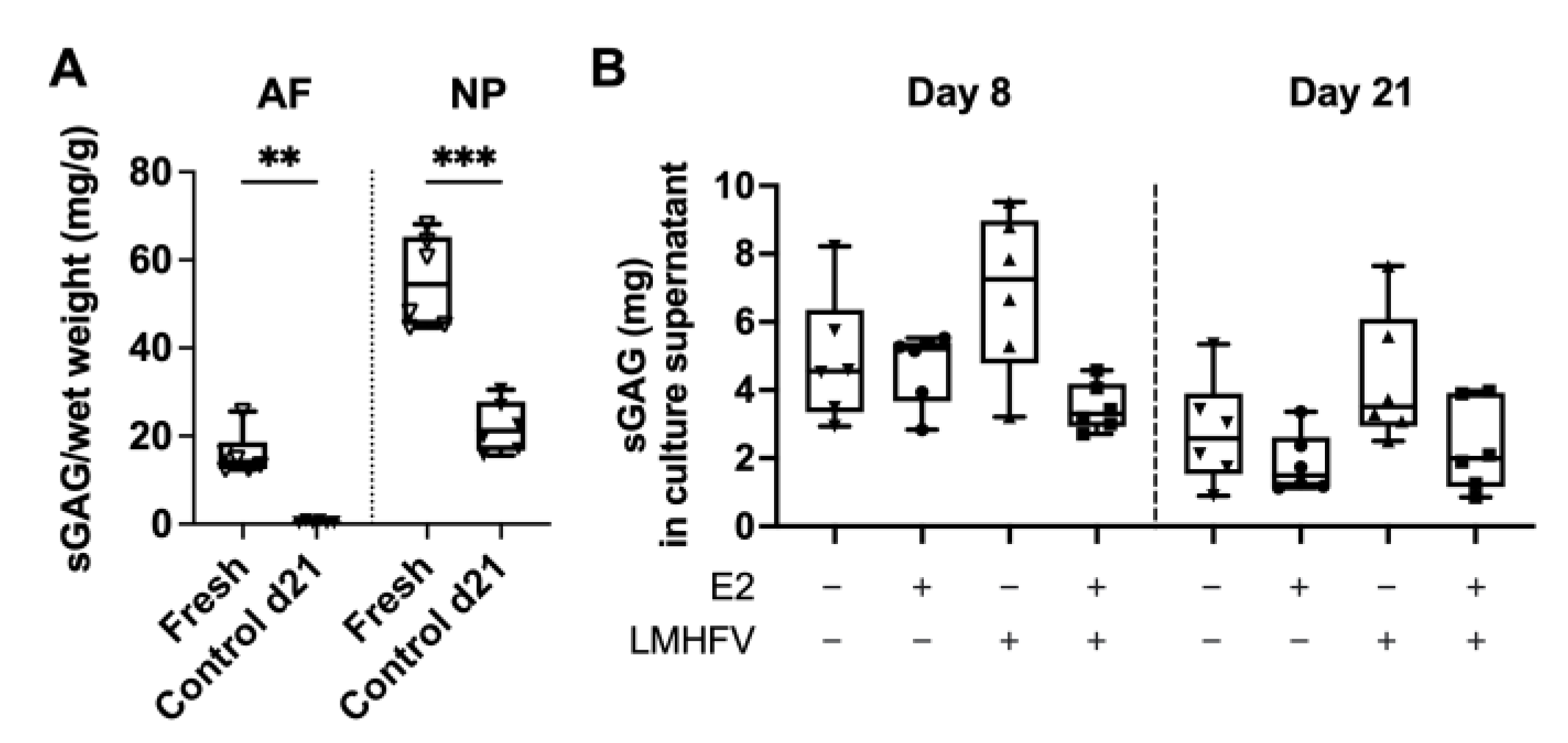
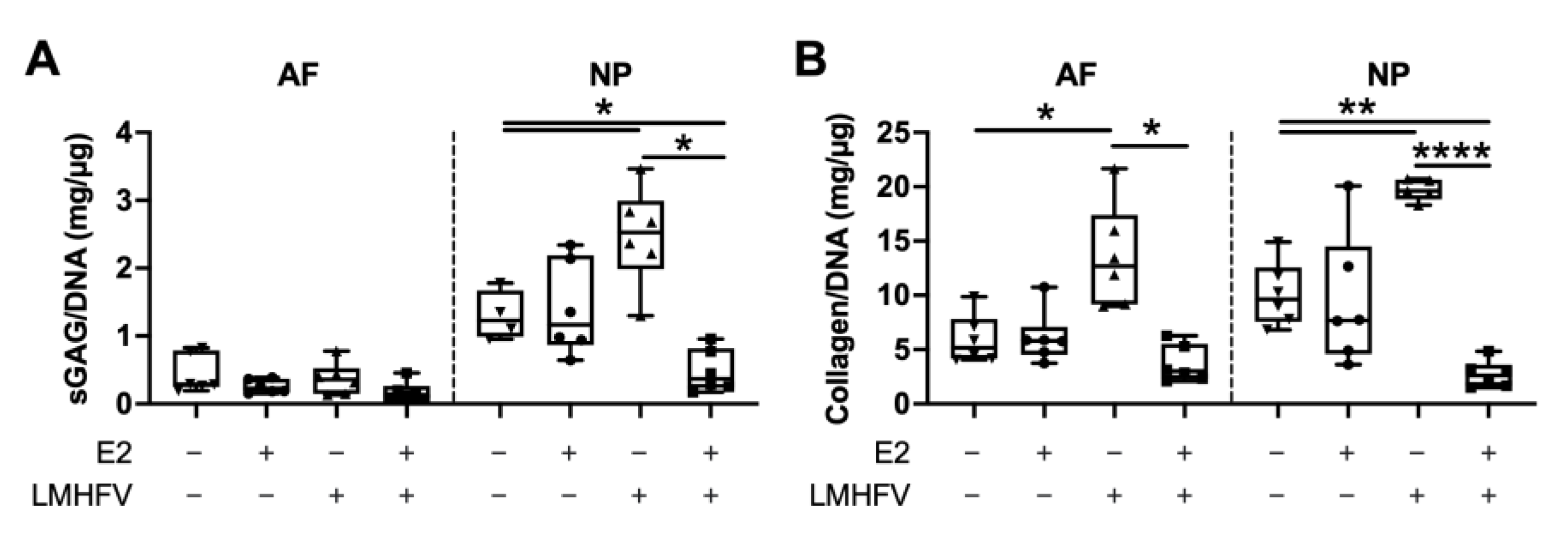
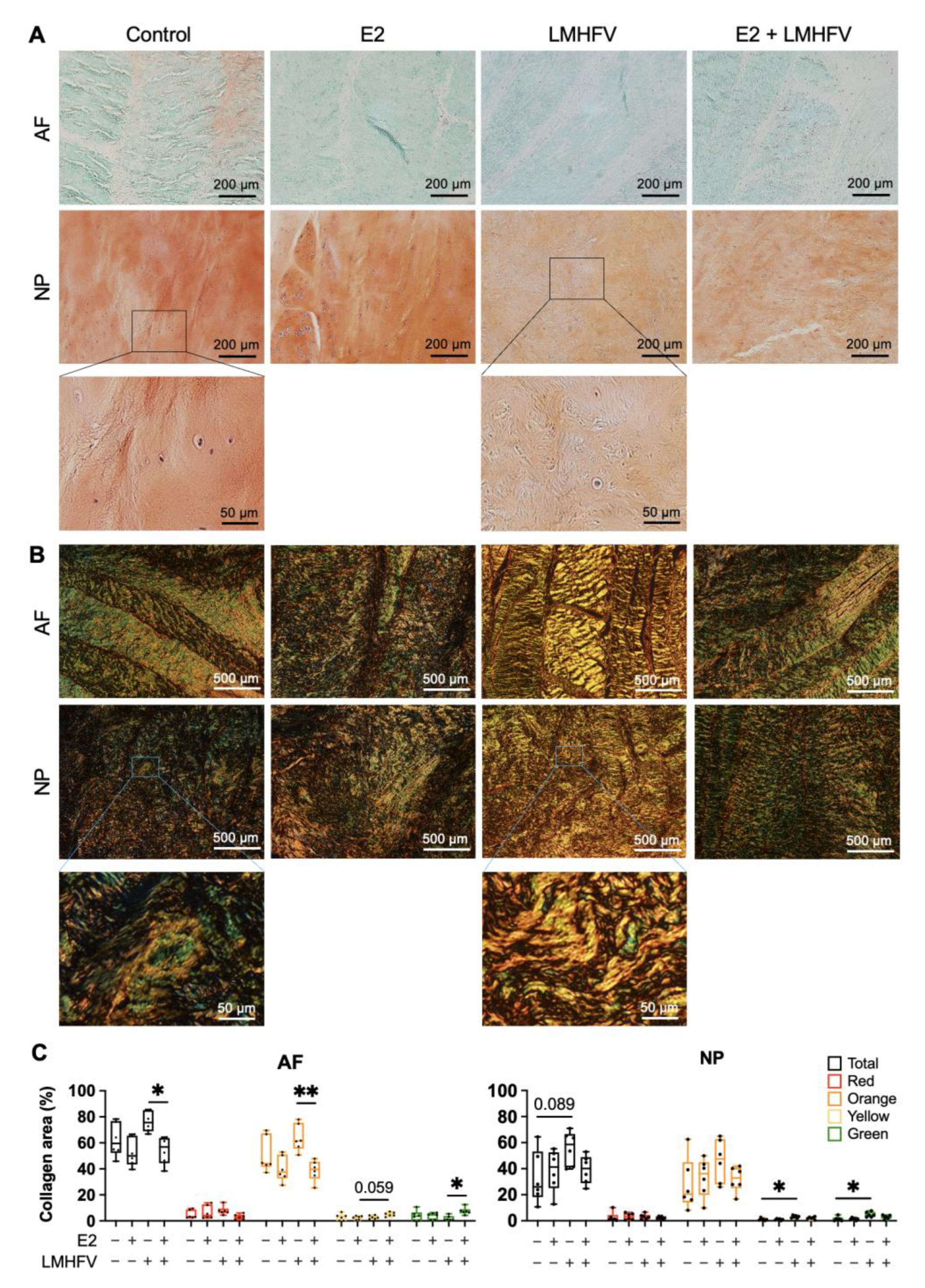
2.2. Effect of Oestrogen and Vibration on Cell Viability, DNA, sGAG and Collagen
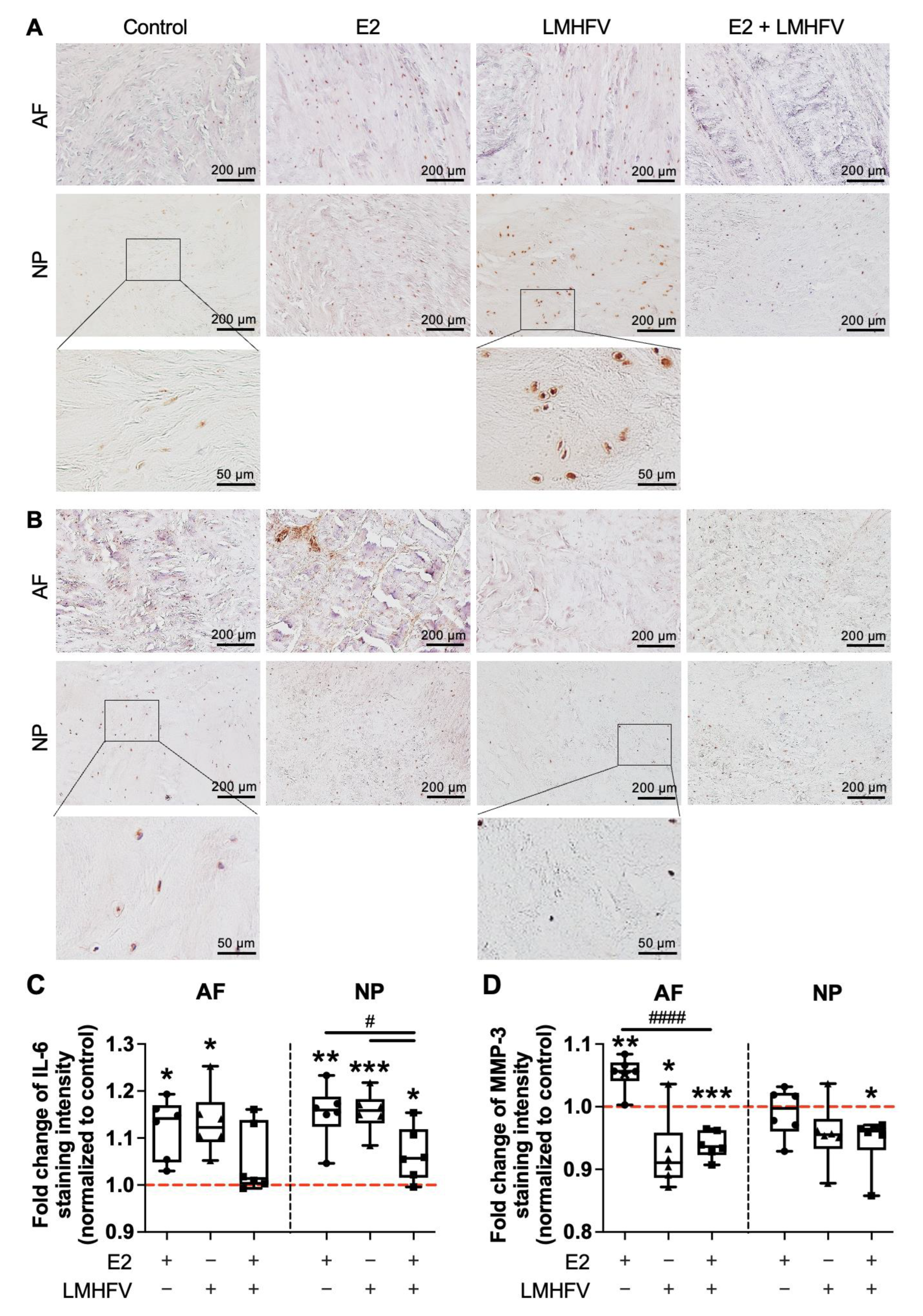
2.3. IL-6 and MMP-3 Production
3. Discussion
4. Materials and Methods
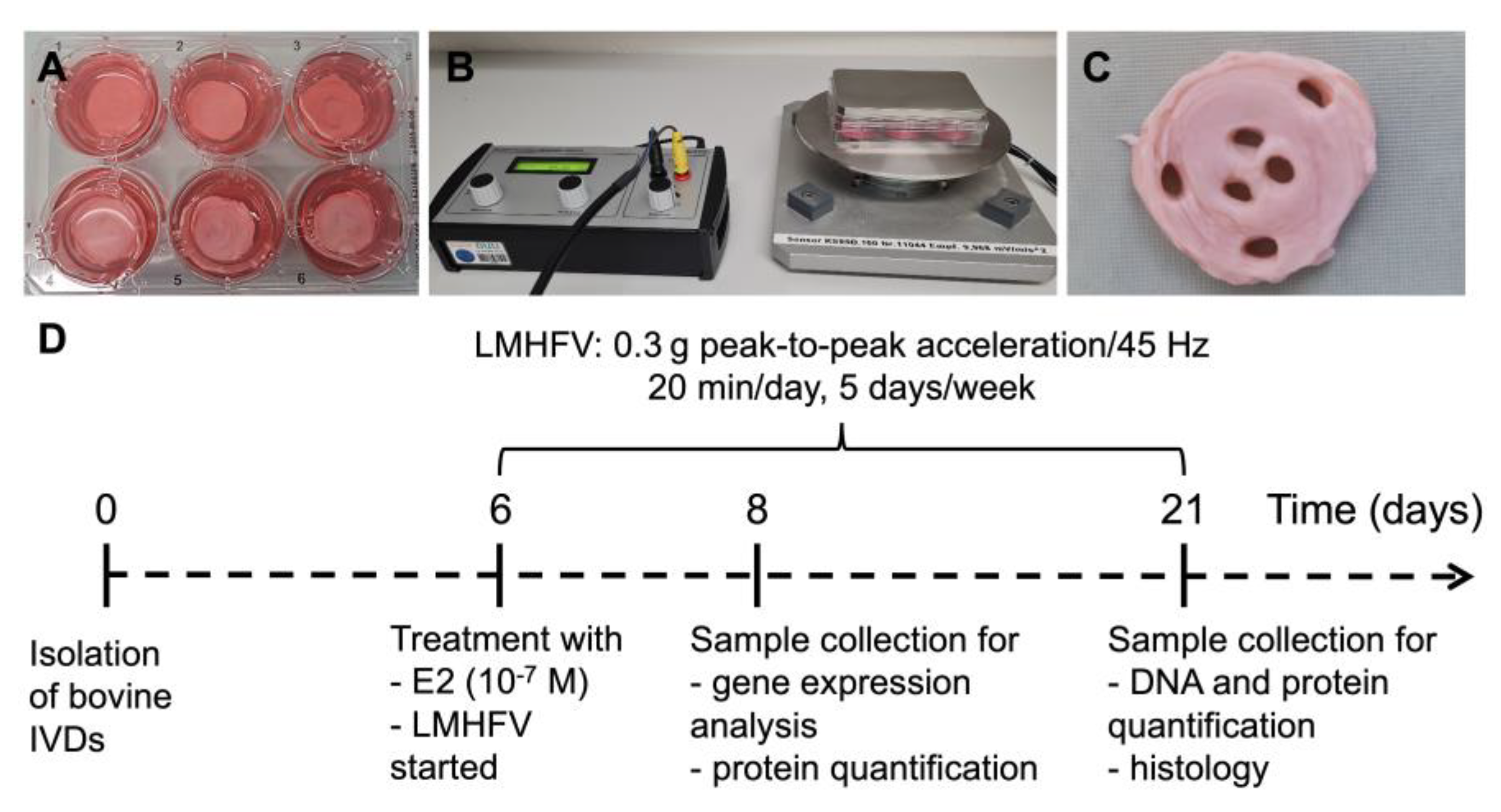
4.1. IVD Isolation and Organ Culture Model
4.2. Gene Expression Analysis
4.3. Cell Viability
4.4. PGE2 Quantification in Culture Supernatants
4.5. DNA, sGAG and Collagen Quantification in AF and NP Tissues
4.6. Histology
4.7. Immunohistochemistry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Basal Expression of Oestrogen Receptors ESR1 and ESR2
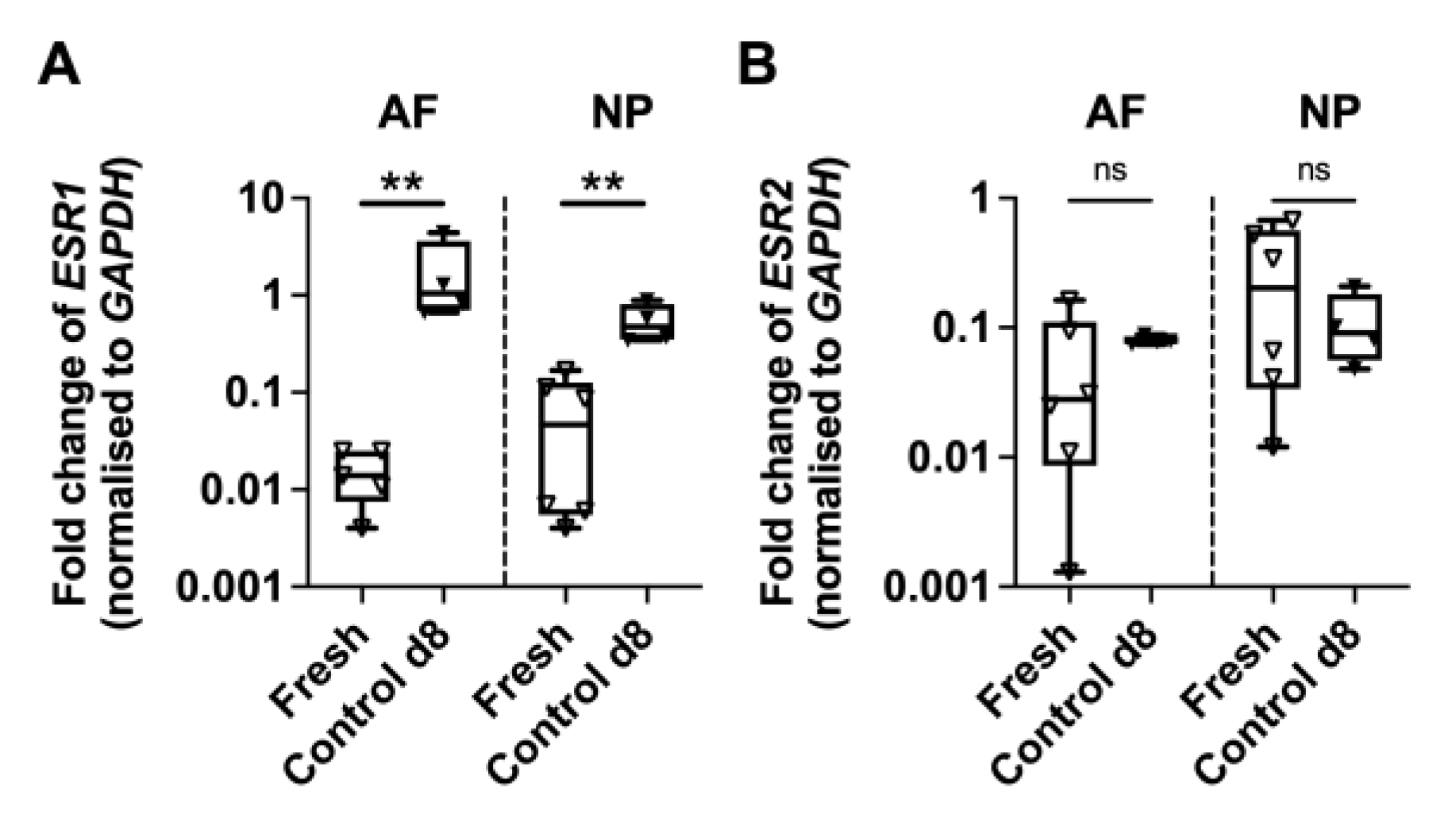
Appendix A.2. Cell Apoptosis Detection in AF and NP at Day 21 of Organ Culture

Appendix A.3. IL-6 and MMP-3 Detection in the Entire IVD

References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.J.; Barber, R.M.; Foreman, K.J.; Abbasoglu Ozgoren, A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef] [Green Version]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef]
- Lou, C.; Chen, H.L.; Feng, X.Z.; Xiang, G.H.; Zhu, S.P.; Tian, N.F.; Jin, Y.L.; Fang, M.Q.; Wang, C.; Xu, H.Z. Menopause is associated with lumbar disc degeneration: A review of 4230 intervertebral discs. Climacteric 2014, 17, 700–704. [Google Scholar] [CrossRef]
- Lou, C.; Chen, H.; Mei, L.; Yu, W.; Zhu, K.; Liu, F.; Chen, Z.; Xiang, G.; Chen, M.; Weng, Q.; et al. Association between menopause and lumbar disc degeneration: An MRI study of 1,566 women and 1,382 men. Menopause 2017, 24, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2022, e1231. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. J. Orthop. Transl. 2015, 3, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.J. Menopause as a potential cause for higher prevalence of low back pain in women than in age-matched men. J. Orthop. Transl. 2017, 8, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.J.; Káplár, Z.; Deng, M.; Leung, J.C.S. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J. Orthop. Transl. 2017, 11, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Wáng, Y.X. Continued progression of lumbar disc degeneration in postmenopausal women. Climacteric 2015, 18, 435. [Google Scholar] [CrossRef]
- Wei, A.; Shen, B.; Williams, L.A.; Bhargav, D.; Yan, F.; Chong, B.H.; Diwan, A.D. Expression and functional roles of estrogen receptor GPR30 in human intervertebral disc. J. Steroid Biochem. Mol. Biol. 2016, 158, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, H.E.; Yamaguchi, D.; Ingram, J.; Leslie, K.; Huang, W.; Miller, T.A.; Hanley, E.N., Jr. Expression and localization of estrogen receptor-beta in annulus cells of the human intervertebral disc and the mitogenic effect of 17-beta-estradiol in vitro. BMC Musculoskelet. Disord. 2002, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, B.; Yuan, Y.; Liu, X.; Zhang, Y.; Liu, H.; Shen, X.; Liu, B.; Chang, L. Protective effect of estrogen against intervertebral disc degeneration is attenuated by miR-221 through targeting estrogen receptor α. Acta Biochim. Biophys. Sin. 2018, 50, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.D.; Ma, L.; Gu, T.X.; Ding, W.Y.; Zhang, F.; Shen, Y.; Zhang, Y.Z.; Yang, D.L.; Zhang, D.; Sun, Y.P.; et al. 17β-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis 2014, 19, 789–800. [Google Scholar] [CrossRef]
- Yang, S.D.; Yang, D.L.; Sun, Y.P.; Wang, B.L.; Ma, L.; Feng, S.Q.; Ding, W.Y. 17β-estradiol protects against apoptosis induced by interleukin-1β in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis 2015, 20, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, S.D.; Huo, X.W.; Yang, D.L.; Ma, L.; Ding, W.Y. 17β-Estradiol inhibits intervertebral disc degeneration by down-regulating MMP-3 and MMP-13 and up-regulating type II collagen in a rat model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Koike, T.; Mikami, T.; Shida, M.; Habuchi, O.; Kitagawa, H. Chondroitin sulfate-E mediates estrogen-induced osteoanabolism. Sci. Rep. 2015, 5, 8994. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, H. Biochemical and immunological study of lumbar disc degeneration. Nihon Seikeigeka Gakkai Zasshi 1985, 59, 1119–1131. [Google Scholar]
- Yuan, H.-Y.; Tang, Y.; Liang, Y.-X.; Lei, L.; Xiao, G.-B.; Wang, S.; Xia, Z.-L. Matrix Metalloproteinase-3 and Vitamin D Receptor Genetic Polymorphisms, and Their Interactions with Occupational Exposure in Lumbar Disc Degeneration. J. Occup. Health 2010, 52, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawilla, N.H.; Darweesh, H.; Mansour, N.; Helal, S.; Taha, F.M.; Awadallah, M.; El Shazly, R. Matrix metalloproteinase-3, vitamin D receptor gene polymorphisms, and occupational risk factors in lumbar disc degeneration. J. Occup. Rehabil. 2014, 24, 370–381. [Google Scholar] [CrossRef]
- Weber-Rajek, M.; Mieszkowski, J.; Niespodziński, B.; Ciechanowska, K. Whole-body vibra-tion exercise in postmenopausal osteoporosis. Przegląd Menopauzalny 2015, 14, 41–47. [Google Scholar]
- Haffner-Luntzer, M.; Lackner, I.; Liedert, A.; Fischer, V.; Ignatius, A. Effects of low-magnitude high-frequency vibration on osteoblasts are dependent on estrogen receptor α signaling and cytoskeletal remodeling. Biochem. Biophys. Res. Commun. 2018, 503, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, E.; Liedert, A.; Heilmann, A.; Wehner, T.; Bindl, R.; Fischer, L.; Haffner-Luntzer, M.; Jakob, F.; Schinke, T.; Amling, M.; et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Models Mech. 2015, 8, 93–104. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.R.; Patel, P.; Pest, M.A.; Ratneswaran, A.; Lalli, G.; Beaucage, K.L.; Backler, G.B.; Kamphuis, M.P.; Esmail, Z.; Lee, J.; et al. Repeated exposure to high-frequency low-amplitude vibration induces degeneration of murine intervertebral discs and knee joints. Arthritis Rheumatol. 2015, 67, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; Veras, M.A.; Yeung, C.; Lalli, G.; Patel, P.; Leitch, K.M.; Holdsworth, D.W.; Dixon, S.J.; Séguin, C.A. Whole-body vibration of mice induces progressive degeneration of intervertebral discs associated with increased expression of Il-1β and multiple matrix degrading enzymes. Osteoarthr. Cartil. 2017, 25, 779–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Gan, Y.; Xu, Y.; Wang, L.; Ouyang, B.; Zhang, C.; Luo, L.; Zhao, C.; Zhou, Q. 17beta-estradiol Attenuates TNF-α-Induced Premature Senescence of Nucleus Pulposus Cells through Regulating the ROS/NF-κB Pathway. Int. J. Biol. Sci. 2017, 13, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehrle, E.; Wehner, T.; Heilmann, A.; Bindl, R.; Claes, L.; Jakob, F.; Amling, M.; Ignatius, A. Distinct frequency dependent effects of whole-body vibration on non-fractured bone and fracture healing in mice. J. Orthop. Res. 2014, 32, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Nerlich, A.; Mittermaier, N.; Weiler, C.; Lumenta, C.; Wuertz, K.; Boos, N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 2009, 18, 1573–1586. [Google Scholar] [CrossRef] [Green Version]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Xu, Y.; Gan, Y.; Wang, L.; Ouyang, B.; Zhang, C.; Luo, L.; Zhao, C.; Zhou, Q. Estrogen Enhances Matrix Synthesis in Nucleus Pulposus Cell through the Estrogen Receptor β-p38 MAPK Pathway. Cell. Physiol. Biochem. 2016, 39, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Hua, Y.; Kong, G.; Wu, X.; Huang, Z.; Huang, Z.; Liu, J.; Yang, Z.; Zhu, Q. Estrogen Deficiency Exacerbates Intervertebral Disc Degeneration Induced by Spinal Instability in Rats. Spine 2019, 44, E510–E519. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; Patel, P.; Beaucage, K.L.; Xiao, Y.; Bacher, C.; Siqueira, W.L.; Holdsworth, D.W.; Dixon, S.J.; Séguin, C.A. Acute vibration induces transient expression of anabolic genes in the murine intervertebral disc. Arthritis Rheum. 2013, 65, 1853–1864. [Google Scholar] [CrossRef] [Green Version]
- Berthois, Y.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Phenol red in tissue culture media is a weak estrogen: Implications concerning the study of estrogen-responsive cells in culture. Proc. Natl. Acad. Sci. USA 1986, 83, 2496–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, G.Q.; Boldt, A.; Nagl, I.; Pereira, C.L.; Benz, K.; Wilke, H.J.; Ignatius, A.; Barbosa, M.A.; Gonçalves, R.M.; Neidlinger-Wilke, C. A degenerative/proinflammatory intervertebral disc organ culture: An ex vivo model for anti-inflammatory drug and cell therapy. Tissue Eng. Part C Methods 2016, 22, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gehlen, Y.; Heizmann, F.; Grad, S.; Alini, M.; Richards, R.G.; Kubosch, D.; Südkamp, N.; Izadpanah, K.; Kubosch, E.J.; et al. Preclinical ex-vivo testing of anti-inflammatory drugs in a bovine intervertebral degenerative disc model. Front. Bioeng. Biotechnol. 2020, 8, 583. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, S.; Menage, J.; Sivan, S.; Urban, J.P. Bovine explant model of degeneration of the intervertebral disc. BMC Musculoskelet. Disord. 2008, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Song, X.X.; Yu, Y.J.; Li, X.F.; Liu, Z.D.; Yu, B.W.; Guo, Z. Estrogen receptor expression in lumbar intervertebral disc of the elderly: Gender- and degeneration degree-related variations. Jt. Bone Spine 2014, 81, 250–253. [Google Scholar] [CrossRef]
- Song, X.X.; Shi, S.; Guo, Z.; Li, X.F.; Yu, B.W. Estrogen receptors involvement in intervertebral discogenic pain of the elderly women: Colocalization and correlation with the expression of Substance P in nucleus pulposus. Oncotarget 2017, 8, 38136–38144. [Google Scholar] [CrossRef] [Green Version]
- Song, M.X.; Ma, X.X.; Wang, C.; Wang, Y.; Sun, C.; Xu, D.R.; Zhu, K.; Li, G.H.; Zhao, H.; Zhang, C. Protective effect of estrogen receptors (ERα/β) against the intervertebral disc degeneration involves activating CCN5 via the promoter. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1811–1820. [Google Scholar]
- Wang, H.; Li, Z.; Huo, Y.; Tian, T.; Yang, D.; Ma, L.; Yang, S.; Ding, W. 17β-Estradiol alleviates intervertebral disc degeneration by inhibiting NF-κB signal pathway. Life Sci. 2021, 284, 119874. [Google Scholar] [CrossRef]
- Gao, X.W.; Su, X.T.; Lu, Z.H.; Ou, J. 17β-Estradiol prevents extracellular matrix degradation by downregulating MMP3 expression via PI3K/Akt/FOXO3 pathway. Spine 2020, 45, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Guo, X.; Zhang, F.; Zheng, L.; Ding, W.; Yang, S. Resveratrol combined with 17β-estradiol prevents IL-1β induced apoptosis in human nucleus pulposus via the PI3K/AKT/Mtor and PI3K/AKT/GSK-3β pathway. J. Investig. Surg. 2021, 34, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.T.; Yang, S.D.; Zhang, F.; Liu, S.; Yang, D.L.; Ma, L.; Wang, H.; Ding, W.Y. 17β-Estradiol protects against interleukin-1β-induced apoptosis in rat nucleus pulposus cells via the mTOR/caspase-3 pathway. Mol. Med. Rep. 2019, 20, 1523–1530. [Google Scholar] [CrossRef] [Green Version]
- Song, X.X.; Jin, L.Y.; Li, X.F.; Luo, Y.; Yu, B.W. Substance P Mediates Estrogen Modulation Proinflammatory Cytokines Release in Intervertebral Disc. Inflammation 2021, 44, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.Q.; Yong, Z.; Kuhn, A.; Riegger, J.; Goncalves, R.M.; Ruf, M.; Mauer, U.M.; Huber-Lang, M.; Ignatius, A.; Brenner, R.E.; et al. Interleukin-1β and cathepsin D modulate formation of the terminal complement complex in cultured human disc tissue. Eur. Spine J. 2021, 30, 2247–2256. [Google Scholar] [CrossRef]
- Patterson, F.; Miralami, R.; Tansey, K.E.; Prabhu, R.K.; Priddy, L.B. Deleterious effects of whole-body vibration on the spine: A review of in vivo, ex vivo, and in vitro models. Anim. Models Exp. Med. 2021, 4, 77–86. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 2004, 204, 47–54. [Google Scholar] [CrossRef]
- Terajima, M.; Perdivara, I.; Sricholpech, M.; Deguchi, Y.; Pleshko, N.; Tomer, K.B.; Yamauchi, M. Glycosylation and cross-linking in bone type I collagen. J. Biol. Chem. 2014, 289, 22636–22647. [Google Scholar] [CrossRef] [Green Version]
- Holguin, N.; Muir, J.; Rubin, C.; Judex, S. Short applications of very low-magnitude vibrations attenuate expansion of the intervertebral disc during extended bed rest. Spine J. 2009, 9, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger-Wilke, C.; Ekkerlein, A.; Goncalves, R.M.; Ferreira, J.R.; Ignatius, A.; Wilke, H.J.; Teixeira, G.Q. Mesenchymal stem cell secretome decreases the inflammatory response in annulus fibrosus organ cultures. Eur. Cells Mater. 2021, 42, 1–19. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Albini, A.; Vené, R.; Benelli, R.; Noonan, D.; Weigert, C.; Weiler, C.; Lichtinghagen, R.; Jochum, M.; Nerlich, A.G. Cell density-dependent regulation of matrix metalloproteinase and TIMP expression in differently tumorigenic breast cancer cell lines. Exp. Cell Res. 2005, 305, 83–98. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Saggese, T.; Teixeira, G.Q.; Wade, K.; Moll, L.; Ignatius, A.; Wilke, H.J.; Goncalves, R.M.; Neidlinger-Wilke, C. Georg Schmorl prize of the German Spine Society (DWG) 2018: Combined inflammatory and mechanical stress weakens the annulus fibrosus: Evidences from a loaded bovine AF organ culture. Eur. Spine J. 2019, 28, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.T.; Park, J.S.; Kim, K.S.; Li, J.; Attallah-Wasif, E.S.; Hutton, W.C.; Boden, S.D. ISSLS Prize Winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine 2004, 29, 2603–2611. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Barrett, A.J.; Lazarus, G.S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem. J. 1974, 137, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Nimni, M.E. Collagen: Structure, function, and metabolism in normal and fibrotic tissues. Semin. Arthritis Rheum. 1983, 13, 1–86. [Google Scholar] [CrossRef]
- Ziff, M.; Kibrick, A.; Dresner, E.; Gribetz, H.J. Excretion of hydroxyproline in patients with rheumatic and non-rheumatic diseases. J. Clin. Investig. 1956, 35, 579–587. [Google Scholar] [CrossRef]
- Pereira, C.L.; Teixeira, G.Q.; Ribeiro-Machado, C.; Caldeira, J.; Costa, M.; Figueiredo, F.; Fernandes, R.; Aguiar, P.; Grad, S.; Barbosa, M.A.; et al. Mesenchymal Stem/Stromal Cells seeded on cartilaginous endplates promote Intervertebral Disc Regeneration through Extracellular Matrix Remodeling. Sci. Rep. 2016, 6, 33836. [Google Scholar] [CrossRef]
- Bezci, S.E.; Werbner, B.; Zhou, M.; Malollari, K.G.; Dorlhiac, G.; Carraro, C.; Streets, A.; O’Connell, G.D. Radial variation in biochemical composition of the bovine caudal intervertebral disc. JOR Spine 2019, 2, e1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiordalisi, M.F.; Ferreira, J.R.; Pinto, M.L.; Ribeiro-Machado, C.; Teixeira Pinto, M.; Oliveira, M.J.; Barbosa, M.A.; Madeira Gonçalves, R.; Caldeira, J. The impact of matrix age on intervertebral disc regeneration. Biomater. Adv. 2022, 143, 213192. [Google Scholar] [CrossRef] [PubMed]



| Gene | Sequence (Forward and Reverse Primer) | Product Size (bp) |
|---|---|---|
| ACAN | fw: 5′-ACA GCG CCT ACC AAG ACA AG-3′ rev: 5′-ACG ATG CCT TTT ACC ACG AC-3′ | 155 |
| COL1A1 | fw: 5′-TGA GAG AGG GGT TGT TGG AC-3′ rev: 5′-AGG TTC ACC CTT CAC ACC TG-3′ | 142 |
| COL2A1 | 5′-CCT GTA GGA CCT TTG GGT CA-3′ 5′-ATA GCG CCG TTG TGT AGG AC-3′ | 145 |
| ESR1 | fw: 5′-GCC TCA AAT CCA TCA TCT TGC T-3′ rev: 5′-CGG TGG ATG TGG TCC TTC TC-3′ | 100 |
| ESR2 | fw: 5′-CTC CTG GAC ACC TCT CTC CTT TAG-3′ rev: 5′-GGT TTC ACG CCA AGG ACT CTT-3′ | 85 |
| GAPDH | fw: 5′-ACC CAG AAG ACT GTG GAT GG-3′ rev: 5′-CAA CAG ACA CGT TGG GAG TG-3′ | 178 |
| MMP1 | fw: 5′-ATG CTG TTT TCC AGA AAG GTG G-3′ rev: 5′-TCA GGA AAC ACC TTC CAC AGA C-3′ | 193 |
| MMP3 | fw: 5′-CTG CGG ATA CTT CCA CAG GT-3′ rev: 5′-ATG GAT GAG CAG GGA AAC AC-3′ | 198 |
| PT53 | fw: 5′-ATT TAC GCG CGG AGT ATT TG-3′ rev: 5′-CCA GTG TGA TGA TGG TGA GG-3′ | 174 |
| PTGS2 | Bt03214492_m1 | |
| TIMP1 | fw: 5′-GCT GGA CAT TGG AGG AAA GA-3′ rev: 5′-CGT CCG GAG AGG AGA TGT AG-3′ | 209 |
| TIMP2 | fw: 5′-TGA GAG AGG GGT TGT TGG AC-3′ rev: 5′-AGG TTC ACC CTT CAC ACC TG-3′ | 142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widmayer, F.; Neidlinger-Wilke, C.; Witz, F.; Jansen, J.U.; Ignatius, A.; Haffner-Luntzer, M.; Teixeira, G.Q. Oestrogen and Vibration Improve Intervertebral Disc Cell Viability and Decrease Catabolism in Bovine Organ Cultures. Int. J. Mol. Sci. 2023, 24, 6143. https://doi.org/10.3390/ijms24076143
Widmayer F, Neidlinger-Wilke C, Witz F, Jansen JU, Ignatius A, Haffner-Luntzer M, Teixeira GQ. Oestrogen and Vibration Improve Intervertebral Disc Cell Viability and Decrease Catabolism in Bovine Organ Cultures. International Journal of Molecular Sciences. 2023; 24(7):6143. https://doi.org/10.3390/ijms24076143
Chicago/Turabian StyleWidmayer, Franziska, Cornelia Neidlinger-Wilke, Fiona Witz, Jan U. Jansen, Anita Ignatius, Melanie Haffner-Luntzer, and Graciosa Q. Teixeira. 2023. "Oestrogen and Vibration Improve Intervertebral Disc Cell Viability and Decrease Catabolism in Bovine Organ Cultures" International Journal of Molecular Sciences 24, no. 7: 6143. https://doi.org/10.3390/ijms24076143





