Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of K. pinnata Extract
4.2. Determination of Chemical Composition of K. pinnata
4.3. Preparation of Metformin Solutions
4.4. Combinatorial K. pinnata and Metformin Preparations
4.5. Preparation of Cultured Human Skeletal Muscle Cells
4.6. Induction of Oxidative Stress on HSMM Cell Culture
4.7. Determination of Cell Concentration
4.8. Measurement of Superoxide Dismutase Activity
4.9. Measurement of Catalase Activity
4.10. Determination of Reduced Glutathione
4.11. Determination of Malondialdehyde (MDA)
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health. Diabetes. 2017. Available online: https://medlineplus.gov/diabetes.html (accessed on 14 October 2017).
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Med. 2017, 130, S40–S50. [Google Scholar] [CrossRef]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Matsumoto, K.; Nishikawa, T.; Suefuji, M.; Nakamaru, K.; Hirashima, Y.; Kawashima, J.; Kawashima, T.; Ichinose, K.; Brownlee, M.; et al. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem. Biophys. Res. Commun. 2003, 300, 216–222. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.F.H.; Klein, H.H. The treatment of type 2 diabetes. Dtsch. Arztebl. Int. 2014, 111, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Cawich, S.O.; Harnarayan, P.; Budhooram, S.; Bobb, N.J.; Islam, S.; Naraynsingh, V. Wonder of Life (Kalanchoe pinnata) leaves to treat diabetic foot infections in Trinidad & Tobago: A case control study. Trop. Dr. 2014, 44, 209–213. [Google Scholar] [CrossRef]
- Joseph, B.; Sridhar, S.; Sankarganesh, J.; Edwin, B.T. Rare Medicinal Plant-Kalanchoe pinnata. Res. J. Microbiol. 2011, 6, 322–327. [Google Scholar] [CrossRef]
- Pattewar, S.V. Kalanchoe pinnata: Phytochemical and Pharmacological Profile. Int. J. Phytopharm. 2012, 2, 993–1000. [Google Scholar] [CrossRef]
- Uchegbu, R.I.; Ahuchaogu, A.A.; Amanze, K.O.; Ibe, C.O. Chemical Constituents Analysis of the Leaves of Bryophyllum pinnatum by GC-MS. AASCIT J. Chem. 2017, 3, 19–22. [Google Scholar]
- Eruygur, N.; Ucar, E.; Ataş, M.; Ergul, M.; Ergul, M.; Sozmen, F. Determination of biological activity of Tragopogon porrifolius and Polygonum cognatum consumed intensively by people in Sivas. Toxicol. Rep. 2019, 7, 59–66. [Google Scholar] [CrossRef]
- El-Hamamsy, M.H.R.I. Potential Antimycobacterial Agents Targeting Dihydrofolate Reductase; 2005; Available from ProQuest Dissertations & Theses Global. (301702923); Available online: https://manowar.tamucc.edu/login?url=https://www.proquest.com/dissertations-theses/potential-antimycobacterial-agent-targeting/docview/301702923/se-2 (accessed on 15 June 2022).
- El-Hamamsy, M.H.R.I.; Smith, A.W.; Thompson, A.S.; Threadgill, M.D. Structure-based design, synthesis and preliminary evaluation of selective inhibitors of dihydrofolate reductase from Mycobacterium tuberculosis. Bioorg. Med. Chem. 2007, 15, 4552–4576. [Google Scholar] [CrossRef] [PubMed]
- Ramon, P.; Sparks, J.; Omoruyi, F. Effect of Combined K. pinnata and Metformin Preparation on Inflammatory Cytokines in Normal and Diabetic Skeletal Muscle Cells. J. Med. Food 2021, 24, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V.K. Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract and its validation using a computational simulation approach. Inform. Med. Unlocked 2019, 14, 6–14. [Google Scholar] [CrossRef]
- Hernández-Caballero, M.E.; Sierra-Ramírez, J.A.; Villalobos-Valencia, R.; Seseña-Méndez, E. Potential of Kalanchoe pinnata as a Cancer Treatment Adjuvant and an Epigenetic Regulator. Molecules 2022, 27, 6425. [Google Scholar] [CrossRef]
- Sajan, M.P.; Bandyopadhyay, G.; Miura, A.; Standaert, M.L.; Nimal, S.; Longnus, S.L.; Van Obberghen, E.; Hainault, I.; Foufelle, F.; Kahn, R.; et al. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E179–E192. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2021, 146, 112560. [Google Scholar] [CrossRef]
- A Complete Guide to EGCG. Available online: https://compoundingrxusa.com/blog/your-guide-to-egcg-epigallocatechin-3-gallate/ (accessed on 21 June 2022).
- Forester, S.C.; Gu, Y.; Lambert, J.D. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol. Nutr. Food Res. 2012, 56, 1647–1654. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, C.J.; Huang, L.H.; Chen, I.J.; Chiu, J.P.; Hsu, C.H. Effects of Green Tea Extract on Insulin Resistance and Glucagon-Like Peptide 1 in Patients with Type 2 Diabetes and Lipid Abnormalities: A Randomized, Double-Blinded, and Placebo-Controlled Trial. PLoS ONE 2014, 9, e91163. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, A.Y.; Quilantang, N.G.; Geraldino, P.J.L.; Cho, E.J.; Lee, S. Anti-oxidant activity of avicularin and isovitexin from Lespedeza cuneate. J. Appl. Biol. Chem. 2019, 62, 143–147. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Chen, S.; Wu, Q. Avicularin inhibits cell proliferation and induces cell apoptosis in cutaneous squamous cell carcinoma. Exp. Ther. Med. 2019, 19, 1065–1071. [Google Scholar] [CrossRef]
- Zhu, X.; Ouyang, W.; Miao, J.; Xiong, P.; Feng, K.; Li, M.; Cao, Y.; Xiao, H. Dietary Avicularin Alleviated Type 2 Diabetes in Mice. FASEB J. 2017, 31, 46.7. [Google Scholar] [CrossRef]
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015, 28, 744–750. [Google Scholar] [CrossRef]
- de Araújo, E.; Guerra, G.; Araújo, D.; de Araújo, A.; Fernandes, J.; Júnior, R.D.A.; da Silva, V.; de Carvalho, T.; Ferreira, L.; Zucolotto, S. Gastroprotective and Antioxidant Activity of Kalanchoe brasiliensis and Kalanchoe pinnata Leaf Juices against Indomethacin and Ethanol-Induced Gastric Lesions in Rats. Int. J. Mol. Sci. 2018, 19, 1265. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Choi, E.-M.; Kwon, M.; Chon, S.; Oh, S.; Woo, J.-T.; Kim, S.W.; Kim, J.-W.; Kim, Y.S. Kaempferol attenuates 2-deoxy-d-ribose-induced oxidative cell damage in MC3T3-E1 osteoblastic cells. Biol. Pharm. Bull. 2009, 32, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Bolaños, J.P. Glutathione and γ-glutamylcysteine in hydrogen peroxide detoxification. Methods Enzym. 2013, 527, 129–144. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox Control of Skeletal Muscle Regeneration. Antioxid. Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef]
- Chukwunonso Obi, B.; Chinwuba Okoye, T.; Okpashi, V.E.; Nonye Igwe, C.; Olisah Alumanah, E. Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J. Diabetes Res. 2016, 2016, 1635361. [Google Scholar] [CrossRef]
- Menon, N.; Sparks, J.; Omoruyi, F.O. Oxidative Stress Parameters and Erythrocyte Membrane Adenosine Triphosphatase Activities in Streptozotocin-induced Diabetic Rats Administered Aqueous Preparation of Kalanchoe pinnata Leaves. Pharmacogn. Res. 2016, 8, 85–88. [Google Scholar] [CrossRef]
- Phung, C.D.; Ezieme, J.A.; Turrens, J.F. Hydrogen peroxide metabolism in skeletal muscle mitochondria. Arch. Biochem. Biophys. 1994, 315, 479–482. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Cohen, G.; Hochstein, P. Glutathione Peroxidase: The Primary Agent for the Elimination of Hydrogen Peroxide in Erythrocytes. Biochemistry 1963, 2, 1420–1428. [Google Scholar] [CrossRef]
- Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules 2021, 26, 5041. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M. The diverse benefits of glutathione: A key antioxidant for reversing chronic illness. Altern. Complement. Ther. 2005, 11, 241–245. [Google Scholar] [CrossRef]
- De Mattia, G.; Bravi, M.; Laurenti, O.; Cassone-Faldetta, M.; Armiento, A.; Ferri, C.; Balsano, F. Influence of reduced glutathione infusion on glucose metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism 1998, 47, 993–997. [Google Scholar] [CrossRef]
- Njålsson, R.; Norgren, S. Physiological and pathological aspects of GSH metabolism. Acta Paediatr. Int. J. Paediatr. 2005, 94, 132–137. [Google Scholar] [CrossRef]
- Harlalka, G.; Patil, C.; Patil, M. Protective effect of Kalanchoe pinnata pers. (Crassulaceae) on gentamicin-induced nephrotoxicity in rats. Indian J. Pharmacol. 2007, 39, 201. [Google Scholar] [CrossRef]
- Aiyegoro, O.A.; Okoh, A.I. Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef]
- Check Out This Tea Brewing Temperature Guide. Available online: https://www.thespruceeats.com/tea-brewing-temperature-guide-766367 (accessed on 3 March 2023).
- Miranda, A.F.; Nette, E.G.; Khan, S.; Brockbank, K.; Schonberg, M. Alteration of myoblast phenotype by dimethyl sulfoxide. Proc. Natl. Acad. Sci. USA 1978, 75, 3826–3830. [Google Scholar] [CrossRef]
- Klaren, W.D.; Rusyn, I. High-Content Assay Multiplexing for Muscle Toxicity Screening in Human-Induced Pluripotent Stem Cell-Derived Skeletal Myoblasts. ASSAY Drug Dev. Technol. 2018, 16, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Wageesha, N.D.A. ResearchGate. 2014. Available online: https://www.researchgate.net/post/What_must_be_the_maximum_final_DMSO_in_a_cell_culture_plate_24_well_plate_if_I_have_to_dissolve_my_compound_in_DMSO (accessed on 8 March 2020).
- Chien, S.-W.; Kuo, D.-Y.; Liao, J.-M.; Wang, P.S.; Yu, C.-H. Growth Modulation of Diabetic Factors and Antidiabetic Drugs on Prostate Cancer Cell Lines. Chin. J. Physiol. 2016, 59, 109–118. [Google Scholar] [CrossRef]
- Yue, W.; Zheng, X.; Lin, Y.; Yang, C.S.; Xu, Q.; Carpizo, D.; Huang, H.; DiPaola, R.S.; Tan, X.-L. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget 2015, 6, 21208–21224. [Google Scholar] [CrossRef]
- Śmieszek, A.; Czyrek, A.; Basinska, K.; Trynda, J.; Skaradzińska, A.; Siudzińska, A.; Marędziak, M.; Marycz, K. Effect of Metformin on Viability, Morphology, and Ultrastructure of Mouse Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells and Balb/3T3 Embryonic Fibroblast Cell Line. BioMed Res. Int. 2015, 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xia, D.; Pan, Z.; Xu, D.; Zhou, Y.; Wu, Y.; Cai, N.; Tang, Q.; Wang, C.; Yan, M.; et al. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis. 2016, 7, e2441. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wondisford, F.E. Metformin action: Concentrations matter. Cell Metab. 2015, 21, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chowdhury, A.; Das, J.; Karmakar, U.K.; Shill, M.C. Assessment of cytotoxicity and antibacterial activities of ethanolic extracts of Kalanchoe pinnata Lin. (Family: Crassulacease) leaves and stems. Int. J. Pharm. Sci. Res. 2011, 15, 2605–2609. [Google Scholar] [CrossRef]
- Chowdhury, K.; Huq, M.; Ali, M.; Huq, I.; Royhan, M.; Adnan, M.; Chy, M.N.U.; Kabir, M.I.; Auniq, R.B.J.; Uddin, M.R.; et al. Antioxidant, cytotoxic and thrombolytic activity of leaves of Kalanchoe pinnata (LAM.) PERS. J. Pharmacogn. Phytochem. 2016, 5, 309–315. [Google Scholar]
- Waititu, K.; Jerono, C.; Kituku, D.; Nzuve, M.; Mambo, F.; Ngugi, P.; Mwethera, P. Phytochemical Composition of Kalanchoe pinnata and Bidens pilosa Leaves Associated with Management of Diabetes. Biomed. Biotechnol. 2018, 6, 15–20. [Google Scholar] [CrossRef]
- Cayman Chemical. Superoxide Dismutase Assay Kit; Cayman Chemical: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Nguyen, K.; Sparks, J.; Omoruyi, F.O. Investigation of the cytotoxicity, antioxidative and immune-modulatory effects of Ligusticum porteri (Osha) root extract on human peripheral blood lymphocytes. J. Integr. Med. 2016, 14, 465–472. [Google Scholar] [CrossRef]
- Abcam. Lipid Peroxidation (MDA) Assay Kit (Colorimetric/Fluorometric); Abcam: Cambridge, UK, 2016. [Google Scholar]
- R&D Systems Inc. TBARS Assay; R&D Systems Inc.: Minneapolis, MN, USA, 2013. [Google Scholar]
- Stanbio Total Protein. Stanbio Laboratory: Boerne, TX, USA, 2017. Available online: http://www.vitroscience.cl/pdf/stanbio/Proteina_Total_(LCR_Orina).pdf (accessed on 15 June 2017).

| Treatment | Day 1 (24 h) | Day 3 (72 h) | |||||
|---|---|---|---|---|---|---|---|
| Metformin (µm) | K. pinnata (µg/mL) | HSMM Cell Viability (% of Control) | DHSMM Cell Viability (% of Control) | Stress-Induced Cell Viability (% of Control) | HSMM Cell Viability (% of Control) | DHSMM Cell Viability (% of Control) | Stress-Induced Cell Viability (% of Control) |
| 5000 | 0 | 98.6 ± 0.907 Aa | 99.27 ± 2.78 Aa | 97.90 ± 0.004 Aa | 86.92 ± 0.242 Aa | 103.90 ± 0.007 Ba | 75.22 ± 0.081 Ca |
| 200 | 25 | 97.49 ± 0.907 Aa | 98.9 ± 2.78 Aa | 100.95 ± 0.068 Ab | 104.93 ± 0.015 Ac | 104.95 ± 0.006 Aa | 63.47 ± 0.048 Bc |
| 150 | 50 | 98.05 ± 0.907 Aa | 94.9 ± 2.78 Aa | 100.66 ± 0.03 Ab | 88.54 ± 0.104 Aa | 104.66 ± 0.006 Ba | 63.25 ± 0.078 Cc |
| 100 | 100 | 97.02 ± 0.907 Aa | 94.9 ± 2.78 Aa | 101.02 ± 0.15 Ab | 100.13 ± 0.143 Ac | 105.02 ± 0.002 Ba | 58.02 ± 0.067 Cc |
| 50 | 150 | 95.76 ± 0.907 Aa | 96.5 ± 2.78 Aa | 96.48 ± 0.016 Ac | 99.34 ± 0.156 Ac | 101.48 ± 0.007 Bb | 48.91 ± 0.125 Cd |
| 30 | 200 | 96.39 ± 0.907 Aa | 94.7 ± 2.78 Aa | 95.10 ± 0.045 Ac | 98.10 ± 0.166 Ac | 102.10 ± 0.006 Bb | 44.65 ± 0.048 Cd |
| 10 | 300 | 94.12 ± 0.907 Aa | 80 ± 2.78 Ab | 95.67 ± 0.046 Ac | 82.25 ± 0.315 Ab | 102.67 ± 0.005 Bb | 40.19 ± 0.10 Cd |
| 0 | 400 | 90.7 ± 0.907 Ab | 80 ± 2.78 Ab | 99.82 ± 0.042 Aa | 83.41 ± 0.292 Ab | 101.82 ± 0.01 Bb | 20.81 ± 0.014 Cb |
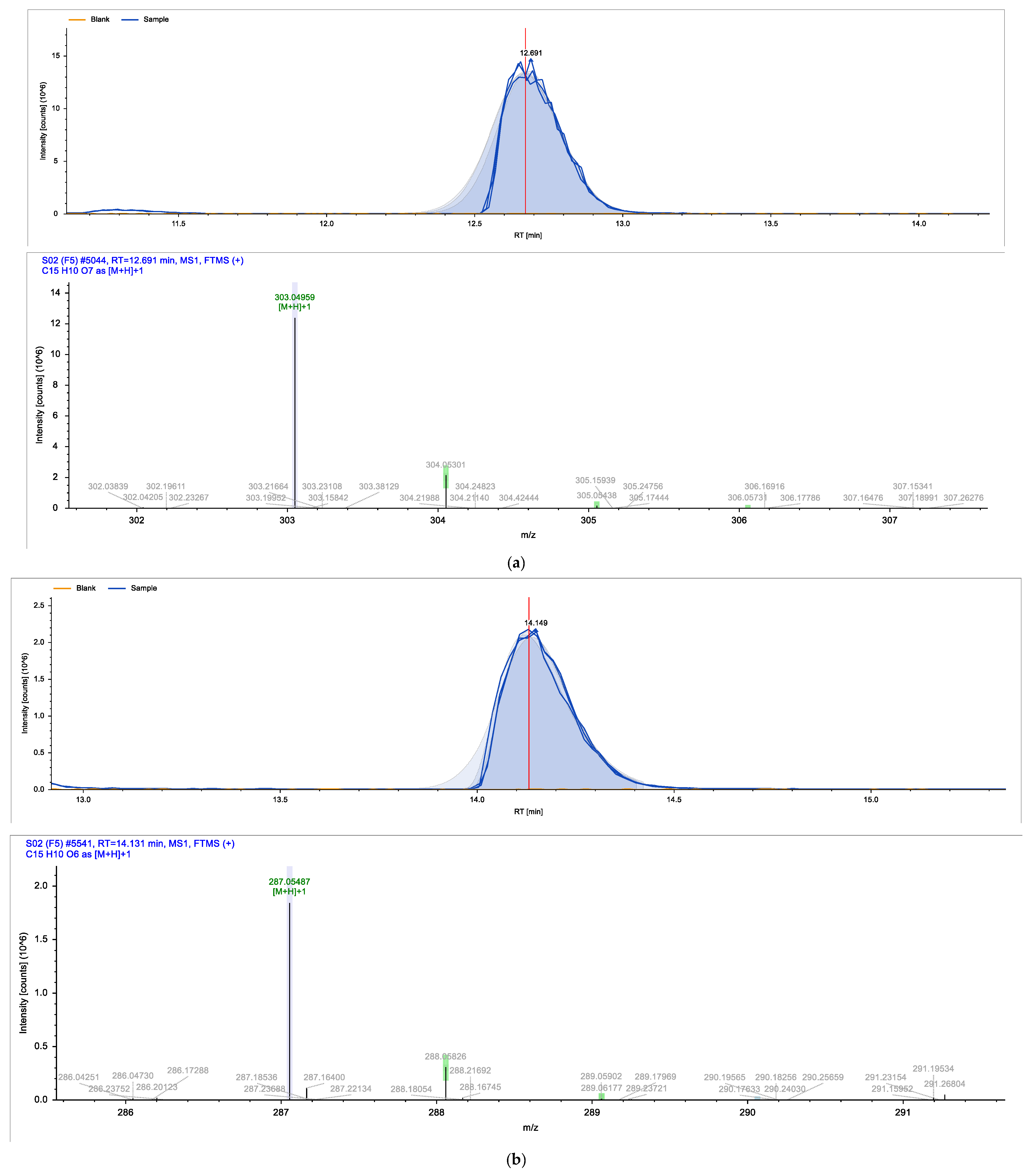
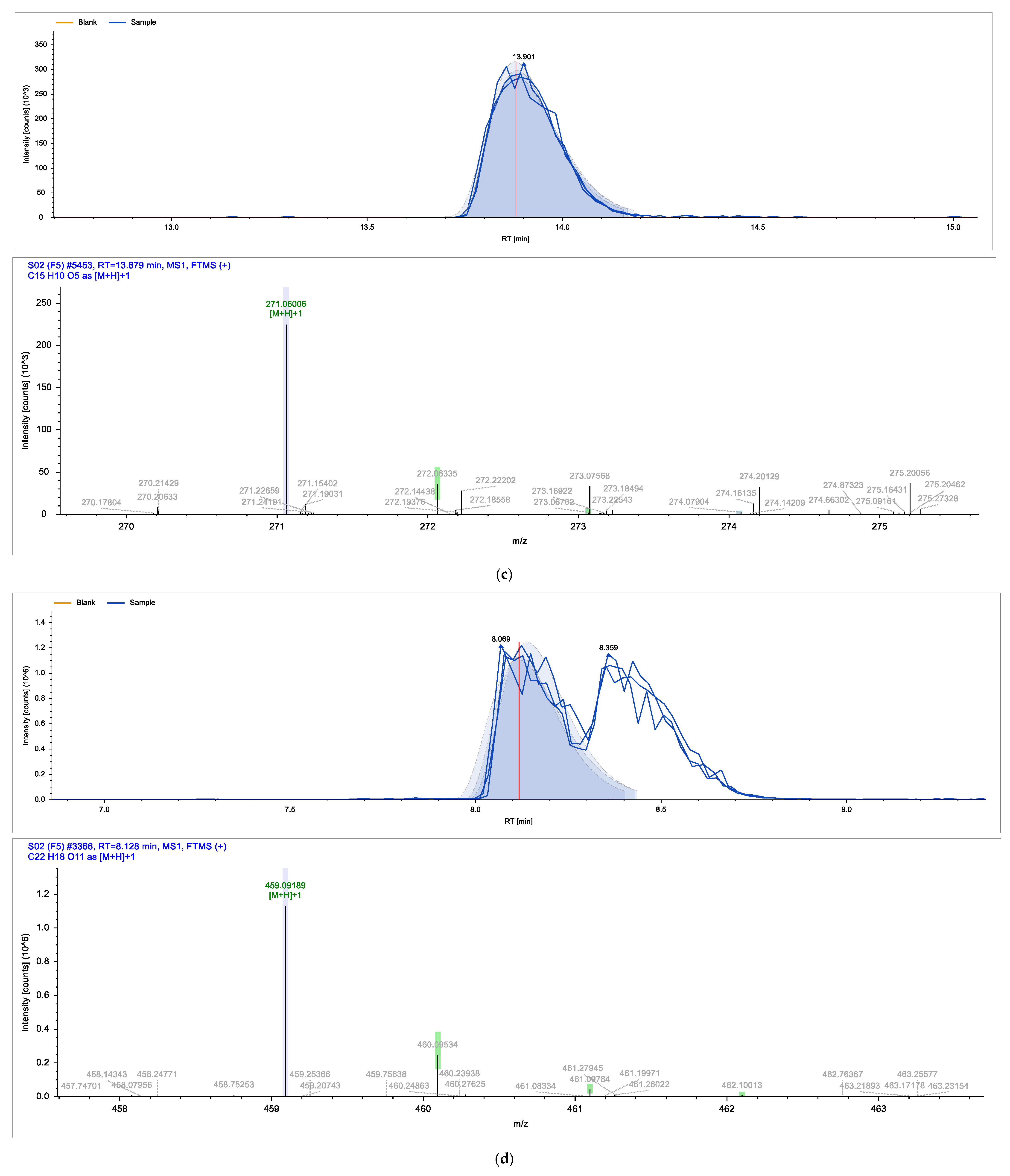
| Identified Compound | Retention Time (min) | Chemical Formula | Molecular Weight (g/mol) |
|---|---|---|---|
| (2R,3R)-Taxifolin | 6.449 | C15H12O7 | 304.25 |
| Epigallocatechin gallate | 8.128 | C22H18O11 | 458.37 |
| Luteolin-7-glucoside | 9.817 | C21H20O11 | 448.37 |
| Scutellarin | 9.822 | C21H18O12 | 462.36 |
| Myricetin | 9.909 | C15H10O8 | 318.24 |
| Avicularin | 9.989 | C20H18O11 | 434.35 |
| Hispidulin 7-glucuronide | 10.698 | C22H20O12 | 476.4 |
| Genistin | 10.71 | C21H20O10 | 432.37 |
| Baicalin | 10.764 | C21H18O11 | 446.36 |
| Prunin | 10.791 | C21H22O10 | 434.4 |
| Juglanin | 10.805 | C20H18O10 | 418.35 |
| Tricin 5-glucoside | 11.117 | C23H24O12 | 492.4 |
| Afzelin | 11.289 | C21H20O10 | 432.38 |
| Eriodictyol | 12.546 | C15H12O6 | 288.25 |
| Quercetin | 12.691 | C15H10O7 | 302.24 |
| Apigenin | 13.879 | C15H10O5 | 270.05 |
| Chalconaringenin | 13.971 | C15H12O5 | 272.26 |
| Kaempferol | 14.131 | C15H10O6 | 286.23 |
| Compound Name | Compound Structure | Chemical Class |
|---|---|---|
| Quercetin |  | Flavonol |
| Kaempferol |  | Flavonol |
| Apigenin | 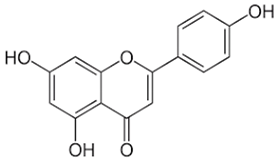 | Flavone |
| Epigallocatechin gallate (EGCG) | 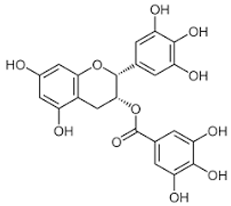 | Catechin |
| Avicularin | 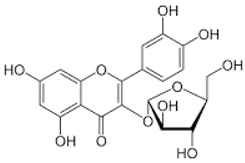 | Flavonol |
| Metformin (μM) | K. pinnata (μg/mL) | HSMM SOD Activity (U/mg of Protein/min) | Stress-Induced SOD Activity (U/mg of Protein/min) | DHSMM SOD Activity (U/mg of Protein/min) |
|---|---|---|---|---|
| Control (0) | Control (0) | 60.48 ± 4.68 Aa | 57.68 ± 7.63 Aa | 40.35 ± 10.95 Aa |
| 5000 | 0 | 137.81 ±15.80 Ab | 77.89 ± 12.31 Bb | 65.91 ± 11.48 Bb |
| 0 | 400 | 71.90 ± 12.94 Ac | 83.89 ± 17.68 Ab | 59.92 ± 14.63 Ac |
| 200 | 25 | 68.91 ± 3.34 Ac | 59.92 ± 17.30 Aa | 44.94 ± 14.31 Aa |
| 150 | 50 | 59.92 ± 6.40 Aa | 56.92 ± 11.83 Aa | 59.92 ± 12.06 Ac |
| 100 | 100 | 86.88 ± 10.91 ABd | 107.85 ± 24.48 Ac | 59.92 ± 14.35 Bc |
| 50 | 150 | 62.91 ± 5.57 Aa | 56.92 ± 8.04 Aa | 56.92 ± 13.16 Ac |
| 30 | 200 | 68.91 ± 9.12 Ac | 59.92 ± 13.68 Aa | 47.93 ± 6.61 Aa |
| 10 | 300 | 71.9 ± 6.97 Ac | 59.92 ± 12.06 Aa | 56.92 ± 10.20 Ac |
| Metformin (μM) | K. pinnata (μg/mL) | HSMM CAT Activity (U/mg of Protein/min) | Stress-Induced CAT Activity (U/mg of Protein/min) | DHSMM CAT Activity (U/mg of Protein/min) |
|---|---|---|---|---|
| Control (0) | Control (0) | 50.32 ± 3.36 Aa | 10.15 ± 4.12 Ba | 30.26 ± 3.36 Ca |
| 5000 | 0 | 53.86 ±3.37 Aa | 22.13 ± 0.79 Bb | 38.06 ± 6.56 Cb |
| 0 | 400 | 86.88 ± 2.16 Ab | 50.83 ± 9.23 Bc | 66.27 ± 0.12 Cc |
| 200 | 25 | 55.22 ± 3.49 Aa | 17.74 ± 2.35 Bb | 45.65 ± 0.22 Ad |
| 150 | 50 | 64.72 ± 0.30 Ac | 44.35 ± 9.22 Bd | 62.5 ± 3.58 Ae |
| 100 | 100 | 79.48 ± 7.61 Ab | 30.83 ± 0.65 Be | 58.36 ± 0.16 Ce |
| 50 | 150 | 64.48 ± 4.41 Ac | 13.11 ± 2.43 Ba | 41.94 ± 0.97 Cd |
| 30 | 200 | 64.60 ± 1.67 Ac | 42.93 ± 5.55 Bd | 53.24 ± 1.36 ABf |
| 10 | 300 | 80.34 ± 1.17 Ab | 22.93 ± 2.28 Bb | 44.91 ± 1.68 Cd |
| Metformin (μM) | K. pinnata (μg/mL) | HSMM (GSH) (μM) | Stress-Induced (GSH) (μM) | DHSMM (GSH) (μM) |
|---|---|---|---|---|
| Control (0) | Control (0) | 2.12 ± 0.04 Aa | 3.11 ± 0.03 Ba | 5.25 ± 0.03 Ca |
| 5000 | 0 | 13.33 ± 0 Ab | 10.00 ± 0.10 Bb | 13.89 ± 0.03 Cb |
| 0 | 400 | 2.22 ± 0.02 Aa | 3.89 ± 0.02 Ba | 21.67 ± 0 Cc |
| 200 | 25 | 6.11 ± 0.05 Ac | 5.00 ± 0.06 Bc | 11.67 ± 0 Cb |
| 150 | 50 | 6.66 ± 0.06 Ac | 10.00 ± 0.03 Bb | 15.00 ± 0.03 Cb |
| 100 | 100 | 17.22 ± 0.25 Ad | 9.44 ± 0.07 Bd | 11.11 ± 0.05 Cb |
| 50 | 150 | 17.77 ± 0.06 Ad | 22.22 ± 0.03 Be | 23.89 ± 0.07 Cc |
| 30 | 200 | 9.44 ± 0.02 Ae | 11.67 ± 0.03 Bf | 14.44 ± 0.03 Cb |
| 10 | 300 | 8.89 ± 0.02 Ae | 11.67 ± 0.05 Bf | 10.56 ± 0.07 Cb |
| Metformin (μM) | K. pinnata (μg/mL) | HSMM (MDA) (µM) | Stress-Induced (MDA) (µM) | DHSMM (MDA) (µM) |
|---|---|---|---|---|
| Control (0) | Control (0) | 52.35 ± 1.25 Aa | 62.32 ± 1.1 Ba | 63.45 ± 0.98 Ba |
| 5000 | 0 | 25.15 ±0.11 Ab | 23.63 ± 0.24 Ab | 21.42 ± 0.30 Bb |
| 0 | 400 | 21.51 ± 0.64 Ac | 18.26 ± 0.88 Bc | 15.71 ± 0.50 Ce |
| 200 | 25 | 22.63 ± 0.47 Ac | 21.21 ± 0.69 Ae | 21.55 ± 1.15 Ab |
| 150 | 50 | 21.55 ± 0.11 Ac | 20.39 ± 0.30 ABe | 19.35 ± 0.22 Bc |
| 100 | 100 | 24.32 ± 0.61 Ab | 22.07 ± 0.49 Bd | 20.73 ± 0.24 Bd |
| 50 | 150 | 22.42 ± 0.28 Ac | 20.77 ± 0.24 ABe | 19.26 ± 0.13 Bc |
| 30 | 200 | 22.25 ± 0.13 Ac | 20.47 ± 0.46 ABe | 18.87 ± 0.27 Bc |
| 10 | 300 | 21.38 ± 0.16 Ac | 19.91 ± 0.30 ABe | 18.31 ± 0.50 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramon, P.; Bergmann, D.; Abdulla, H.; Sparks, J.; Omoruyi, F. Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 6211. https://doi.org/10.3390/ijms24076211
Ramon P, Bergmann D, Abdulla H, Sparks J, Omoruyi F. Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells. International Journal of Molecular Sciences. 2023; 24(7):6211. https://doi.org/10.3390/ijms24076211
Chicago/Turabian StyleRamon, Pedro, Daniela Bergmann, Hussain Abdulla, Jean Sparks, and Felix Omoruyi. 2023. "Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells" International Journal of Molecular Sciences 24, no. 7: 6211. https://doi.org/10.3390/ijms24076211
APA StyleRamon, P., Bergmann, D., Abdulla, H., Sparks, J., & Omoruyi, F. (2023). Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells. International Journal of Molecular Sciences, 24(7), 6211. https://doi.org/10.3390/ijms24076211






