Polymer Translocation and Nanopore Sequencing: A Review of Advances and Challenges
Abstract
1. Introduction

2. Polymer Translocation: Highlights from Theoretical Development
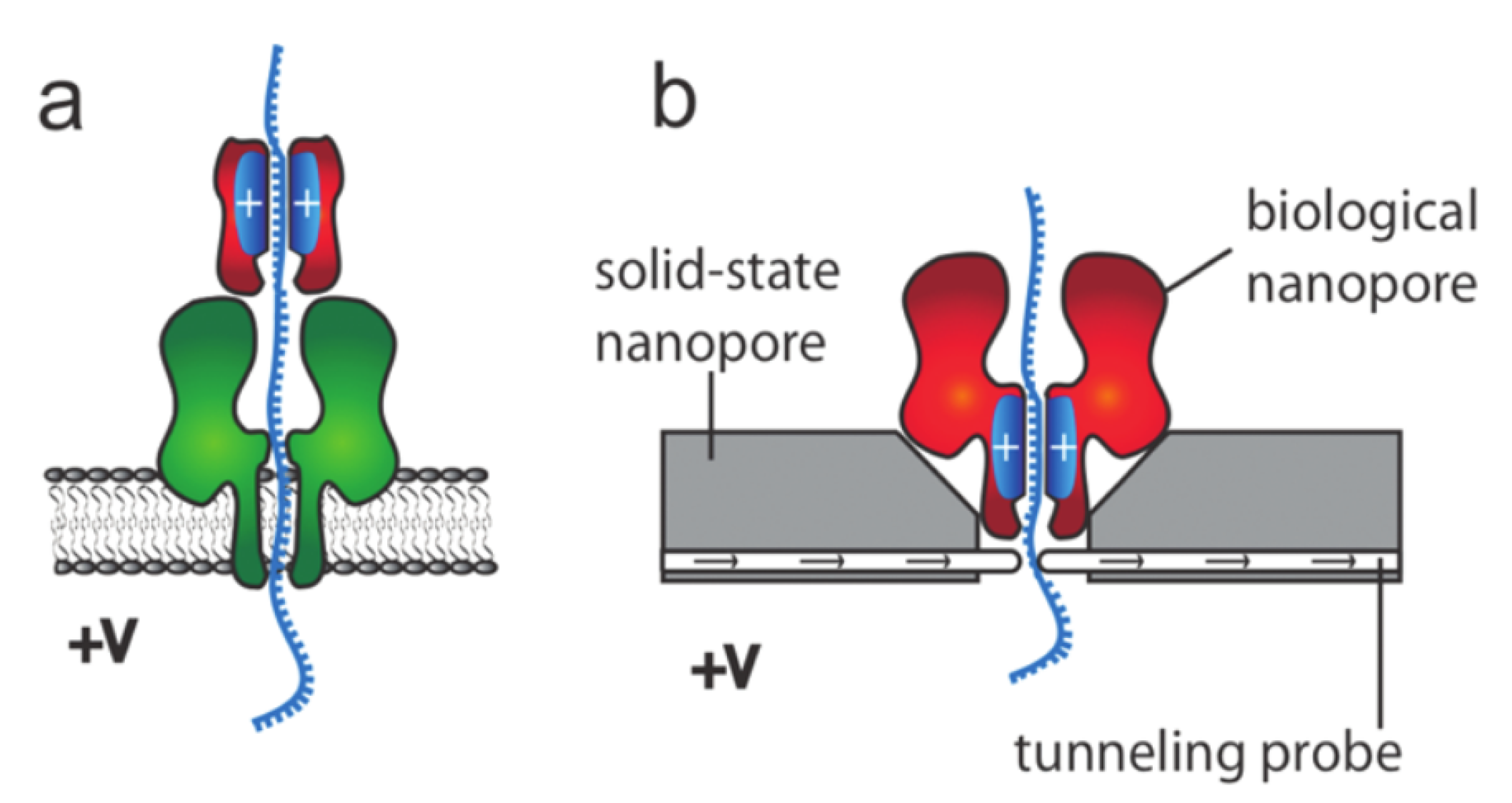
3. Nanopores: A Brief Overview
4. Controlling the Speed of Translocation
5. Summary and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberts, B. Molecular Biology of the Cell; WW Norton & Company: New York, NY, USA, 2017. [Google Scholar]
- De Gennes, P.G.; Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Vanderzande, C. Lattice Models of Polymers; Number 11; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Reisner, W.; Pedersen, J.N.; Austin, R.H. DNA confinement in nanochannels: Physics and biological applications. Rep. Prog. Phys. 2012, 75, 106601. [Google Scholar] [CrossRef] [PubMed]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Hanover, J.; Liepins, A. The ion channel behavior of the nuclear pore complex. J. Membr. Biol. 1995, 146, 239–251. [Google Scholar] [PubMed]
- Yusupova, G.Z.; Yusupov, M.M.; Cate, J.; Noller, H.F. The path of messenger RNA through the ribosome. Cell 2001, 106, 233–241. [Google Scholar] [CrossRef]
- Kasianowicz, J.J.; Brandin, E.; Branton, D.; Deamer, D.W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA 1996, 93, 13770–13773. [Google Scholar] [CrossRef]
- Goto, Y.; Akahori, R.; Yanagi, I.; Takeda, K.I. Solid-state nanopores towards single-molecule DNA sequencing. J. Hum. Genet. 2020, 65, 69–77. [Google Scholar] [CrossRef]
- Shen, B.; Piskunen, P.; Nummelin, S.; Liu, Q.; Kostiainen, M.A.; Linko, V. Advanced DNA Nanopore Technologies. ACS Appl. Bio. Mater. 2020, 3, 5606–5619. [Google Scholar] [CrossRef]
- Mohammadi, M.M.; Bavi, O. DNA sequencing: An overview of solid-state and biological nanopore-based methods. Biophys. Rev. 2021, 14, 99–110. [Google Scholar] [CrossRef]
- Tsutsui, J.M.; Xie, F.; Porter, R.T. The use of microbubbles to target drug delivery. Cardiovasc. Ultrasound 2004, 2, 23. [Google Scholar] [CrossRef]
- Hanss, B.; Leal-Pinto, E.; Bruggeman, L.A.; Copeland, T.D.; Klotman, P.E. Identification and characterization of a cell membrane nucleic acid channel. Proc. Natl. Acad. Sci. USA 1998, 95, 1921–1926. [Google Scholar]
- Wang, W.; van Niekerk, E.; Willis, D.E.; Twiss, J.L. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev. Neurobiol. 2007, 67, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Craighead, H.G. Separation of long DNA molecules in a microfabricated entropic trap array. Science 2000, 288, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.W.; Rodrigues, C.G.; Stanford, V.M.; Rubinson, K.A.; Krasilnikov, O.V.; Kasianowicz, J.J. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc. Natl. Acad. Sci. USA 2007, 104, 8207–8211. [Google Scholar] [CrossRef]
- Morris, J.R.; Boutell, C.; Keppler, M.; Densham, R.; Weekes, D.; Alamshah, A.; Butler, L.; Galanty, Y.; Pangon, L.; Kiuchi, T.; et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 2009, 462, 886–890. [Google Scholar] [CrossRef]
- Hirschhorn, J.N. Genomewide association studies—Illuminating biologic pathways. N. Engl. J. Med. 2009, 360, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.; et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef]
- Venta, K.; Shemer, G.; Puster, M.; Rodriguez-Manzo, J.A.; Balan, A.; Rosenstein, J.K.; Shepard, K.; Drndic, M. Differentiation of short, single-stranded DNA homopolymers in solid-state nanopores. ACS Nano 2013, 7, 4629–4636. [Google Scholar] [CrossRef]
- Palyulin, V.V.; Ala-Nissila, T.; Metzler, R. Polymer translocation: The first two decades and the recent diversification. Soft Matter 2014, 10, 9016–9037. [Google Scholar] [CrossRef]
- Afrasiabian, N.; Denniston, C. The journey of a single polymer chain to a nanopore. Soft Matter 2020, 16, 9101–9112. [Google Scholar] [CrossRef]
- Seth, S.; Bhattacharya, A. How capture affects polymer translocation in a solitary nanopore. J. Chem. Phys. 2022, 156, 244902. [Google Scholar] [CrossRef]
- Mayer, S.F.; Cao, C.; Dal Peraro, M. Biological nanopores for single-molecule sensing. iScience 2022, 25. [Google Scholar] [CrossRef] [PubMed]
- Dubbeldam, J.; Rostiashvili, V.G.; Milchev, A.; Vilgis, T.A. Driven translocation of a polymer: Fluctuations at work. Phys. Rev. E 2013, 87, 032147. [Google Scholar] [CrossRef]
- Derrington, I.M.; Butler, T.Z.; Collins, M.D.; Manrao, E.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Nanopore DNA sequencing with MspA. Proc. Natl. Acad. Sci. USA 2010, 107, 16060–16065. [Google Scholar] [CrossRef] [PubMed]
- Peskin, C.S.; Odell, G.M.; Oster, G.F. Cellular motions and thermal fluctuations: The Brownian ratchet. Biophys. J. 1993, 65, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, A.; Skolnick, J. Spontaneous translocation of a polymer across a curved membrane. Phys. Rev. Lett. 1995, 74, 2142. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef]
- Sung, W.; Park, P. Polymer translocation through a pore in a membrane. Phys. Rev. Lett. 1996, 77, 783. [Google Scholar] [CrossRef]
- Muthukumar, M. Polymer translocation through a hole. J. Chem. Phys. 1999, 111, 10371–10374. [Google Scholar] [CrossRef]
- Chuang, J.; Kantor, Y.; Kardar, M. Anomalous dynamics of translocation. Phys. Rev. E 2001, 65, 011802. [Google Scholar] [CrossRef]
- Luo, K.; Ala-Nissila, T.; Ying, S.C. Polymer translocation through a nanopore: A two-dimensional Monte Carlo study. J. Chem. Phys. 2006, 124, 034714. [Google Scholar] [CrossRef]
- Panja, D.; Barkema, G.T.; Ball, R.C. Anomalous dynamics of unbiased polymer translocation through a narrow pore. J. Physics: Condens. Matter 2007, 19, 432202. [Google Scholar] [CrossRef]
- Panja, D.; Barkema, G.T.; Ball, R.C. Polymer translocation out of planar confinements. J. Phys. Condens. Matter 2008, 20, 075101. [Google Scholar] [CrossRef]
- Luo, K.; Ollila, S.T.; Huopaniemi, I.; Ala-Nissila, T.; Pomorski, P.; Karttunen, M.; Ying, S.C.; Bhattacharya, A. Dynamical scaling exponents for polymer translocation through a nanopore. Phys. Rev. E 2008, 78, 050901. [Google Scholar] [CrossRef]
- Gauthier, M.; Slater, G. Molecular dynamics simulation of a polymer chain translocating through a nanoscopic pore. Eur. Phys. J. E 2008, 25, 17–23. [Google Scholar] [CrossRef]
- Panja, D.; Barkema, G.T.; Kolomeisky, A.B. Through the eye of the needle: Recent advances in understanding biopolymer translocation. J. Phys. Condens. Matter 2013, 25, 413101. [Google Scholar] [CrossRef]
- Panja, D.; Barkema, G.T. Rouse modes of self-avoiding flexible polymers. J. Chem. Phys. 2009, 131, 154903. [Google Scholar] [CrossRef]
- Panja, D.; Barkema, G.T. Simulations of two-dimensional unbiased polymer translocation using the bond fluctuation model. J. Chem. Phys. 2010, 132, 014902. [Google Scholar] [CrossRef]
- Luo, M.B.; Cao, W.P. Influence of polymer-pore interaction on the translocation of a polymer through a nanopore. Phys. Rev. E 2012, 86, 031914. [Google Scholar] [CrossRef]
- Brandani, G.; Baldovin, F.; Orlandini, E.; Stella, A.L. Finite-size scaling in unbiased translocation dynamics. J. Stat. Mech. Theory Exp. 2014, 2014, P05019. [Google Scholar] [CrossRef]
- Mohanta, D.; Giri, D.; Kumar, S. Effect of solvent gradient inside the entropic trap on polymer migration. Phys. Rev. E 2022, 105, 024135. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S. Polymer in a pore: Effect of confinement on the free energy barrier. Phys. A Stat. Mech. Appl. 2018, 499, 216–223. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Giri, D.; Nath, S. Statistical mechanics of a polymer chain attached to the interface of a cone-shaped channel. EPL Europhys. Lett. 2017, 118, 28001. [Google Scholar] [CrossRef]
- Abdolvahab, R.H.; Ejtehadi, M.R.; Metzler, R. Sequence dependence of the binding energy in chaperone-driven polymer translocation through a nanopore. Phys. Rev. E 2011, 83, 011902. [Google Scholar] [CrossRef] [PubMed]
- de Haan, H.W.; Slater, G.W. Using an incremental mean first passage approach to explore the viscosity dependent dynamics of the unbiased translocation of a polymer through a nanopore. J. Chem. Phys. 2012, 136, 204902. [Google Scholar] [CrossRef]
- de Haan, H.W.; Slater, G.W. Memory effects during the unbiased translocation of a polymer through a nanopore. J. Chem. Phys. 2012, 136, 154903. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, K. Dynamics of polymer translocation through a nanopore induced by different sizes of crowding agents. J. Chem. Phys. 2013, 138, 204903. [Google Scholar] [CrossRef]
- de Haan, H.W.; Slater, G.W. Translocation of a polymer through a nanopore across a viscosity gradient. Phys. Rev. E 2013, 87, 042604. [Google Scholar] [CrossRef]
- Malgaretti, P.; Oshanin, G. Polymer Translocation Across a Corrugated Channel: Fick–Jacobs Approximation Extended Beyond the Mean First-Passage Time. Polymers 2019, 11, 251. [Google Scholar] [CrossRef]
- Tan, F.; Chen, Y.; Zhao, N. Effects of active crowder size and activity–crowding coupling on polymer translocation. Soft Matter 2021, 17, 1940–1954. [Google Scholar] [CrossRef]
- Khalilian, H.; Sarabadani, J.; Ala-Nissila, T. Polymer translocation in an environment of active rods. arXiv 2022, arXiv:2211.07114. [Google Scholar]
- Fazli, Z.; Naji, A. Rectification of polymer translocation through nanopores by chiral and nonchiral active particles. arXiv 2022, arXiv:2207.07628. [Google Scholar] [CrossRef] [PubMed]
- Kantor, Y.; Kardar, M. Anomalous dynamics of forced translocation. Phys. Rev. E 2004, 69, 021806. [Google Scholar] [CrossRef] [PubMed]
- Huopaniemi, I.; Luo, K.; Ala-Nissila, T.; Ying, S.C. Langevin dynamics simulations of polymer translocation through nanopores. J. Chem. Phys. 2006, 125, 124901. [Google Scholar] [CrossRef] [PubMed]
- Dubbeldam, J.; Milchev, A.; Rostiashvili, V.; Vilgis, T.A. Polymer translocation through a nanopore: A showcase of anomalous diffusion. Phys. Rev. E 2007, 76, 010801. [Google Scholar] [CrossRef]
- Dubbeldam, J.; Milchev, A.; Rostiashvili, V.; Vilgis, T.A. Driven polymer translocation through a nanopore: A manifestation of anomalous diffusion. EPL Europhys. Lett. 2007, 79, 18002. [Google Scholar] [CrossRef]
- Sakaue, T. Nonequilibrium dynamics of polymer translocation and straightening. Phys. Rev. E 2007, 76, 021803. [Google Scholar] [CrossRef]
- Vocks, H.; Panja, D.; Barkema, G.T.; Ball, R.C. Pore-blockade times for field-driven polymer translocation. J. Phys. Condens. Matter 2008, 20, 095224. [Google Scholar] [CrossRef]
- Gauthier, M.G.; Slater, G.W. A Monte Carlo algorithm to study polymer translocation through nanopores. II. Scaling laws. J. Chem. Phys. 2008, 128, 05B619. [Google Scholar] [CrossRef]
- Luo, K.; Ala-Nissila, T.; Ying, S.C.; Metzler, R. Driven polymer translocation through nanopores: Slow-vs.-Fast dynamics. EPL Europhys. Lett. 2010, 88, 68006. [Google Scholar] [CrossRef]
- Adhikari, R.; Bhattacharya, A. Driven translocation of a semi-flexible chain through a nanopore: A Brownian dynamics simulation study in two dimensions. J. Chem. Phys. 2013, 138, 204909. [Google Scholar] [CrossRef]
- Sakaue, T. Sucking genes into pores: Insight into driven translocation. Phys. Rev. E 2010, 81, 041808. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Sakaue, T. Dynamical diagram and scaling in polymer driven translocation. Eur. Phys. J. E 2011, 34, 135. [Google Scholar] [CrossRef] [PubMed]
- Katkar, H.H.; Muthukumar, M. Role of non-equilibrium conformations on driven polymer translocation. J. Chem. Phys. 2018, 148, 024903. [Google Scholar] [CrossRef]
- Saito, T.; Sakaue, T. Process time distribution of driven polymer transport. Phys. Rev. E 2012, 85, 061803. [Google Scholar] [CrossRef]
- Sakaue, T. Dynamics of polymer translocation: A short review with an introduction of weakly-driven regime. Polymers 2016, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Sarabadani, J.; Ala-Nissila, T. Theory of pore-driven and end-pulled polymer translocation dynamics through a nanopore: An overview. J. Physics: Condens. Matter 2018, 30, 274002. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; An, L.; Shi, A.C. Polymer Translocation Time. J. Phys. Chem. Lett. 2021, 12, 11534–11542. [Google Scholar] [CrossRef]
- Nikoofard, N.; Khalilian, H.; Fazli, H. Directed translocation of a flexible polymer through a cone-shaped nano-channel. J. Chem. Phys. 2013, 139, 074901. [Google Scholar] [CrossRef] [PubMed]
- Nikoofard, N.; Fazli, H. A flexible polymer confined inside a cone-shaped nano-channel. Soft Matter 2015, 11, 4879–4887. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Menard, L.D.; Panyukov, S.; Rubinstein, M.; Ramsey, J.M. Enhanced nanochannel translocation and localization of genomic DNA molecules using three-dimensional nanofunnels. Nat. Commun. 2017, 8, 807. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, K.; Singh, S.; Foster, D. Polymer in wedge-shaped confinement: Effect on the θ temperature. Phys. Rev. E 2020, 101, 030502. [Google Scholar] [CrossRef] [PubMed]
- Polson, J.M.; Heckbert, D.R. Polymer translocation into cavities: Effects of confinement geometry, crowding, and bending rigidity on the free energy. Phys. Rev. E 2019, 100, 012504. [Google Scholar] [CrossRef] [PubMed]
- Ambjörnsson, T.; Metzler, R. Chaperone-assisted translocation. Phys. Biol. 2004, 1, 77. [Google Scholar] [CrossRef]
- Yu, W.; Ma, Y.; Luo, K. Translocation of stiff polymers through a nanopore driven by binding particles. J. Chem. Phys. 2012, 137, 244905. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Luo, K. Chaperone-assisted translocation of a polymer through a nanopore. J. Am. Chem. Soc. 2011, 133, 13565–13570. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; Bhattacharya, A. Translocation of a semiflexible polymer through a nanopore in the presence of attractive binding particles. Phys. Rev. E 2015, 92, 032711. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, H.; Sarabadani, J.; Ala-Nissila, T. Polymer translocation through a nanopore assisted by an environment of active rods. Phys. Rev. Res. 2021, 3, 013080. [Google Scholar] [CrossRef]
- Grosberg, A.Y.; Nechaev, S.; Tamm, M.; Vasilyev, O. How long does it take to pull an ideal polymer into a small hole? Phys. Rev. Lett. 2006, 96, 228105. [Google Scholar] [CrossRef]
- Huopaniemi, I.; Luo, K.; Ala-Nissila, T.; Ying, S.C. Polymer translocation through a nanopore under a pulling force. Phys. Rev. E 2007, 75, 061912. [Google Scholar] [CrossRef]
- Panja, D.; Barkema, G.T. Passage times for polymer translocation pulled through a narrow pore. Biophys. J. 2008, 94, 1630–1637. [Google Scholar] [CrossRef]
- Sarabadani, J.; Ghosh, B.; Chaudhury, S.; Ala-Nissila, T. Dynamics of end-pulled polymer translocation through a nanopore. EPL Europhys. Lett. 2018, 120, 38004. [Google Scholar] [CrossRef]
- Menais, T. Polymer translocation under a pulling force: Scaling arguments and threshold forces. Phys. Rev. E 2018, 97, 022501. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.; Tatek, Y.B. End-Pulled Translocation of a Star Polymer out of a Confining Cylindrical Cavity. Macromol. Theory Simul. 2021, 30, 2000090. [Google Scholar] [CrossRef]
- He, Y.D.; Qian, H.J.; Lu, Z.Y.; Li, Z.S. Polymer translocation through a nanopore in mesoscopic simulations. Polymer 2007, 48, 3601–3606. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Deng, M.; Liang, H. Effects of electrostatic interactions on the translocation of polymers through a narrow pore under different solvent conditions: A dissipative particle dynamics simulation study. Macromol. Theory Simulations 2012, 21, 120–129. [Google Scholar] [CrossRef]
- Luo, M.B.; Tsehay, D.A.; Sun, L.Z. Temperature dependence of the translocation time of polymer through repulsive nanopores. J. Chem. Phys. 2017, 147, 034901. [Google Scholar] [CrossRef]
- Kwon, S.; Sung, B.J. Effects of solvent quality and non-equilibrium conformations on polymer translocation. J. Chem. Phys. 2018, 149, 244907. [Google Scholar] [CrossRef]
- Yang, K.; Vishnyakov, A.; Neimark, A.V. Polymer Translocation through a Nanopore: DPD Study. J. Phys. Chem. B 2013, 117, 3648–3658. [Google Scholar] [CrossRef]
- Moisio, J.E.; Piili, J.; Linna, R.P. Driven polymer translocation in good and bad solvent: Effects of hydrodynamics and tension propagation. Phys. Rev. E 2016, 94, 022501. [Google Scholar] [CrossRef]
- Luo, M.B.; Wu, F.; Zhang, S.; Sun, L.Z. Effect of temperature on the escape of charged polymer chain from a repulsive nanopore. Mol. Simul. 2019, 45, 1044–1050. [Google Scholar] [CrossRef]
- Kapahnke, F.; Schmidt, U.; Heermann, D.W.; Weiss, M. Polymer translocation through a nanopore: The effect of solvent conditions. J. Chem. Phys. 2010, 132, 164904. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Zhuo, B.Y.; Yang, X.; Luo, M.B. Simulation study for the pulling translocation of a polymer globule. Polym. J. 2021, 53, 1047–1056. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Liu, Y.; Liang, H. Flow-induced translocation of polymers through a fluidic channel: A dissipative particle dynamics simulation study. J. Chem. Phys. 2011, 134, 134906. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Binder, K. Out-of-equilibrium characteristics of a forced translocating chain through a nanopore. Phys. Rev. E 2010, 81, 041804. [Google Scholar] [CrossRef] [PubMed]
- Rowghanian, P.; Grosberg, A.Y. Force-driven polymer translocation through a nanopore: An old problem revisited. J. Phys. Chem. B 2011, 115, 14127–14135. [Google Scholar] [CrossRef]
- Ikonen, T.; Bhattacharya, A.; Ala-Nissila, T.; Sung, W. Influence of non-universal effects on dynamical scaling in driven polymer translocation. J. Chem. Phys. 2012, 137, 085101. [Google Scholar] [CrossRef]
- Sarabadani, J.; Ikonen, T.; Ala-Nissila, T. Iso-flux tension propagation theory of driven polymer translocation: The role of initial configurations. J. Chem. Phys. 2014, 141, 214907. [Google Scholar] [CrossRef]
- Ikonen, T.; Bhattacharya, A.; Ala-Nissila, T.; Sung, W. Influence of pore friction on the universal aspects of driven polymer translocation. EPL Europhys. Lett. 2013, 103, 38001. [Google Scholar] [CrossRef]
- Sarabadani, J.; Ikonen, T.; Ala-Nissila, T. Theory of polymer translocation through a flickering nanopore under an alternating driving force. J. Chem. Phys. 2015, 143, 074905. [Google Scholar] [CrossRef]
- Sarabadani, J.; Ikonen, T.; Mökkönen, H.; Ala-Nissila, T.; Carson, S.; Wanunu, M. Driven translocation of a semi-flexible polymer through a nanopore. Sci. Rep. 2017, 7, 7423. [Google Scholar] [CrossRef]
- Ghosh, B.; Sarabadani, J.; Chaudhury, S.; Ala-Nissila, T. Pulling a folded polymer through a nanopore. J. Phys. Condens. Matter 2020, 33, 015101. [Google Scholar] [CrossRef] [PubMed]
- Sarabadani, J.; Buyukdagli, S.; Ala-Nissila, T. Pulling a DNA molecule through a nanopore embedded in an anionic membrane: Tension propagation coupled to electrostatics. J. Phys. Condens. Matter 2020, 32, 385101. [Google Scholar] [CrossRef] [PubMed]
- Sarabadani, J.; Metzler, R.; Ala-Nissila, T. Driven polymer translocation into a channel: Isoflux tension propagation theory and Langevin dynamics simulations. Phys. Rev. Res. 2022, 4, 033003. [Google Scholar] [CrossRef]
- Bonet Avalos, J.; Rubi, J.; Bedeaux, D. Dynamics of rodlike polymers in dilute solution. Macromolecules 1993, 26, 2550–2561. [Google Scholar] [CrossRef]
- Chen, K.; Jou, I.; Ermann, N.; Muthukumar, M.; Keyser, U.F.; Bell, N.A.W. Dynamics of driven polymer transport through a nanopore. Nat. Phys. 2021, 17, 1043–1049. [Google Scholar] [CrossRef]
- Deamer, D.W.; Branton, D. Characterization of Nucleic Acids by Nanopore Analysis. Accounts Chem. Res. 2002, 35, 817–825. [Google Scholar] [CrossRef]
- Meller, A. Dynamics of polynucleotide transport through nanometre-scale pores. J. Phys. Condens. Matter 2003, 15, R581. [Google Scholar] [CrossRef]
- Olasagasti, F.; Lieberman, K.R.; Benner, S.; Cherf, G.M.; Dahl, J.M.; Deamer, D.W.; Akeson, M. Replication of individual DNA molecules under electronic control using a protein nanopore. Nat. Nanotechnol. 2010, 5, 798–806. [Google Scholar] [CrossRef]
- Mathé, J.; Aksimentiev, A.; Nelson, D.R.; Schulten, K.; Meller, A. Orientation discrimination of single-stranded DNA inside the α-hemolysin membrane channel. Proc. Natl. Acad. Sci. USA 2005, 102, 12377–12382. [Google Scholar] [CrossRef]
- Wanunu, M. Nanopores: A journey towards DNA sequencing. Phys. Life Rev. 2012, 9, 125–158. [Google Scholar] [CrossRef]
- Bhatti, H.; Jawed, R.; Ali, I.; Iqbal, K.; Han, Y.; Lu, Z.; Liu, Q. Recent advances in biological nanopores for nanopore sequencing, sensing and comparison of functional variations in MspA mutants. RSC Adv. 2021, 11, 28996–29014. [Google Scholar] [CrossRef] [PubMed]
- Agah, S.; Zheng, M.; Pasquali, M.; Kolomeisky, A.B. DNA sequencing by nanopores: Advances and challenges. J. Phys. D Appl. Phys. 2016, 49, 413001. [Google Scholar] [CrossRef]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-beam sculpting at nanometre length scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Storm, A.J.; Chen, J.H.; Ling, X.S.; Zandbergen, H.W.; Dekker, C. Fabrication of solid-state nanopores with single-nanometre precision. Nat. Mater. 2003, 2, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Kwok, H.; Briggs, K.; Tabard-Cossa, V. Nanopore Fabrication by Controlled Dielectric Breakdown. PLoS ONE 2014, 9, e92880. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, I.; Akahori, R.; Hatano, T.; Takeda, K.I. Fabricating nanopores with diameters of sub-1 nm to 3 nm using multilevel pulse-voltage injection. Sci. Rep. 2014, 4, 5000. [Google Scholar] [CrossRef] [PubMed]
- Waugh, M.; Briggs, K.; Gunn, D.; Gibeault, M.; King, S.; Ingram, Q.; Jimenez, A.M.; Berryman, S.; Lomovtsev, D.; Andrzejewski, L.; et al. Solid-state nanopore fabrication by automated controlled breakdown. Nat. Protoc. 2020, 15, 122–143. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yamazaki, H.; Ren, R.; Wanunu, M.; Ivanov, A.P.; Edel, J.B. Solid-state nanopore sensors. Nat. Rev. Mater. 2020, 5, 931–951. [Google Scholar] [CrossRef]
- Lee, K.; Park, K.B.; Kim, H.J.; Yu, J.S.; Chae, H.; Kim, H.M.; Kim, K.B. Recent Progress in Solid-State Nanopores. Adv. Mater. 2018, 30, 1704680. [Google Scholar] [CrossRef]
- Merchant, C.A.; Healy, K.; Wanunu, M.; Ray, V.; Peterman, N.; Bartel, J.; Fischbein, M.D.; Venta, K.; Luo, Z.; Johnson, A.T.C.; et al. DNA Translocation through Graphene Nanopores. Nano Lett. 2010, 10, 2915–2921. [Google Scholar] [CrossRef]
- Wanunu, M.; Morrison, W.; Rabin, Y.; Grosberg, A.Y.; Meller, A. Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient. Nat. Nanotechnol. 2010, 5, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, T.; Zrehen, A.; Girsault, A.; Meller, A. Optically-Monitored Nanopore Fabrication Using a Focused Laser Beam. Sci. Rep. 2018, 8, 9765. [Google Scholar] [CrossRef]
- Larkin, J.; Henley, R.; Bell, D.C.; Cohen-Karni, T.; Rosenstein, J.K.; Wanunu, M. Slow DNA Transport through Nanopores in Hafnium Oxide Membranes. ACS Nano 2013, 7, 10121–10128. [Google Scholar] [CrossRef]
- Danda, G.; Drndić, M. Two-dimensional nanopores and nanoporous membranes for ion and molecule transport. Curr. Opin. Biotechnol. 2019, 55, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Brilmayer, R.; Förster, C.; Zhao, L.; Andrieu-Brunsen, A. Recent trends in nanopore polymer functionalization. Curr. Opin. Biotechnol. 2020, 63, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Pardehkhorram, R.; Andrieu-Brunsen, A. Pushing the limits of nanopore transport performance by polymer functionalization. Chem. Commun. 2022, 58, 5188–5204. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.R.; Scott, A.; Rotem, D.; Mehta, K.K.; Bayley, H.; Dekker, C. Hybrid pore formation by directed insertion of α-haemolysin into solid-state nanopores. Nat. Nanotechnol. 2010, 5, 874–877. [Google Scholar] [CrossRef]
- Hernández-Ainsa, S.; Bell, N.A.W.; Thacker, V.V.; Göpfrich, K.; Misiunas, K.; Fuentes-Perez, M.E.; Moreno-Herrero, F.; Keyser, U.F. DNA Origami Nanopores for Controlling DNA Translocation. ACS Nano 2013, 7, 6024–6030. [Google Scholar] [CrossRef]
- Ding, T.; Yang, J.; Pan, V.; Zhao, N.; Lu, Z.; Ke, Y.; Zhang, C. DNA nanotechnology assisted nanopore-based analysis. Nucleic Acids Res. 2020, 48, 2791–2806. [Google Scholar] [CrossRef]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA Origami: Scaffolds for Creating Higher Order Structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef]
- Piskunen, P.; Nummelin, S.; Shen, B.; Kostiainen, M.A.; Linko, V. Increasing Complexity in Wireframe DNA Nanostructures. Molecules 2020, 25, 1823. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, M.; Singh, S.L.; Bharadwaj, A.S.; Kishore, V.; Singh, A.V. Self-Assembly of DNA-Grafted Colloids: A Review of Challenges. Micromachines 2022, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Cairns-Gibson, D.F.; Cockroft, S.L. Functionalised nanopores: Chemical and biological modifications. Chem. Sci. 2022, 13, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Fologea, D.; Uplinger, J.; Thomas, B.; McNabb, D.S.; Li, J. Slowing DNA translocation in a solid-state nanopore. Nano Lett. 2005, 5, 1734–1737. [Google Scholar] [CrossRef]
- Kowalczyk, S.W.; Wells, D.B.; Aksimentiev, A.; Dekker, C. Slowing down DNA Translocation through a Nanopore in Lithium Chloride. Nano Lett. 2012, 12, 1038–1044. [Google Scholar] [CrossRef]
- Liang, X.; Hu, Q.; Wang, Q.; Li, W.; Zhang, T.; Yang, S.; Sun, M.; Bleuel, M.; Yao, G. Changes in Nanoscale Pore Structures and Mesopore Connectivity as a Result of Artificial Maturation of a Source Rock from the Shanxi Formation, Ordos Basin. Energy Fuels 2022, 36, 9967–9981. [Google Scholar] [CrossRef]
- Luan, B.; Aksimentiev, A. Control and reversal of the electrophoretic force on DNA in a charged nanopore. J. Phys. Condens. Matter 2010, 22, 454123. [Google Scholar] [CrossRef]
- Wanunu, M.; Sutin, J.; McNally, B.; Chow, A.; Meller, A. DNA Translocation Governed by Interactions with Solid-State Nanopores. Biophys. J. 2008, 95, 4716–4725. [Google Scholar] [CrossRef]
- Zou, A.; Xiu, P.; Ou, X.; Zhou, R. Spontaneous Translocation of Single-Stranded DNA in Graphene–MoS2 Heterostructure Nanopores: Shape Effect. J. Phys. Chem. B 2020, 124, 9490–9496. [Google Scholar] [CrossRef]
- Karmi, A.; Sakala, G.P.; Rotem, D.; Reches, M.; Porath, D. Durable, Stable, and Functional Nanopores Decorated by Self-Assembled Dipeptides. ACS Appl. Mater. Interfaces 2020, 12, 14563–14568. [Google Scholar] [CrossRef]
- Carlsen, A.T.; Zahid, O.K.; Ruzicka, J.A.; Taylor, E.W.; Hall, A.R. Selective detection and quantification of modified DNA with solid-state nanopores. Nano Lett. 2014, 14, 5488–5492. [Google Scholar] [CrossRef]
- Ying, Y.L.; Li, D.W.; Li, Y.; Lee, J.S.; Long, Y.T. Enhanced translocation of poly (dt) 45 through an α-hemolysin nanopore by binding with antibody. Chem. Commun. 2011, 47, 5690–5692. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, D.V.; Jonsson, M.P.; Dekker, C. Temperature dependence of DNA translocations through solid-state nanopores. Nanotechnology 2015, 26, 234004. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Z.; Cao, W.P.; Luo, M.B. Free energy landscape for the translocation of polymer through an interacting pore. J. Chem. Phys. 2009, 131, 194904. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.N.; Muthukumar, M.; Meller, A. pH Tuning of DNA Translocation Time through Organically Functionalized Nanopores. ACS Nano 2013, 7, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Keyser, U.F.; Koeleman, B.N.; Van Dorp, S.; Krapf, D.; Smeets, R.M.; Lemay, S.G.; Dekker, N.H.; Dekker, C. Direct force measurements on DNA in a solid-state nanopore. Nat. Phys. 2006, 2, 473–477. [Google Scholar] [CrossRef]
- Keyser, U.F. Optical tweezers for mechanical control over DNA in a nanopore. In Nanopore-Based Technology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 115–134. [Google Scholar]
- Keyser, U.; Van der Does, J.; Dekker, C.; Dekker, N. Optical tweezers for force measurements on DNA in nanopores. Rev. Sci. Instruments 2006, 77, 105105. [Google Scholar] [CrossRef]
- Peng, H.; Ling, X.S. Reverse DNA translocation through a solid-state nanopore by magnetic tweezers. Nanotechnology 2009, 20, 185101. [Google Scholar] [CrossRef]
- Willmott, G.; Vogel, R.; Yu, S.; Groenewegen, L.; Roberts, G.; Kozak, D.; Anderson, W.; Trau, M. Use of tunable nanopore blockade rates to investigate colloidal dispersions. J. Phys. Condens. Matter 2010, 22, 454116. [Google Scholar] [CrossRef]
- Spiering, A.; Getfert, S.; Sischka, A.; Reimann, P.; Anselmetti, D. Nanopore translocation dynamics of a single DNA-bound protein. Nano Lett. 2011, 11, 2978–2982. [Google Scholar] [CrossRef]
- Bulushev, R.D.; Marion, S.; Radenovic, A. Relevance of the Drag Force during Controlled Translocation of a DNA–Protein Complex through a Glass Nanocapillary. Nano Lett. 2015, 15, 7118–7125. [Google Scholar] [CrossRef] [PubMed]
- Ristic, D.; Wyman, C.; Paulusma, C.; Kanaar, R. The architecture of the human Rad54–DNA complex provides evidence for protein translocation along DNA. Proc. Natl. Acad. Sci. USA 2001, 98, 8454–8460. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, B.; Sakai, H.; Davis, T.A.; Wiedmann, M. A protein complex required for signal-sequence-specific sorting and translocation. Nature 1994, 370, 434–440. [Google Scholar] [CrossRef]
- Bulushev, R.D.; Marion, S.; Petrova, E.; Davis, S.J.; Maerkl, S.J.; Radenovic, A. Single molecule localization and discrimination of dna–protein complexes by controlled translocation through nanocapillaries. Nano Lett. 2016, 16, 7882–7890. [Google Scholar] [CrossRef] [PubMed]
- van den Hout, M.; Vilfan, I.D.; Hage, S.; Dekker, N.H. Direct force measurements on double-stranded RNA in solid-state nanopores. Nano Lett. 2010, 10, 701–707. [Google Scholar] [CrossRef]
- Niknam Hamidabad, M.; Haji Abdolvahab, R. Translocation through a narrow pore under a pulling force. Sci. Rep. 2019, 9, 17885. [Google Scholar] [CrossRef]
- Hsiao, P.Y. Translocation of Charged Polymers through a Nanopore in Monovalent and Divalent Salt Solutions: A Scaling Study Exploring over the Entire Driving Force Regimes. Polymers 2018, 10, 1229. [Google Scholar] [CrossRef]
- de Haan, H.W.; Sean, D.; Slater, G.W. Reducing the variance in the translocation times by prestretching the polymer. Phys. Rev. E 2018, 98, 022501. [Google Scholar] [CrossRef]
- Fiasconaro, A.; Falo, F. Force spectroscopy analysis in polymer translocation. Phys. Rev. E 2018, 98, 062501. [Google Scholar] [CrossRef]
- Fiasconaro, A.; Diez-Senorans, G.; Falo, F. End-pulled polymer translocation through a many-body flexible pore. Polymer 2022, 259, 125305. [Google Scholar] [CrossRef]
- Akahori, R.; Yanagi, I.; Goto, Y.; Harada, K.; Yokoi, T.; Takeda, K.i. Discrimination of three types of homopolymers in single-stranded DNA with solid-state nanopores through external control of the DNA motion. Sci. Rep. 2017, 7, 9073. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.M.; Li, H.; Timp, G. Direct, concurrent measurements of the forces and currents affecting DNA in a nanopore with comparable topography. ACS Nano 2014, 8, 5484–5493. [Google Scholar] [CrossRef]
- Hyun, C.; Kaur, H.; Rollings, R.; Xiao, M.; Li, J. Threading immobilized DNA molecules through a solid-state nanopore at >100 µs per base rate. ACS Nano 2013, 7, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.F.; Kowalczyk, S.W.; Calado, V.E.; Pandraud, G.; Zandbergen, H.W.; Vandersypen, L.M.; Dekker, C. DNA translocation through graphene nanopores. Nano Lett. 2010, 10, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Mirsaidov, U.; Comer, J.; Dimitrov, V.; Aksimentiev, A.; Timp, G. Slowing the translocation of double-stranded DNA using a nanopore smaller than the double helix. Nanotechnology 2010, 21, 395501. [Google Scholar] [CrossRef]
- Comer, J.; Aksimentiev, A. DNA sequence-dependent ionic currents in ultra-small solid-state nanopores. Nanoscale 2016, 8, 9600–9613. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, D.; Zhao, Q. Solid-state nanopore-based DNA single molecule detection and sequencing. Microchim. Acta 2016, 183, 941–953. [Google Scholar] [CrossRef]
- Briggs, K.; Kwok, H.; Tabard-Cossa, V. Automated Fabrication of 2-nm Solid-State Nanopores for Nucleic Acid Analysis. Small 2014, 10, 2077–2086. [Google Scholar] [CrossRef]
- Duleba, D.; Dutta, P.; Denuga, S.; Johnson, R.P. Effect of Electrolyte Concentration and Pore Size on Ion Current Rectification Inversion. ACS Meas. Sci. 2022, 2, 271–277. [Google Scholar] [CrossRef]
- Carson, S.; Wilson, J.; Aksimentiev, A.; Wanunu, M. Smooth DNA transport through a narrowed pore geometry. Biophys. J. 2014, 107, 2381–2393. [Google Scholar] [CrossRef]
- Saha, K.K.; Drndic, M.; Nikolic, B.K. DNA base-specific modulation of microampere transverse edge currents through a metallic graphene nanoribbon with a nanopore. Nano Lett. 2012, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, K.; Bulushev, R.D.; Khlybov, S.; Dumcenco, D.; Kis, A.; Radenovic, A. Identification of single nucleotides in MoS2 nanopores. Nat. Nanotechnol. 2015, 10, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Shankla, M.; Aksimentiev, A. Molecular transport across the ionic liquid–aqueous electrolyte interface in a mos2 nanopore. ACS Appl. Mater. Interfaces 2020, 12, 26624–26634. [Google Scholar] [CrossRef]
- Kumawat, R.L.; Pathak, B. Identifying DNA Nucleotides via Transverse Electronic Transport in Atomically Thin Topologically Defected Graphene Electrodes. ACS Appl. Bio. Mater. 2020, 4, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Z.; Li, H.; Xu, X.; Luo, M.B. Simulation study on the translocation of polyelectrolyte through conical nanopores. J. Phys. Condens. Matter 2018, 30, 495101. [Google Scholar] [CrossRef]
- Sharma, A.; Kapri, R.; Chaudhuri, A. Driven translocation of a semiflexible polymer through a conical channel in the presence of attractive surface interactions. Sci. Rep. 2022, 12, 19081. [Google Scholar] [CrossRef]
- Fragasso, A.; Schmid, S.; Dekker, C. Comparing current noise in biological and solid-state nanopores. ACS Nano 2020, 14, 1338–1349. [Google Scholar] [CrossRef]
- Sun, L.Z.; Cao, W.P.; Wang, C.H.; Xu, X. The translocation dynamics of the polymer through a conical pore: Non-Stuck, weak-stuck, and strong-stuck modes. J. Chem. Phys. 2021, 154, 054903. [Google Scholar] [CrossRef]
- Krasilnikov, O.V.; Rodrigues, C.G.; Bezrukov, S.M. Single polymer molecules in a protein nanopore in the limit of a strong polymer-pore attraction. Phys. Rev. Lett. 2006, 97, 018301. [Google Scholar] [CrossRef]
- Luo, K.; Ala-Nissila, T.; Ying, S.C.; Bhattacharya, A. Influence of polymer-pore interactions on translocation. Phys. Rev. Lett. 2007, 99, 148102. [Google Scholar] [CrossRef]
- Luo, M.B. Translocation of polymer chains through interacting nanopores. Polymer 2007, 48, 7679–7686. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wang, C.; Zhou, Y.L.; Luo, M.B. Effect of attractive polymer-pore interactions on translocation dynamics. J. Chem. Phys. 2009, 130, 054902. [Google Scholar] [CrossRef] [PubMed]
- Piguet, F.; Foster, D. Translocation of short and long polymers through an interacting pore. J. Chem. Physics 2013, 138, 084902. [Google Scholar] [CrossRef]
- Chauhan, K.; Kumar, S. Dynamics of a polymer chain translocating through varying cone-shaped channels. Phys. Rev. E 2021, 103, 042501. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhuri, A.; Kapri, R. Driven translocation of a flexible polymer through an interacting conical pore. arXiv 2021, arXiv:2102.08067. [Google Scholar]
- Jung, S.W.; Kim, H.S.; Cho, A.E.; Kim, Y.H. Nitrogen doping of carbon nanoelectrodes for enhanced control of DNA translocation dynamics. ACS Appl. Mater. Interfaces 2018, 10, 18227–18236. [Google Scholar] [CrossRef]
- Si, W.; Zhang, Y.; Sha, J.; Chen, Y. Controllable and reversible DNA translocation through a single-layer molybdenum disulfide nanopore. Nanoscale 2018, 10, 19450–19458. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Matsui, K.; Yanagi, I.; Takeda, K.I. Silicon nitride nanopore created by dielectric breakdown with a divalent cation: Deceleration of translocation speed and identification of single nucleotides. Nanoscale 2019, 11, 14426–14433. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Kang, J.; Lee, T.G.; Kim, H.M.; Kim, K.B. Surface modification of solid-state nanopore by plasma-polymerized chemical vapor deposition of poly (ethylene glycol) for stable device operation. Nanotechnology 2020, 31, 185503. [Google Scholar] [CrossRef]
- Wei, G.; Hu, R.; Li, Q.; Lu, W.; Liang, H.; Nan, H.; Lu, J.; Li, J.; Zhao, Q. Oligonucleotide Discrimination Enabled by Tannic Acid-Coordinated Film-Coated Solid-State Nanopores. Langmuir 2022, 38, 6443–6453. [Google Scholar] [CrossRef]
- Yu, Y.s.; Lu, X.; Ding, H.M.; Ma, Y.Q. Computational investigation on DNA sequencing using functionalized graphene nanopores. Phys. Chem. Chem. Phys. 2018, 20, 9063–9069. [Google Scholar] [CrossRef]
- Barati Farimani, A.; Dibaeinia, P.; Aluru, N.R. DNA origami–graphene hybrid nanopore for DNA detection. ACS Appl. Mater. Interfaces 2017, 9, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Restrepo, M.; Mikhailova, E.; Bayley, H.; Maglia, G. Controlled translocation of individual DNA molecules through protein nanopores with engineered molecular brakes. Nano Lett. 2011, 11, 746–750. [Google Scholar] [CrossRef]
- Wong, C.T.A.; Muthukumar, M. Polymer translocation through α-hemolysin pore with tunable polymer-pore electrostatic interaction. J. Chem. Phys. 2010, 133, 07B607. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.J.; Muthukumar, M. Electrostatic control of polymer translocation speed through α-hemolysin protein pore. Macromolecules 2016, 49, 9132–9138. [Google Scholar] [CrossRef]
- Cohen, J.A.; Chaudhuri, A.; Golestanian, R. Stochastic sensing of polynucleotides using patterned nanopores. Phys. Rev. X 2012, 2, 021002. [Google Scholar] [CrossRef]
- Katkar, H.; Muthukumar, M. Effect of charge patterns along a solid-state nanopore on polyelectrolyte translocation. J. Chem. Phys. 2014, 140, 04B602_1. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.C.; Wu, F.; Luo, M.B. Simulation on the translocation of homopolymers through sandwich-like compound channels. J. Chem. Phys. 2015, 143, 12B632_1. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.C.; Zhang, S.; Luo, M.B. Translocation of diblock copolymer through compound channels: A Monte Carlo simulation study. Macromolecules 2014, 47, 7215–7220. [Google Scholar] [CrossRef]
- Orekhov, P.S.; Kholina, E.G.; Bozdaganyan, M.E.; Nesterenko, A.M.; Kovalenko, I.B.; Strakhovskaya, M.G. Molecular mechanism of uptake of cationic photoantimicrobial phthalocyanine across bacterial membranes revealed by molecular dynamics simulations. J. Phys. Chem. B 2018, 122, 3711–3722. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhuri, A.; Kapri, R. Sequencing of semiflexible polymers of varying bending rigidity using patterned pores. J. Chem. Phys. 2018, 148, 164901. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.L.; Sun, L.Z.; Chen, Y.C.; Luo, M.B. Simulation study on the migration of diblock copolymers in periodically patterned slits. J. Chem. Phys. 2019, 150, 164904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, Y.; Dunphy, D.R.; Adams, D.P.; Hodges, C.; Liu, N.; Zhang, N.; Xomeritakis, G.; Jin, X.; Aluru, N.; et al. DNA translocation through an array of kinked nanopores. Nat. Mater. 2010, 9, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Luo, K. Dynamics of polymer translocation through kinked nanopores. J. Chem. Phys. 2015, 142, 084901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, W.; Luo, K. Surface-adsorption-induced polymer translocation through a nanopore: Effects of the adsorption strength and the surface corrugation. Phys. Rev. E 2015, 92, 022603. [Google Scholar] [CrossRef] [PubMed]
- Bianco, V.; Malgaretti, P. Non-monotonous polymer translocation time across corrugated channels: Comparison between Fick-Jacobs approximation and numerical simulations. J. Chem. Phys. 2016, 145, 114904. [Google Scholar] [CrossRef]
- Nagarajan, K.; Chen, S.B. Polyelectrolyte translocation through a tortuous nanopore. J. Phys. Chem. B 2019, 123, 9031–9037. [Google Scholar] [CrossRef]
- Simon, S.M.; Peskin, C.S.; Oster, G.F. What drives the translocation of proteins? Proc. Natl. Acad. Sci. USA 1992, 89, 3770–3774. [Google Scholar] [CrossRef]
- Neupert, W.; Brunner, M. The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 2002, 3, 555–565. [Google Scholar] [CrossRef]
- Zandi, R.; Reguera, D.; Rudnick, J.; Gelbart, W.M. What drives the translocation of stiff chains? Proc. Natl. Acad. Sci. USA 2003, 100, 8649–8653. [Google Scholar] [CrossRef]
- Abdolvahab, R.H. Non-equilibrium effects in chaperone-assisted translocation of a stiff polymer. Phys. Lett. A 2018, 382, 162–167. [Google Scholar] [CrossRef]
- Pu, M.; Jiang, H.; Hou, Z. Polymer translocation through nanopore into active bath. J. Chem. Phys. 2016, 145, 174902. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Luo, K. Polymer translocation through a nanopore driven by binding particles: Influence of chain rigidity. Phys. Rev. E 2014, 90, 042708. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.C. Translocation of heterogeneous flexible polymers assisted by binding particles. Chin. J. Polym. Sci. 2020, 38, 784–790. [Google Scholar] [CrossRef]
- Molcrette, B.; Chazot-Franguiadakis, L.; Liénard, F.; Balassy, Z.; Freton, C.; Grangeasse, C.; Montel, F. Experimental study of a nanoscale translocation ratchet. Proc. Natl. Acad. Sci. USA 2022, 119, e2202527119. [Google Scholar] [CrossRef]
- Fulton, A.B. How crowded is the cytoplasm? Cell 1982, 30, 345–347. [Google Scholar] [CrossRef]
- Gopinathan, A.; Kim, Y.W. Polymer translocation in crowded environments. Phys. Rev. Lett. 2007, 99, 228106. [Google Scholar] [CrossRef]
- Cao, W.P.; Sun, L.Z.; Wang, C.; Luo, M.B. Monte Carlo simulation on polymer translocation in crowded environment. J. Chem. Phys. 2011, 135, 174901. [Google Scholar] [CrossRef]
- Dubbeldam, J.L.; Rostiashvili, V.G.; Vilgis, T.A. Driven translocation of a polymer: Role of pore friction and crowding. J. Chem. Phys. 2014, 141, 124112. [Google Scholar] [CrossRef]
- Gao, Q.C.; Li, Z.Y.; Xu, Y.W.; Guo, C.; Hou, J.X. Polymer translocation of linear polymer and ring polymer influenced by crowding. Mod. Phys. Lett. B 2019, 33, 1950318. [Google Scholar] [CrossRef]
- Chau, C.C.; Radford, S.E.; Hewitt, E.W.; Actis, P. Macromolecular crowding enhances the detection of DNA and proteins by a solid-state nanopore. Nano Lett. 2020, 20, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Hamidabad, M.N.; Asgari, S.; Abdolvahab, R.H. Nanoparticle-assisted polymer translocation through a nanopore. Polymer 2020, 204, 122847. [Google Scholar] [CrossRef]
- Umeta, D.T.; Asfaw, S.N.; Didu, S.H.; Feyisa, C.G.; Feyisa, D.K. Monte Carlo Simulation of Static and Dynamic Properties of Linear Polymer in a Crowded Environment. Adv. Polym. Technol. 2022, 2022. [Google Scholar] [CrossRef]
- Squires, A.H.; Hersey, J.S.; Grinstaff, M.W.; Meller, A. A nanopore–nanofiber mesh biosensor to control DNA translocation. J. Am. Chem. Soc. 2013, 135, 16304–16307. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Haga, T.; Yanagi, I.; Yokoi, T.; Takeda, K.I. Deceleration of single-stranded DNA passing through a nanopore using a nanometre-sized bead structure. Sci. Rep. 2015, 5, 16640. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Jiang, A.; Kuo, C.; Nazarian, R.; Li, K.; Ma, A.; Siegal, B.; Toh, C.; Schmidt, J.J. Improved measurement of proteins using a solid-state nanopore coupled with a hydrogel. ACS Sens. 2020, 5, 370–376. [Google Scholar] [CrossRef]
- Uplinger, J.; Thomas, B.; Rollings, R.; Fologea, D.; McNabb, D.; Li, J. K+, Na+, and Mg2+ on DNA translocation in silicon nitride nanopores. Electrophoresis 2012, 33, 3448–3457. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Sha, J.; Ni, Z.; Yi, H.; Chen, Y. Nanopore detection of DNA molecules in magnesium chloride solutions. Nanoscale Res. Lett. 2013, 8, 245. [Google Scholar] [CrossRef]
- Hsiao, P.Y. Translocation of a Polyelectrolyte through a Nanopore in the Presence of Trivalent Counterions: A Comparison with the Cases in Monovalent and Divalent Salt Solutions. ACS Omega 2020, 5, 19805–19819. [Google Scholar] [CrossRef]
- Bloomfield, V.A. DNA condensation by multivalent cations. Biopolym. Orig. Res. Biomol. 1997, 44, 269–282. [Google Scholar] [CrossRef]
- Luan, B.; Aksimentiev, A. DNA attraction in monovalent and divalent electrolytes. J. Am. Chem. Soc. 2008, 130, 15754–15755. [Google Scholar] [CrossRef] [PubMed]
- Hatlo, M.M.; Panja, D.; van Roij, R. Translocation of DNA molecules through nanopores with salt gradients: The role of osmotic flow. Phys. Rev. Lett. 2011, 107, 068101. [Google Scholar] [CrossRef]
- Bello, J.; Mowla, M.; Troise, N.; Soyring, J.; Borgesi, J.; Shim, J. Increased dwell time and occurrence of dsDNA translocation events through solid state nanopores by LiCl concentration gradients. Electrophoresis 2019, 40, 1082–1090. [Google Scholar] [CrossRef]
- Le, A.T.; Krylova, S.M.; Krylov, S.N. Ideal-filter capillary electrophoresis: A highly efficient partitioning method for selection of protein binders from oligonucleotide libraries. Electrophoresis 2019, 40, 2553–2564. [Google Scholar] [CrossRef]
- Vu, T.; Borgesi, J.; Soyring, J.; D’Alia, M.; Davidson, S.L.; Shim, J. Employing LiCl salt gradient in the wild-type α-hemolysin nanopore to slow down DNA translocation and detect methylated cytosine. Nanoscale 2019, 11, 10536–10545. [Google Scholar] [CrossRef]
- Charron, M.; Philipp, L.; He, L.; Tabard-Cossa, V. Elucidating the dynamics of polymer transport through nanopores using asymmetric salt concentrations. Nano Res. 2022, 15, 9943–9953. [Google Scholar] [CrossRef]
- Sakaue, T.; Saito, T.; Wada, H. Dragging a polymer in a viscous fluid: Steady state and transient. Phys. Rev. E 2012, 86, 011804. [Google Scholar] [CrossRef]
- Hu, H.X.; Wu, F.; Yang, X.; Wang, C.; Luo, M.B. Effect of Solvent Viscosity on the Driven Translocation of Charged Polymers through Nanopores. Chin. J. Polym. Sci. 2022, 40, 532–540. [Google Scholar] [CrossRef]
- Yeh, L.H.; Zhang, M.; Joo, S.W.; Qian, S. Slowing down DNA translocation through a nanopore by lowering fluid temperature. Electrophoresis 2012, 33, 3458–3465. [Google Scholar] [CrossRef]
- Payet, L.; Martinho, M.; Merstorf, C.; Pastoriza-Gallego, M.; Pelta, J.; Viasnoff, V.; Auvray, L.; Muthukumar, M.; Mathé, J. Temperature effect on ionic current and ssDNA transport through nanopores. Biophys. J. 2015, 109, 1600–1607. [Google Scholar] [CrossRef]
- He, Y.; Tsutsui, M.; Scheicher, R.H.; Bai, F.; Taniguchi, M.; Kawai, T. Thermophoretic manipulation of DNA translocation through nanopores. ACS Nano 2013, 7, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, G.; Ma, J.; Yuan, Z.; Si, W.; Liu, L.; Sha, J.; Chen, Y. Temperature effect on translocation speed and capture rate of nanopore-based DNA detection. Sci. China Technol. Sci. 2015, 58, 519–525. [Google Scholar] [CrossRef]
- Belkin, M.; Aksimentiev, A. Molecular dynamics simulation of DNA capture and transport in heated nanopores. ACS Appl. Mater. Interfaces 2016, 8, 12599–12608. [Google Scholar] [CrossRef]
- Stein, D.; Deurvorst, Z.; van der Heyden, F.H.; Koopmans, W.J.; Gabel, A.; Dekker, C. Electrokinetic concentration of DNA polymers in nanofluidic channels. Nano Lett. 2010, 10, 765–772. [Google Scholar] [CrossRef]
- Wang, C.; Sensale, S.; Pan, Z.; Senapati, S.; Chang, H.C. Slowing down DNA translocation through solid-state nanopores by edge-field leakage. Nature Commun. 2021, 12, 140. [Google Scholar] [CrossRef]
- Bezrukov, S.M.; Vodyanoy, I.; Parsegian, V.A. Counting polymers moving through a single ion channel. Nature 1994, 370, 279–281. [Google Scholar] [CrossRef]
- Buyukdagli, S.; Sarabadani, J.; Ala-Nissila, T. Theoretical modeling of polymer translocation: From the electrohydrodynamics of short polymers to the fluctuating long polymers. Polymers 2019, 11, 118. [Google Scholar] [CrossRef]
- Bell, N.A.; Chen, K.; Ghosal, S.; Ricci, M.; Keyser, U.F. Asymmetric dynamics of DNA entering and exiting a strongly confining nanopore. Nature Commun. 2017, 8, 380. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.L.; Chauhan, K.; Bharadwaj, A.S.; Kishore, V.; Laux, P.; Luch, A.; Singh, A.V. Polymer Translocation and Nanopore Sequencing: A Review of Advances and Challenges. Int. J. Mol. Sci. 2023, 24, 6153. https://doi.org/10.3390/ijms24076153
Singh SL, Chauhan K, Bharadwaj AS, Kishore V, Laux P, Luch A, Singh AV. Polymer Translocation and Nanopore Sequencing: A Review of Advances and Challenges. International Journal of Molecular Sciences. 2023; 24(7):6153. https://doi.org/10.3390/ijms24076153
Chicago/Turabian StyleSingh, Swarn Lata, Keerti Chauhan, Atul S. Bharadwaj, Vimal Kishore, Peter Laux, Andreas Luch, and Ajay Vikram Singh. 2023. "Polymer Translocation and Nanopore Sequencing: A Review of Advances and Challenges" International Journal of Molecular Sciences 24, no. 7: 6153. https://doi.org/10.3390/ijms24076153
APA StyleSingh, S. L., Chauhan, K., Bharadwaj, A. S., Kishore, V., Laux, P., Luch, A., & Singh, A. V. (2023). Polymer Translocation and Nanopore Sequencing: A Review of Advances and Challenges. International Journal of Molecular Sciences, 24(7), 6153. https://doi.org/10.3390/ijms24076153






