Low P-Selectin Glycoprotein Ligand-1 Expression in Neutrophils Associates with Disease Activity and Deregulated NET Formation in Systemic Lupus Erythematosus
Abstract
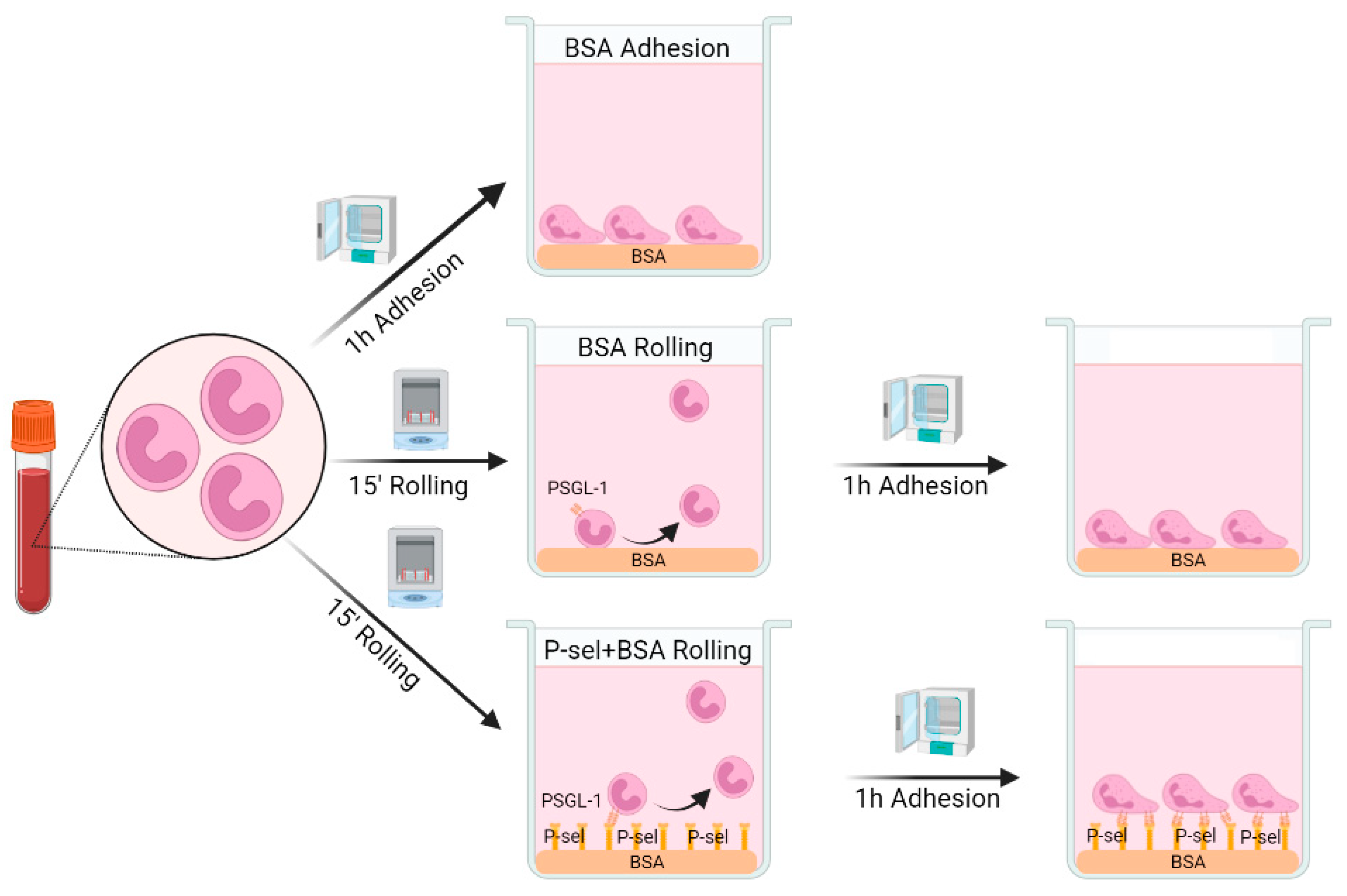
:1. Introduction
2. Results
2.1. PSGL-1 Expression Is Reduced in Neutrophils of Active SLE Patients
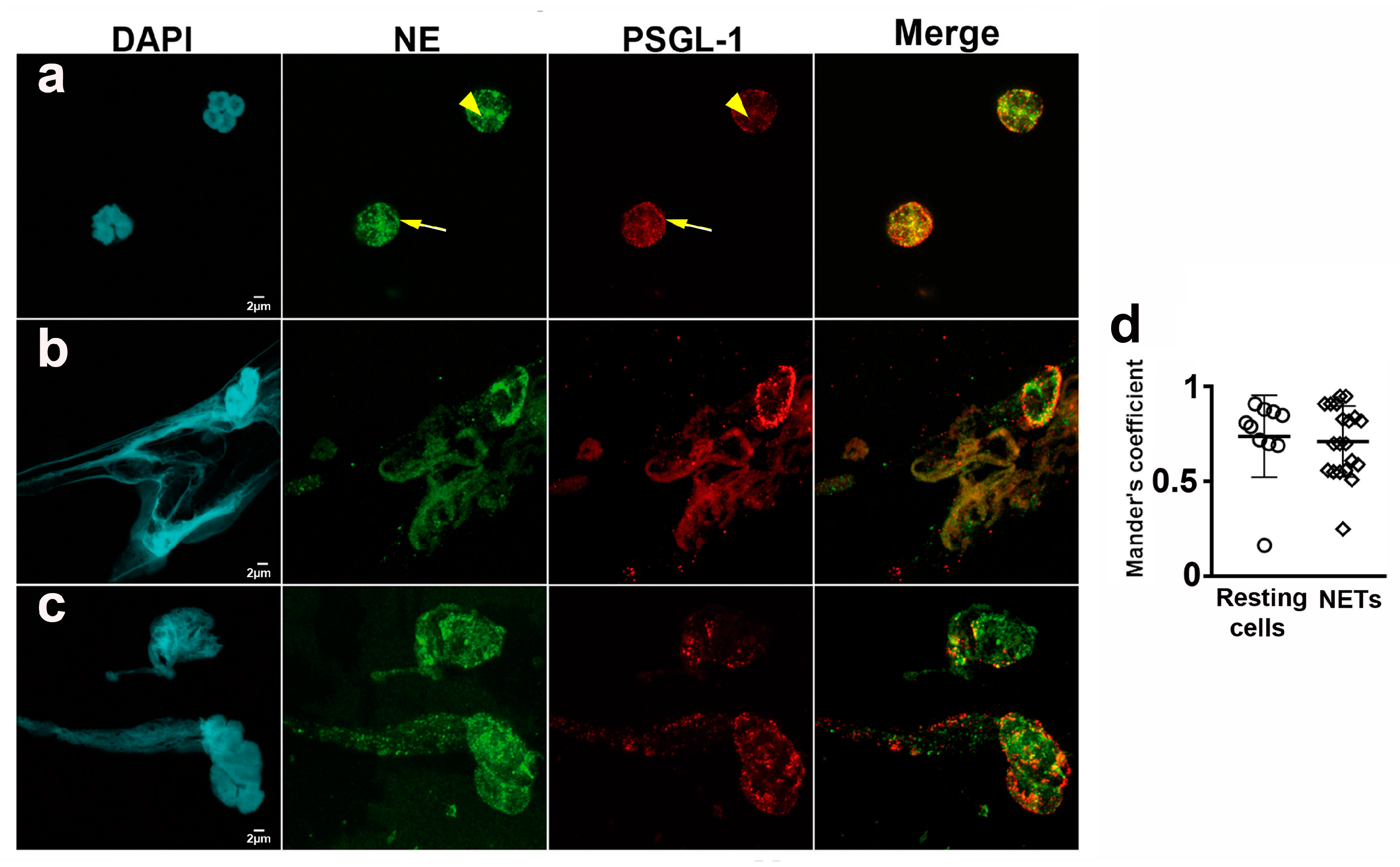
2.2. PSGL-1 Is Located in the NETs
2.3. Neutrophils from SLE Patients Are More Susceptible to Generate NETs
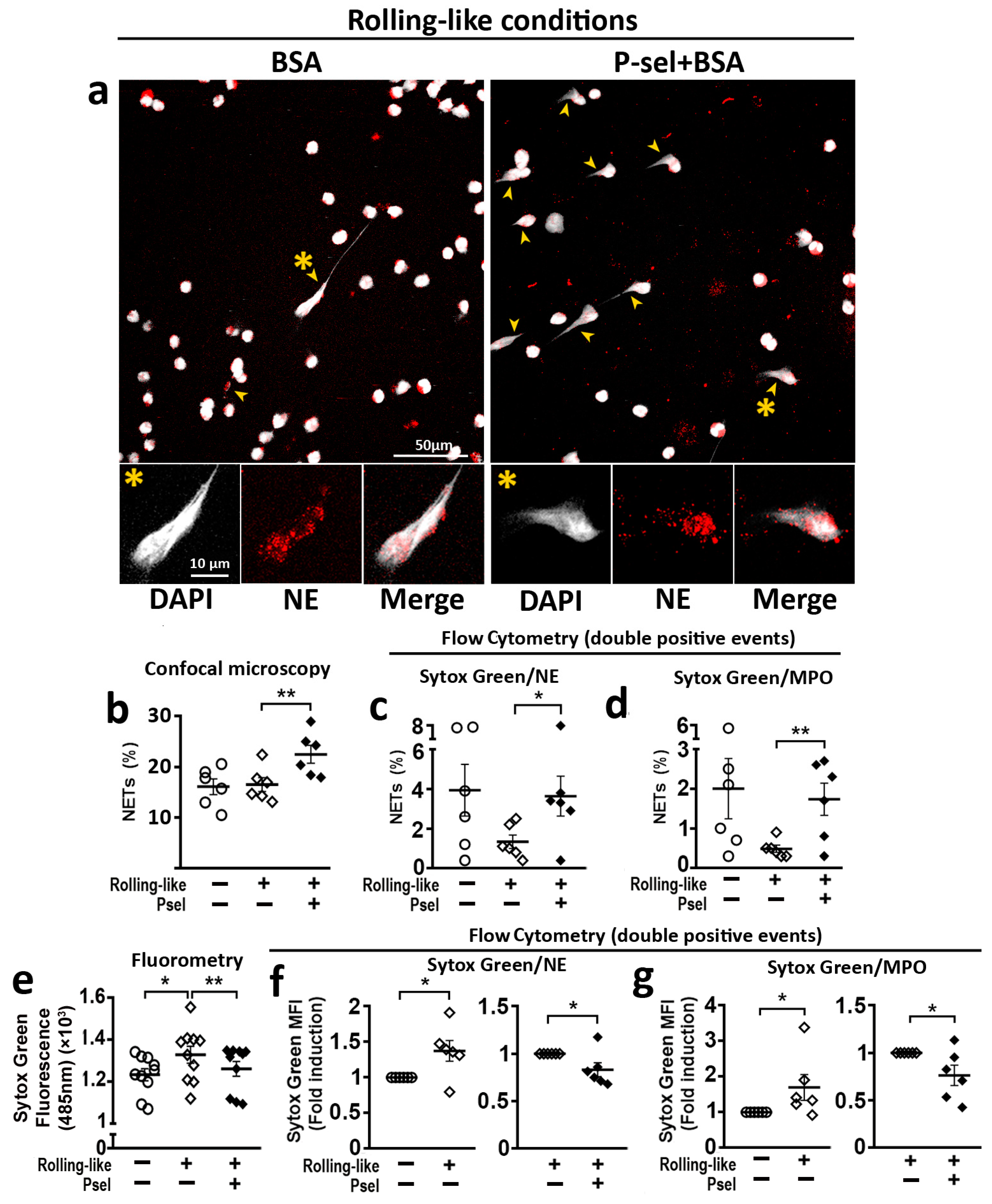
2.4. PSGL-1/P-Selectin Interaction Modulates NET Generation in HD
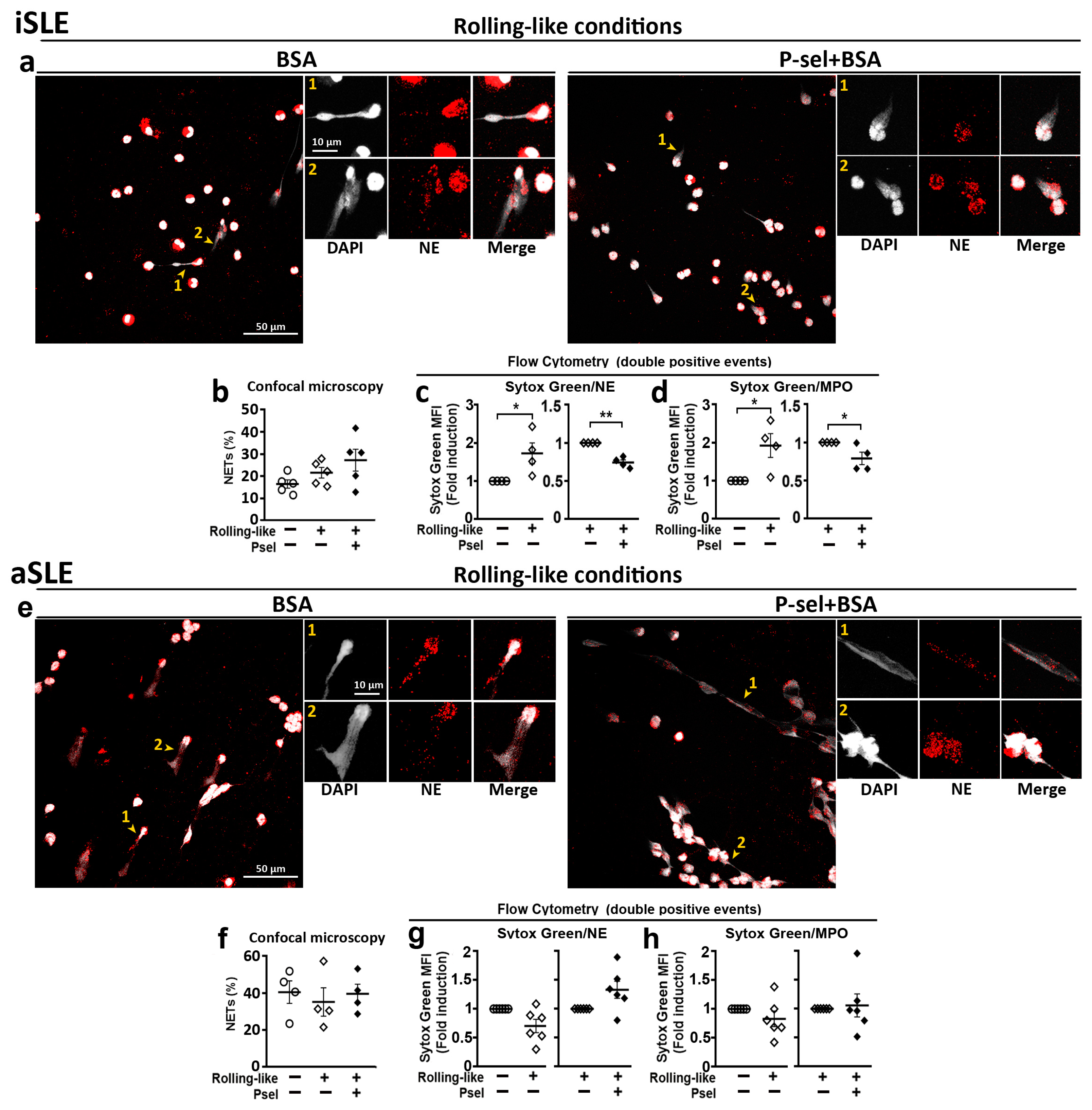
2.5. PSGL-1/P-Selectin Interaction Does Not Control DNA Extrusion in SLE Patients with Active Disease
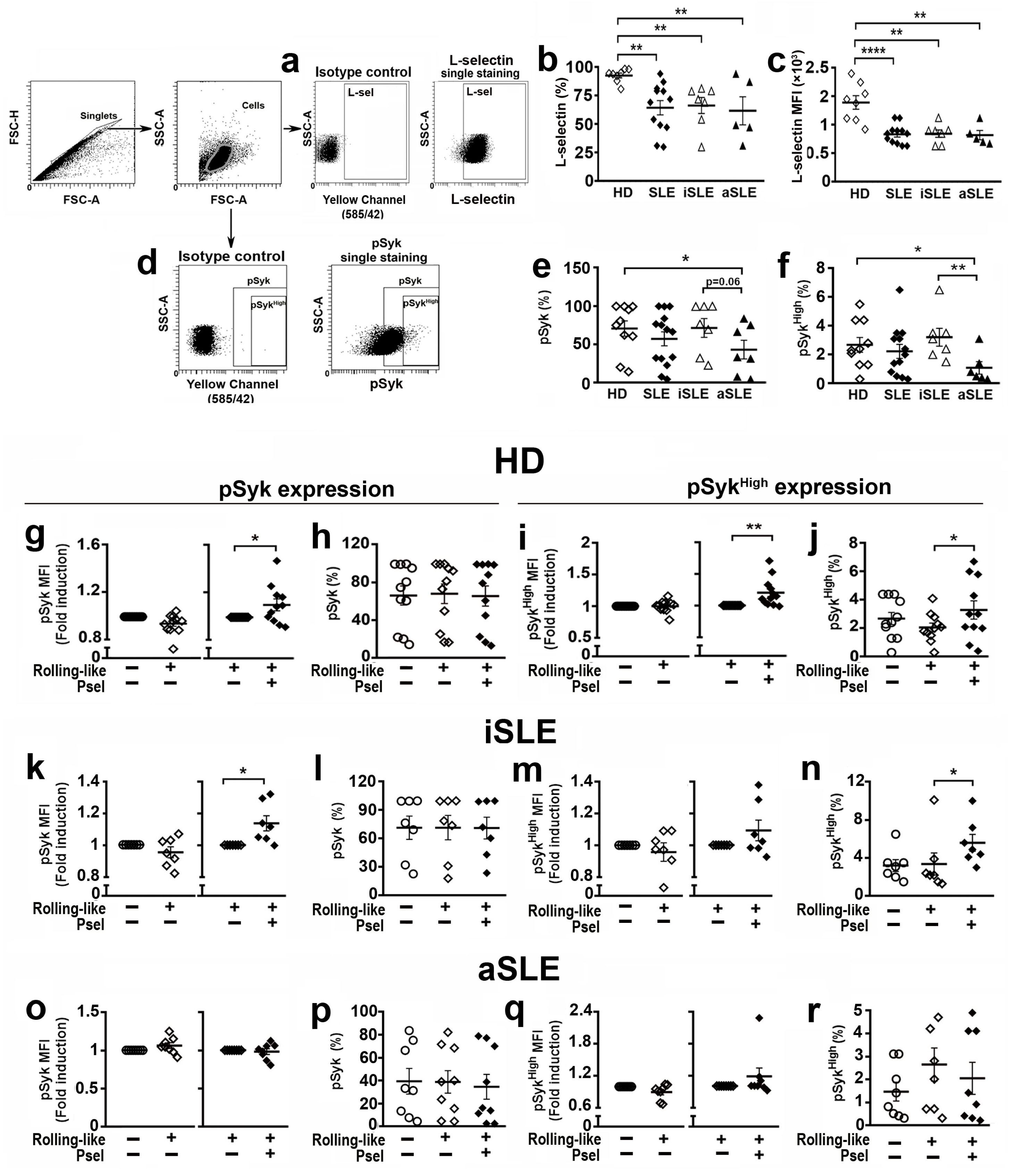
2.6. PSGL-1/P-Selectin Interaction Signaling Is Altered in SLE Patients
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Labeling of Neutrophils for Flow Cytometry
4.3. Neutrophils Isolation
4.4. Rolling-like Assays
4.5. Activation with PMA
4.6. Quantification of NETs by Fluorometry, Confocal Microscopy and Flow Cytometry
4.7. Phospho-Syk Measurement by Flow Cytometry
4.8. Confocal Image Analysis of NETs
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef]
- Fortuna, G.; Brennan, M.T. Systemic lupus erythematosus: Epidemiology, pathophysiology, manifestations, and management. Dent. Clin. N. Am. 2013, 57, 631–655. [Google Scholar] [CrossRef]
- Hakkim, A.; Furnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef] [Green Version]
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693. [Google Scholar] [CrossRef]
- van der Linden, M.; van den Hoogen, L.L.; Westerlaken, G.H.A.; Fritsch-Stork, R.D.E.; van Roon, J.A.G.; Radstake, T.; Meyaard, L. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology 2018, 57, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.; Bonanni, A.; Petretto, A.; Vaglio, A.; Pratesi, F.; Santucci, L.; Migliorini, P.; Bertelli, R.; Galetti, M.; Belletti, S.; et al. Neutrophil Extracellular Traps Profiles in Patients with Incident Systemic Lupus Erythematosus and Lupus Nephritis. J. Rheumatol. 2019, 47, 377–386. [Google Scholar] [CrossRef]
- Tvaroska, I.; Selvaraj, C.; Koca, J. Selectins-The Two Dr. Jekyll and Mr. Hyde Faces of Adhesion Molecules—A Review. Molecules 2020, 25, 2835. [Google Scholar] [CrossRef]
- DeRogatis, J.M.; Viramontes, K.M.; Neubert, E.N.; Tinoco, R. PSGL-1 Immune Checkpoint Inhibition for CD4(+) T Cell Cancer Immunotherapy. Front. Immunol. 2021, 12, 636238. [Google Scholar] [CrossRef]
- Somers, W.S.; Tang, J.; Shaw, G.D.; Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell 2000, 103, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends Immunol 2017, 38, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urzainqui, A.; Serrador, J.M.; Viedma, F.; Yanez-Mo, M.; Rodriguez, A.; Corbi, A.L.; Alonso-Lebrero, J.L.; Luque, A.; Deckert, M.; Vazquez, J.; et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity 2002, 17, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Negoro, P.E.; Xu, S.; Dagher, Z.; Hopke, A.; Reedy, J.L.; Feldman, M.B.; Khan, N.S.; Viens, A.L.; Alexander, N.J.; Atallah, N.J.; et al. Spleen Tyrosine Kinase Is a Critical Regulator of Neutrophil Responses to Candida Species. mBio 2020, 11, e02043-19. [Google Scholar] [CrossRef]
- Nani, S.; Fumagalli, L.; Sinha, U.; Kamen, L.; Scapini, P.; Berton, G. Src family kinases and Syk are required for neutrophil extracellular trap formation in response to beta-glucan particles. J. Innate Immun. 2014, 7, 59–73. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Lv, K.; Zheng, G.; Wang, H.; Chen, S.; Huang, L.; Liu, Y.; Zhang, Y.; Tang, Z.; et al. Asebogenin suppresses thrombus formation via inhibition of Syk phosphorylation. Br. J. Pharmacol. 2023, 180, 287–307. [Google Scholar] [CrossRef]
- Urzainqui, A.; Martinez del Hoyo, G.; Lamana, A.; de la Fuente, H.; Barreiro, O.; Olazabal, I.M.; Martin, P.; Wild, M.K.; Vestweber, D.; Gonzalez-Amaro, R.; et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J. Immunol. 2007, 179, 7457–7465. [Google Scholar] [CrossRef] [Green Version]
- Nunez-Andrade, N.; Lamana, A.; Sancho, D.; Gisbert, J.P.; Gonzalez-Amaro, R.; Sanchez-Madrid, F.; Urzainqui, A. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J. Pathol. 2011, 224, 212–221. [Google Scholar]
- Perez-Frias, A.; Gonzalez-Tajuelo, R.; Nunez-Andrade, N.; Tejedor, R.; Garcia-Blanco, M.J.; Vicente-Rabaneda, E.; Castaneda, S.; Gamallo, C.; Silvan, J.; Esteban-Villafruela, A.; et al. Development of an autoimmune syndrome affecting the skin and internal organs in P-selectin glycoprotein ligand 1 leukocyte receptor-deficient mice. Arthritis Rheumatol. 2014, 66, 3178–3189. [Google Scholar] [CrossRef]
- Gonzalez-Tajuelo, R.; Silvan, J.; Perez-Frias, A.; de la Fuente-Fernandez, M.; Tejedor, R.; Espartero-Santos, M.; Vicente-Rabaneda, E.; Juarranz, A.; Munoz-Calleja, C.; Castaneda, S.; et al. P-Selectin preserves immune tolerance in mice and is reduced in human cutaneous lupus. Sci. Rep. 2017, 7, 41841. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Xie, C.; Wang, H.W.; Zhou, X.J.; Schwartz, N.; Calixto, S.; Mackay, M.; Aranow, C.; Putterman, C.; Mohan, C. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J. Immunol. 2007, 179, 7166–7175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carestia, A.; Kaufman, T.; Rivadeneyra, L.; Landoni, V.I.; Pozner, R.G.; Negrotto, S.; D’Atri, L.P.; Gómez, R.M.; Schattner, M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J. Leukoc. Biol. 2015, 99, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Urzainqui, A.; de Andres, B.; Gonzalez-Tajuelo, R.; Domenech, M.; Gonzalez-Camacho, F.; Sanchez-Madrid, F.; Brown, J.S.; Garcia, E.; Yuste, J. PSGL-1 on Leukocytes is a Critical Component of the Host Immune Response against Invasive Pneumococcal Disease. PLoS Pathog. 2016, 12, e1005500. [Google Scholar] [CrossRef]
- Bestebroer, J.; Poppelier, M.J.; Ulfman, L.H.; Lenting, P.J.; Denis, C.V.; van Kessel, K.P.; van Strijp, J.A.; de Haas, C.J. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 2007, 109, 2936–2943. [Google Scholar] [CrossRef]
- Troese, M.J.; Carlyon, J.A. Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand 1. Infect. Immun. 2009, 77, 4018–4027. [Google Scholar] [CrossRef] [Green Version]
- Herron, M.J.; Nelson, C.M.; Larson, J.; Snapp, K.R.; Kansas, G.S.; Goodman, J.L. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 2000, 288, 1653–1656. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009, 15, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, S.; Waheed, A.A.; Dabbagh, D.; Zhou, Z.; Trinite, B.; Wang, Z.; Yu, J.; Wang, D.; Li, F.; et al. PSGL-1 restricts HIV-1 infectivity by blocking virus particle attachment to target cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9537–9545. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Song, Y.; Zhang, S.; Diao, M.; Huang, S.; Li, S.; Tan, X. PSGL-1 inhibits HIV-1 infection by restricting actin dynamics and sequestering HIV envelope proteins. Cell Discov. 2020, 6, 53–68. [Google Scholar] [CrossRef]
- He, S.; Waheed, A.A.; Hetrick, B.; Dabbagh, D.; Akhrymuk, I.V.; Kehn-Hall, K.; Freed, E.O.; Wu, Y. PSGL-1 Inhibits the Incorporation of SARS-CoV and SARS-CoV-2 Spike Glycoproteins into Pseudovirions and Impairs Pseudovirus Attachment and Infectivity. Viruses 2020, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Stadtmann, A.; Germena, G.; Block, H.; Boras, M.; Rossaint, J.; Sundd, P.; Lefort, C.; Fisher, C.I.; Buscher, K.; Gelschefarth, B.; et al. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J. Exp. Med. 2013, 210, 2171–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlar, R.A.; Husain, S. The enigmas of the lupus anticoagulant: Mechanisms, diagnosis, and management. Curr. Rheumatol. Rep. 2008, 10, 74–80. [Google Scholar] [CrossRef]
- Larosa, M.; Le Guern, V.; Guettrot-Imbert, G.; Morel, N.; Abisror, N.; Morati-Hafsaoui, C.; Orquevaux, P.; Diot, E.; Doria, A.; Sarrot-Reynauld, F.; et al. Evaluation of lupus anticoagulant, damage, and remission as predictors of pregnancy complications in systemic lupus erythematosus: The French GR2 study. Rheumatology 2022, 61, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.J.; Park, D.D.; Park, S.S.; Haller, C.A.; Chen, J.; Dai, E.; Liu, L.; Mandhapati, A.R.; Eradi, P.; Dhakal, B.; et al. A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood 2021, 138, 1182–1193. [Google Scholar] [CrossRef]
- Yago, T.; Liu, Z.; Ahamed, J.; McEver, R.P. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood 2018, 132, 1426–1437. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Knight, J.S.; Hodgin, J.B.; Wang, J.; Guo, C.; Kleiman, K.; Eitzman, D.T. Psgl-1 Deficiency is Protective against Stroke in a Murine Model of Lupus. Sci. Rep. 2016, 6, 28997. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Diaz, C.; Varela-Trinidad, G.U.; Munoz-Sanchez, G.; Solorzano-Castanedo, K.; Avila-Arrezola, K.E.; Iniguez-Gutierrez, L.; Delgado-Rizo, V.; Fafutis-Morris, M. To Trap a Pathogen: Neutrophil Extracellular Traps and Their Role in Mucosal Epithelial and Skin Diseases. Cells 2021, 10, 1469. [Google Scholar] [CrossRef]
- Frangou, E.; Vassilopoulos, D.; Boletis, J.; Boumpas, D.T. An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment. Autoimmun. Rev. 2019, 18, 751–760. [Google Scholar] [CrossRef]
- Panicker, S.R.; Mehta-D’souza, P.; Zhang, N.; Klopocki, A.G.; Shao, B.; McEver, R.P. Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice. Blood 2017, 130, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeRogatis, J.M.; Viramontes, K.M.; Neubert, E.N.; Henriquez, M.L.; Guerrero-Juarez, C.F.; Tinoco, R. Targeting the PSGL-1 Immune Checkpoint Promotes Immunity to PD-1-Resistant Melanoma. Cancer Immunol. Res. 2022, 10, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Pego-Reigosa, J.M.; Nicholson, L.; Pooley, N.; Langham, S.; Embleton, N.; Marjenberg, Z.; Barut, V.; Desta, B.; Wang, X.; Langham, J.; et al. The risk of infections in adult patients with systemic lupus erythematosus: Systematic review and meta-analysis. Rheumatology 2021, 60, 60–72. [Google Scholar] [CrossRef]
- Braegelmann, C.; Holzel, M.; Ludbrook, V.; Dickson, M.; Turan, N.; Ferring-Schmitt, S.; Sternberg, S.; Bieber, T.; Kuhn, A.; Wenzel, J. Spleen tyrosine kinase (SYK) is a potential target for the treatment of cutaneous lupus erythematosus patients. Exp. Dermatol. 2016, 25, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Kavanaugh, A.; Genovese, M.C.; Musser, T.K.; Grossbard, E.B.; Magilavy, D.B. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 2010, 363, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Kunwar, S.; Devkota, A.R.; Ghimire, D.K. Fostamatinib, an oral spleen tyrosine kinase inhibitor, in the treatment of rheumatoid arthritis: A meta-analysis of randomized controlled trials. Rheumatol. Int. 2016, 36, 1077–1087. [Google Scholar] [CrossRef]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Hung, J.C.; Brown, M.L. Radiopharmaceuticals for Imaging of Infectious and Inflammatory Lesions. In Correspondence Continuing Education Courses for Nuclear Pharmacists and Nuclear Medicine Professionals; University of New Mexico: Albuquerque, NM, USA, 1993; Volume 2, pp. 1–25. [Google Scholar]
- Thakur, M.L.; Seifert, C.L.; Madsen, M.T.; McKenney, S.M.; Desai, A.G.; Park, C.H. Neutrophil labeling: Problems and pitfalls. Semin. Nucl. Med. 1984, 14, 107–117. [Google Scholar] [CrossRef]
- Thorson, L.M.; Turkalj, A.; Hung, J.C. In vitro evaluation of neutrophil viability after exposure to a hypotonic medium. Nucl. Med. Commun. 1995, 16, 615–620. [Google Scholar] [CrossRef]
- Silvan, J.; Gonzalez-Tajuelo, R.; Vicente-Rabaneda, E.; Perez-Frias, A.; Espartero-Santos, M.; Munoz-Callejas, A.; Garcia-Lorenzo, E.; Gamallo, C.; Castaneda, S.; Urzainqui, A. Deregulated PSGL-1 Expression in B Cells and Dendritic Cells May Be Implicated in Human Systemic Sclerosis Development. J. Investig. Dermatol. 2018, 138, 2123–2132. [Google Scholar] [CrossRef] [Green Version]
- Henneck, T.; Mergani, A.; Clever, S.; Seidler, A.E.; Brogden, G.; Runft, S.; Baumgartner, W.; Branitzki-Heinemann, K.; von Kockritz-Blickwede, M. Formation of Neutrophil Extracellular Traps by Reduction of Cellular Cholesterol Is Independent of Oxygen and HIF-1alpha. Int. J. Mol. Sci. 2022, 23, 3195. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, T.; Tengstrom, F.C.; Kamerling, S.W.; Pusey, C.D.; Scherer, H.U.; Toes, R.E.; Rabelink, T.J.; van Kooten, C.; Teng, Y.K. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun. Rev. 2016, 15, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavillet, M.; Martinod, K.; Renella, R.; Harris, C.; Shapiro, N.I.; Wagner, D.D.; Williams, D.A. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am. J. Hematol. 2015, 90, 1155–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, S.; Shimizu, S.; Matsuo, J.; Nishibata, Y.; Kusunoki, Y.; Hattanda, F.; Shida, H.; Nakazawa, D.; Tomaru, U.; Atsumi, T.; et al. Measurement of NET formation in vitro and in vivo by flow cytometry. Cytometry A 2017, 91, 822–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneck, E.; Mallek, F.; Schiederich, J.; Kramer, E.; Markmann, M.; Hecker, M.; Sommer, N.; Weissmann, N.; Pak, O.; Michel, G.; et al. Flow Cytometry-Based Quantification of Neutrophil Extracellular Traps Shows an Association with Hypercoagulation in Septic Shock and Hypocoagulation in Postsurgical Systemic Inflammation-A Proof-of-Concept Study. J. Clin. Med. 2020, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]


| SLE Patients | Inactive SLE (n = 33) | Active SLE (n = 14) | p-Value |
|---|---|---|---|
| Female/Male, n (%) | 30 (90)/3 (10) | 13 (92)/1 (8) | |
| Age (years), mean [min–max] | 50.35 [23–83] | 43.28 [19–59] | |
| Disease duration (years), mean [min–max] | 13.79 [1 month–38 years] | 8.57 [1 month–21 years] | |
| ANA + profile, n (%) | 32 (96) | 14 (100) | |
| Anti-ds-DNA + profile, n (%) | 13 (39) | 13 (92) *** | 0.0010 |
| Anti-Sm + profile, n (%) | 5 (15) | 3 (21) | 0.6795 |
| Lupus anticoagulant | 5 (15) | 5 (35) | 0.1373 |
| SLICC/ACR Damage Index (mean [min–max]) | 0.57 [0–4] | 0.71 [0–3] | 0.1436 |
| SLEDAI (mean [min–max]) | 1.11 [0–4] | 14.14 [6–41] **** | <0.0001 |
| Renal manifestations, n (%) | 15 (45) | 12 (85) * | 0.0220 |
| Lung manifestations, n (%) | 5 (15) | 5 (35) | 0.1171 |
| Raynaud´s phenomenon | 6 (18) | 3 (21) | 0.5435 |
| Treatment, n (%) | |||
| Azathioprine | 15 (45) | 8 (57) | |
| Belimumab | 2 (6) | 4 (28) | |
| Glucocorticoids | 29 (87) | 13 (92) | |
| Hydroxychloroquine | 30 (90) | 14 (100) | |
| Methotrexate | 9 (27) | 4 (28) | |
| Mycophenolate mofetil | 4 (11) | 12 (85) | |
| Rituximab | 6 (18) | 5 (35) |
| SLE Patients | Inactive SLE (n = 7) | Active SLE (n = 8) | p-Value |
|---|---|---|---|
| Female/Male, n (%) | 7 (100) | 7 (87)/1 (13) | |
| Age (years), mean [min–max] | 48.85 [24–63] | 29.87 [23–54] | |
| Disease duration (years), mean [min–max] | 25 [5–38 years] | 5.08 [1 month–16 years] | |
| ANA + profile, n (%) | 6 (85) | 8 (100) | |
| Anti-ds-DNA + profile, n (%) | 2 (28) | 8 (100) *** | 0.0070 |
| Anti-Sm + profile, n (%) | 1 (14) | 5 (62) | 0.1189 |
| Lupus anticoagulant | 1 (14) | 2 (25) | 0.5515 |
| SLICC/ACR Damage Index (mean [min–max]) | 0.57 [0–3] | 1 [0–3] | 0.1883 |
| SLEDAI (mean [min–max]) | 2.85 (0–4) | 14 (11–23) *** | 0.0013 |
| Renal manifestations, n (%) | 4 (57) | 7 (87) | 0.2821 |
| Lung manifestations, n (%) | 1 (14) | 3 (37) | 0.3392 |
| Raynaud´s phenomenon | 1 (14) | 2 (25) | 0.5545 |
| Treatment, n (%) | |||
| Azathioprine | 0 (0) | 1 (12) | |
| Glucocorticoids | 3 (42) | 7 (87) | |
| Cyclophosphamide | 0 (0) | 1 (12) | |
| Hydroxychloroquine | 2 (28) | 6 (75) | |
| Methotrexate | 1 (14) | 2 (25) | |
| Mycophenolate mofetil | 3 (42) | 1 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Callejas, A.; González-Sánchez, E.; Silván, J.; San Antonio, E.; González-Tajuelo, R.; Ramos-Manzano, A.; Sánchez-Abad, I.; González-Alvaro, I.; García-Pérez, J.; Tomero, E.G.; et al. Low P-Selectin Glycoprotein Ligand-1 Expression in Neutrophils Associates with Disease Activity and Deregulated NET Formation in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 6144. https://doi.org/10.3390/ijms24076144
Muñoz-Callejas A, González-Sánchez E, Silván J, San Antonio E, González-Tajuelo R, Ramos-Manzano A, Sánchez-Abad I, González-Alvaro I, García-Pérez J, Tomero EG, et al. Low P-Selectin Glycoprotein Ligand-1 Expression in Neutrophils Associates with Disease Activity and Deregulated NET Formation in Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2023; 24(7):6144. https://doi.org/10.3390/ijms24076144
Chicago/Turabian StyleMuñoz-Callejas, Antonio, Elena González-Sánchez, Javier Silván, Esther San Antonio, Rafael González-Tajuelo, Alejandra Ramos-Manzano, Inés Sánchez-Abad, Isidoro González-Alvaro, Javier García-Pérez, Eva G. Tomero, and et al. 2023. "Low P-Selectin Glycoprotein Ligand-1 Expression in Neutrophils Associates with Disease Activity and Deregulated NET Formation in Systemic Lupus Erythematosus" International Journal of Molecular Sciences 24, no. 7: 6144. https://doi.org/10.3390/ijms24076144





