Soybean GmSAUL1, a Bona Fide U-Box E3 Ligase, Negatively Regulates Immunity Likely through Repressing the Activation of GmMPK3
Abstract
1. Introduction
2. Results
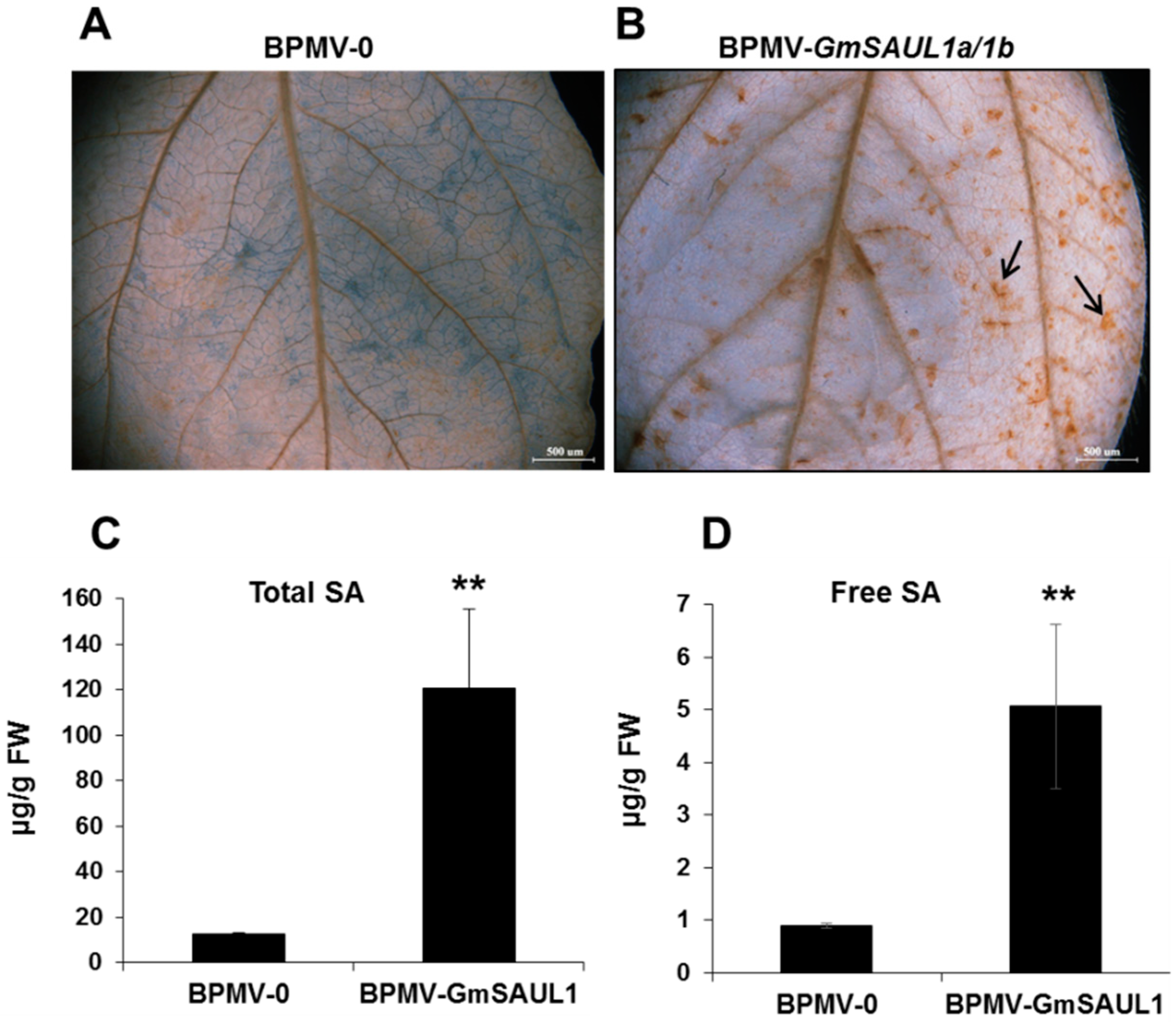
2.1. Silencing GmSAUL1 Results in a Constitutively Activated Immune Responses in Soybean
2.2. Silencing GmSAUL1a/1b Leads to Enhanced Resistance to Biotrophic Bacterial and Viral Pathogens
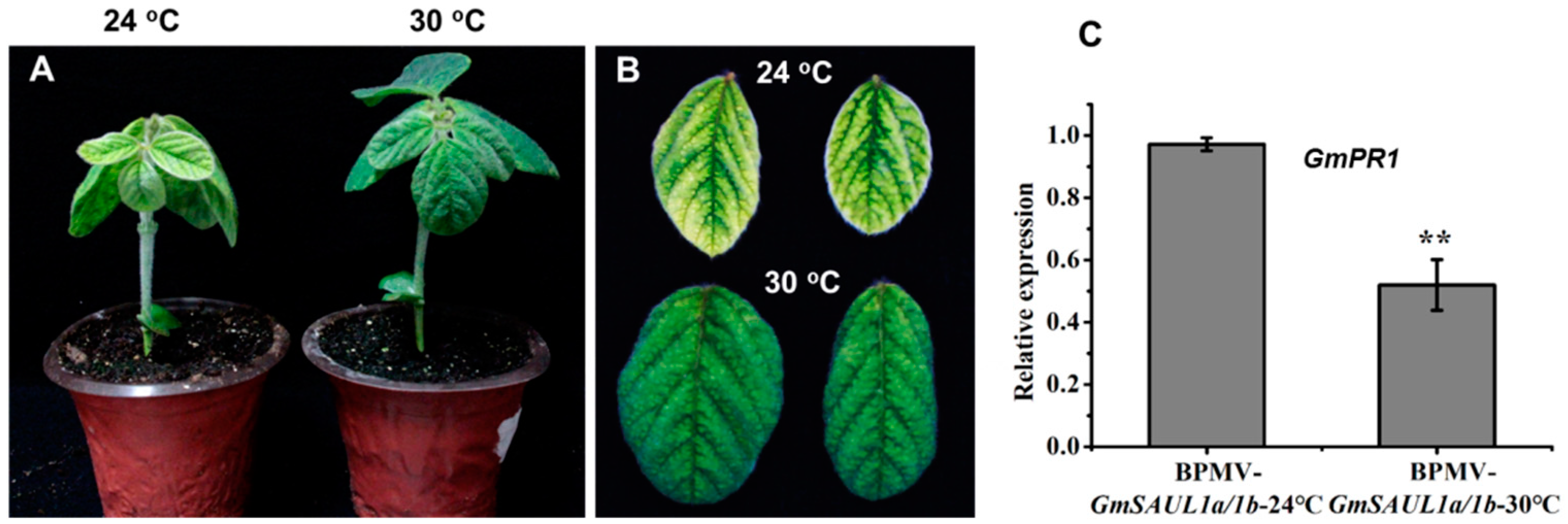
2.3. Autoimmune Phenotype of GmSAUL1a/1b-Silenced Plants Is Significantly Suppressed by Higher Temperature Treatment
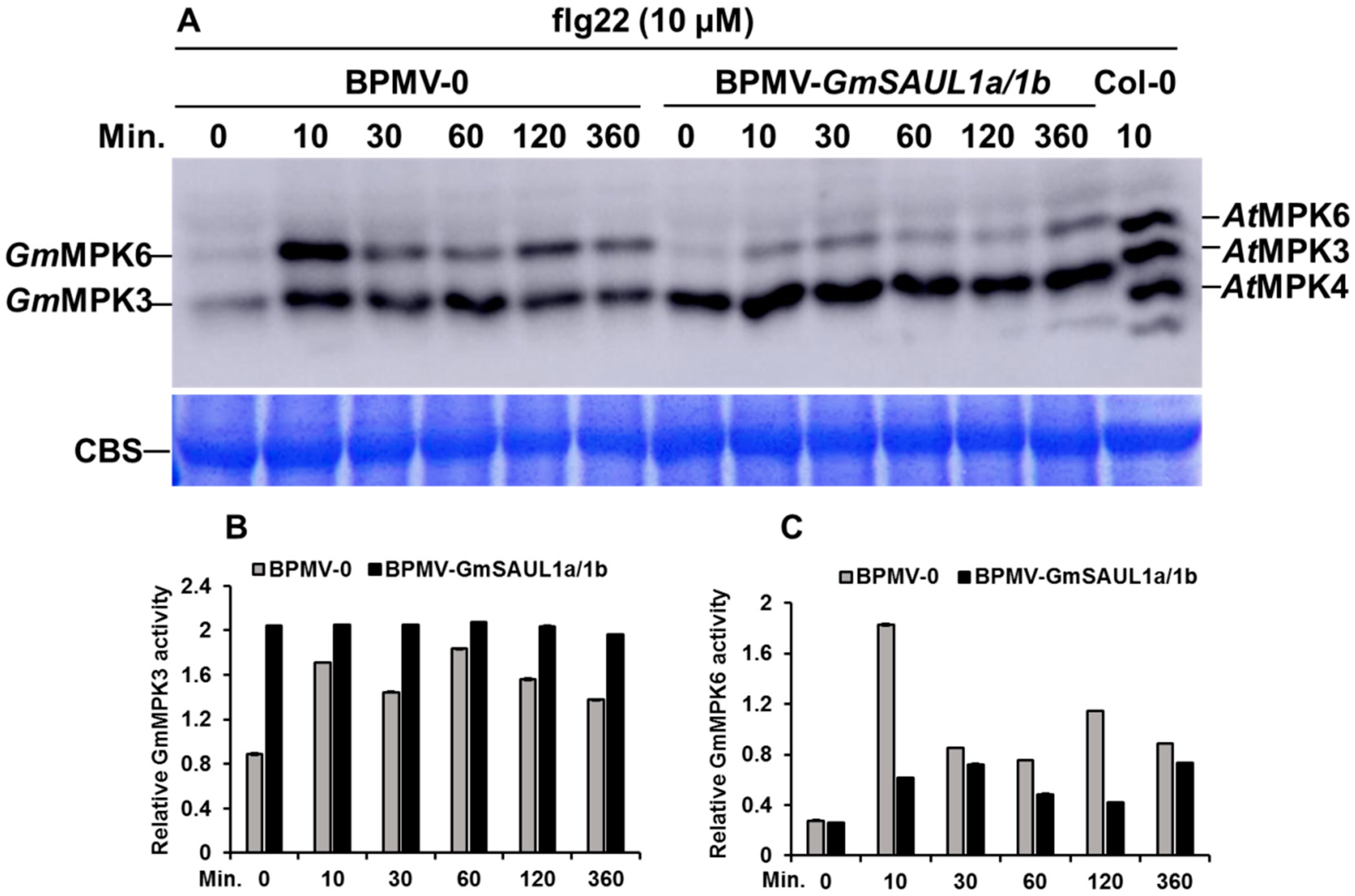
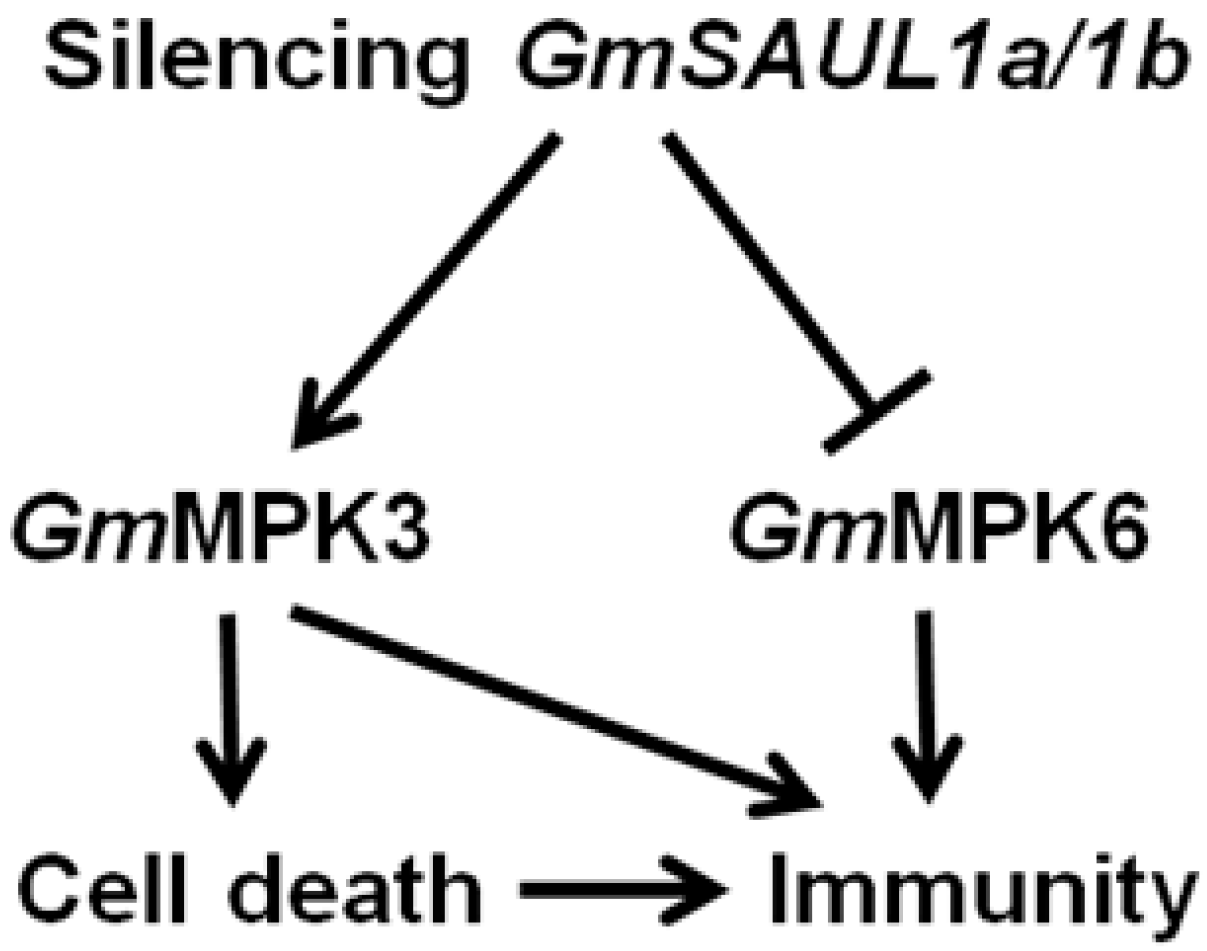
2.4. Silencing GmSAUL1a/1b Exhibits Opposite Effects on the Activation of GmMPK3 and GmMPK6 in Response to flg22 Treatment
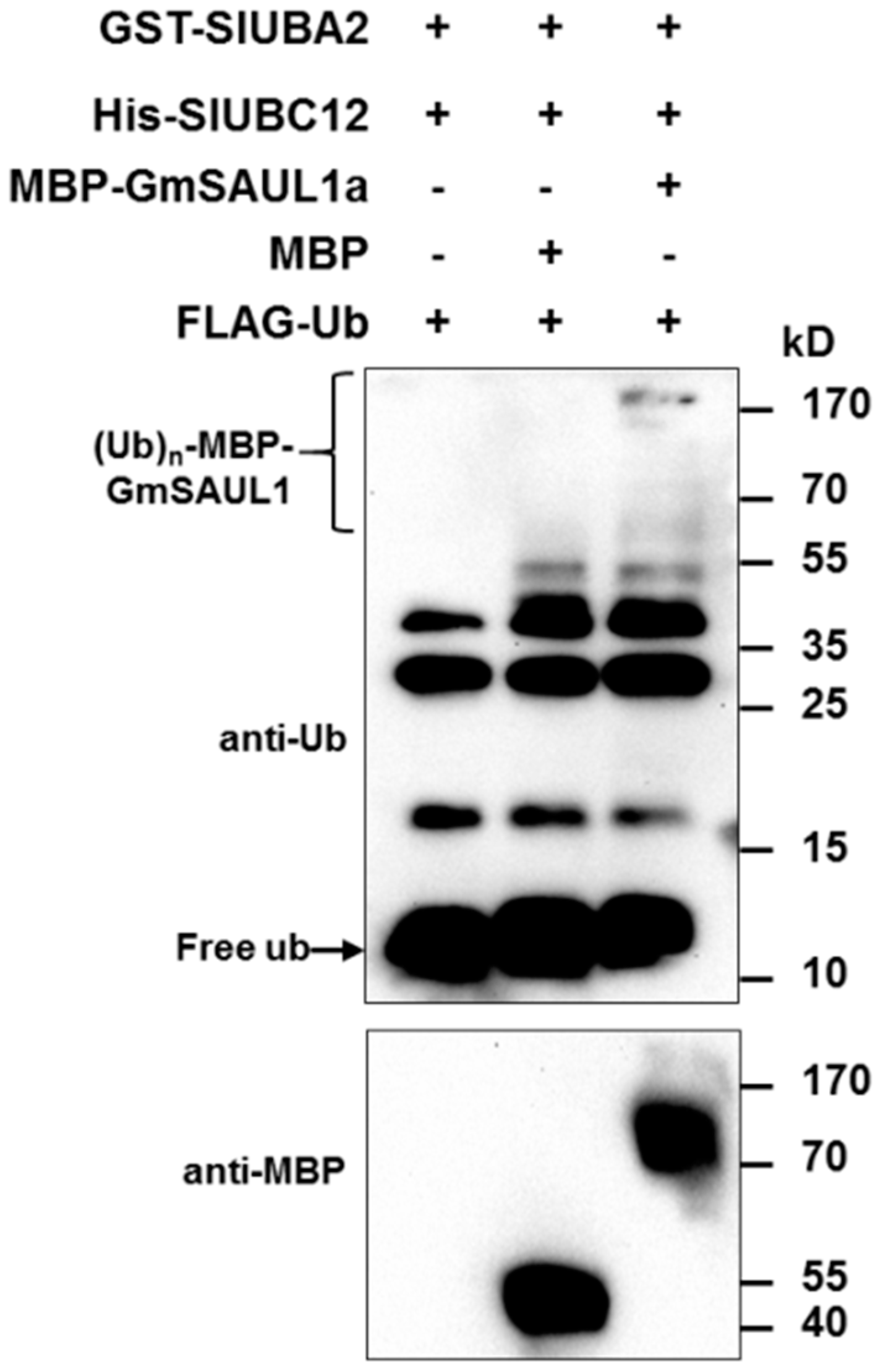
2.5. GmSAUL1a Is a Bona Fide E3 Ubiquitin Ligase
3. Discussion
3.1. Function of SAUL1 Homologs Is Conserved across Plant Species
3.2. Roles of GmSAUL1s in PTI and ETI
3.3. Silencing of GmSAUL1a/1b in Soybean Activates Immunity through Activating GmMPK3
3.4. Conclusions
4. Materials and Methods
4.1. Plant Materials
4.2. BPMV-Mediated VIGS
4.3. RNA Isolation and RT-qPCR
4.4. SMV-N-GUS Inoculation, GUS Staining, and GUS Foci Measurements
4.5. Inoculation of Pseudomonas syringae pv. glycinea (Psg)
4.6. Construction of MBP-GmSAUL1a Fusion Protein and In Vitro Ubiquitination Assay
4.7. MAPK Activity Assay
4.8. Histochemical Assays
4.9. SA Quantification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulze-Lefert, P.; Panstruga, R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci. 2011, 16, 117–125. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.G.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI cross-talk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Tena, G.; Boudsocq, M.; Sheen, J. Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 2011, 14, 519–529. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, L. Crosstalk between ubiquitination and other post-translational protein modifications in plant immunity. Plant Commun. 2020, 25, 100041. [Google Scholar] [CrossRef]
- Smalle, J.; Viestra, R.D. The Ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef]
- Hershko, A.; Clechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Li, X. Ubiquitination in NB-LRR-mediated immunity. Curr. Opin. Plant Biol. 2012, 15, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Vega-Sanchez, M.E.; Zhu, T.; Wang, G.L. Ubiquitination-mediated protein degradation and modification: An emerging theme in plant-microbe interactions. Cell Res. 2006, 16, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zeng, L. Conventional and unconventional ubiquitinnation in plant immunity. Mol. Plant Pathol. 2017, 18, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Estelle, M. The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 2010, 61, 1029–1040. [Google Scholar] [CrossRef]
- Zeng, L.; Qu, S.; Bordeos, A.; Yang, C.; Baraoidan, M.; Yan, H.; Xie, Q.; Nahm, B.H.; Leung, H.; Wang, G.L. Soptted leaf 11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 2004, 16, 2795–2808. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lin, W.; Gao, X.; Wu, S.; Cheng, C.; Avila, J.; Heese, A.; Devarenne, T.P.; He, P.; Shan, L. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 2011, 332, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, M.; Anderson, R.G.; Ichimura, K.; Pecenkova, T.; Reuter, P.; Žársky, V.; McDowell, J.M.; Shirasu, K.; Trujillo, M. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 2012, 24, 4703–4716. [Google Scholar] [CrossRef]
- Raab, S.; Drechsel, G.; Zarepour, M.; Hartung, W.; Koshiba, T.; Bittner, F.; Hoth, S. Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J. 2009, 59, 39–51. [Google Scholar] [CrossRef]
- Vogelmann, K.; Drechsel, G.; Bergler, J.; Subert, C.; Philippar, K.; Soll, J.; Engelmann, J.C.; Engelsdorf, T.; Voll, L.M.; Hoth, S. Early senescence and cell death in Arabidopsis saul1 mutants involves the PAD4-dependent salicylic acid pathway. Plant Physiol. 2012, 159, 1477–1487. [Google Scholar] [CrossRef]
- Disch, E.M.; Tong, M.; Kotur, T.; Koch, G.; Wolf, C.A.; Li, X.; Hoth, S. Membrane-associated ubiquitin ligase SAUL1 suppresses temperature-and humidity-dependent autoimmunity in Arabidopsis. Mol. Plant-Microbe Interact. 2016, 29, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Lee, I.C.; Kim, J.; Kim, J.H.; Chung, E.H.; Kim, H.J.; Park, S.J.; Kim, Y.M.; Kang, S.K.; Nam, H.G.; et al. NORE1/SAUL1 integrates temperature-dependent defense programs involving SGT1b and PAD4 pathways and leaf senescence in Arabidopsis. Physio. Plant. 2016, 158, 180–199. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Kotur, T.; Liang, W.; Vogelmann, K.; Kleine, T.; Leister, D.; Brieske, C.; Yang, S.; Ludke, D.; Wiermer, M.; et al. E3 ligase SAUL1 serves as a positive regulator of PAMP-triggered immunity and its homeostasis is monitored by immune receptor SOC3. New Phytol. 2017, 215, 1516–1532. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; van Wersch, S.; Tong, M.; Li, X. TIR-NB-LRR immune receptor SOC3 pairs with truncated TIR-NB protein CHS1 or TN2 to monitor the homeostasis of E3 ligase SAUL1. New Phytol. 2019, 221, 2054–2066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Liu, J.; Ding, Y.; Wang, S.; Zhang, X.; Liu, Y.; Yang, S. Temperature-dependent autoimmunity mediated by chs1 requires its neighboring TNL gene SOC3. New Phytol. 2016, 213, 1330–1345. [Google Scholar] [CrossRef] [PubMed]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signal-ling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Zhang, S.; Klessig, D.F. Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by Tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 1998, 95, 7433–7438. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y. Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 2001, 13, 1877–1889. [Google Scholar]
- Menke, F.L.; van Pelt, J.A.; Pieterse, C.M.; Klessig, D.F. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 2004, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Liu, Y.; Yang, K.Y.; Han, L.; Mao, G.; Glazebrook, J.; Zhang, S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis MAP Kinase 4 Negatively Regulates Sy Liang, W.stemic Acquired Resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.L.; Zhou, L.; Yun, B.W.; Nielsen, H.B.; Fiil, B.K.; Petersen, K.; Mackinlay, J.; Loake, G.J.; Mundy, J.; Morris, P.C. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol. 2008, 148, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008, 18, 1190–1198. [Google Scholar] [CrossRef]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 2012, 24, 2225–2236. [Google Scholar]
- Pitzschke, A.; Djamei, A.; Bitton, F.; Hirt, H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2009, 2, 120–137. [Google Scholar] [CrossRef]
- Liu, J.Z.; Horstman, H.D.; Braun, E.; Graham, M.A.; Zhang, C.; Navarre, D.; Qiu, W.L.; Lee, Y.; Nettleton, D.; Hill, J.H.; et al. Soybean homologs of MPK4 negatively regulate defense responses and positively regulate growth and development. Plant Physiol. 2001, 157, 1363–1378. [Google Scholar] [CrossRef]
- Liu, J.Z.; Braun, E.; Qiu, W.L.; Shi, Y.F.; Marcelino-Guimars, F.C.; Navarre, D.; Hill, J.H.; Whitham, S.A. Positive and negative roles for soybean MPK6 in regulating defense responses. Mol. Plant Microbe Interact. 2014, 27, 824–834. [Google Scholar] [CrossRef]
- Liu, J.Z.; Graham, M.A.; Pedley, K.F.; Whitham, S.A. Gaining insight into soybean defense responses using functional genomics approaches. Brief. Funct. Genom. 2015, 14, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Fang, Y.; Pang, H. The current status of the soybean-soybean mosaic virus (SMV) pathosystem. Front. Microbiol. 2016, 7, 1906. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Liu, D.D.; Lan, H.J.; Masoud, H.S.; Ye, M.Y.; Dai, X.Y.; Zhong, C.L.; Tian, S.N.; Liu, J.Z. Silencing GmBIR1 in Soybean Results in Activated Defense Responses. Int. J. Mol. Sci. 2022, 23, 7450. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, C.; Li, Z.C.; Wang, Z.R.; Jiang, X.X.; Shi, Y.F.; Tian, S.N.; Braun, E.; Mei, Y.; Qiu, W.L.; et al. The MAPK kinase kinase GmMEKK1 regulates cell death and defense responses. Plant Physiol. 2018, 178, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.N.; Liu, D.D.; Zhong, C.L.; Xu, H.Y.; Yang, S.; Fang, Y.; Ran, J.; Liu, J.Z. Silencing GmFLS2 enhances the susceptibility of soybean to bacterial pathogen through attenuating the activation of GmMAPK signaling pathway. Plant Sci. 2020, 292, 110386. [Google Scholar] [CrossRef]
- Liu, J.Z.; Whitham, S.A. Over-expression of a nuclear-localized DnaJ domain-containing HSP40 from soybean reveals its roles in cell death and disease resistance. Plant J. 2013, 74, 110–121. [Google Scholar] [CrossRef]
- Wang, L.; Eggenberger, A.; Hill, J.; Bogdanove, A.J. Pseudomonas syringae effector avrB confers soybean cultivar-specific avirulence on Soybean mosaic virus adapted for transgene expression but effector avrPto does not. Mol. Plant Microbe Interact. 2006, 19, 304–312. [Google Scholar] [CrossRef]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Whitham, S.; McCormick, S.; Baker, B. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc. Natl. Acad. Sci. USA 1996, 93, 8776–8781. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, W.; Hua, J. Temperature Modulates Plant Defense Responses through NB-LRR Proteins. PLoS Pathog. 2010, 6, e1000844. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Nie, H.; Shen, Q.; Zhang, S.; Lukowitz, W.; Tang, D. EDR1 physically interacts with MKK4/MKK5 and negatively regulates a MAP kinase cascade to modulate plant innate immunity. PLoS Genet. 2014, 10, e1004389. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Genot, B.; Lang, J.; Berriri, S.; Garmier, M.; Gilard, F.; Pateyron, S.; Haustraete, K.; Van Der Straeten, D.; Hirt, H.; Colcombet, J. Constitutively active Arabidopsis MAP Kinase 3 triggers defense responses involving salicylic acid and SUMM2 resistance protein. Plant Physiol. 2017, 174, 1238–1249. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Bruffett, K.; Lee, J.; Hause, G.; Walker, J.; Zhang, S. Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell 2008, 20, 602–613. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Whitham, S.A.; Hill, J.H. Development and use of an efficient DNA-based viral gene silencing vector for soybean. Mol. Plant Microbe Interact. 2009, 22, 123–131. [Google Scholar] [CrossRef]
- Zhang, C.; Bradshaw, J.D.; Whitham, S.A.; Hill, J.H. The development of an efficient multipurpose bean pod mottle virus viral vector set for foreign gene expression and RNA silencing. Plant Physiol. 2010, 153, 52–65. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Liu, J.Z.; Duan, J.; Whitham, S.A.; Qian, W.J. S-nitrosylation inhibits the kinase activity of tomato phosphoinositide-dependent kinase 1 (PDK1). J. Biol. Chem. 2017, 292, 19743–19751. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yang, H.; Zhang, S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 2002, 277, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Zhao, J.; Li, Y.; Wang, J.; Guo, R.; Gan, S.; Liu, C.J.; Zhang, K. S5H/DMR6 Encodes a Salicylic Acid 5-Hydroxylase That Fine-Tunes Salicylic Acid Homeostasis. Plant Physiol. 2017, 175, 1082–1093. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-M.; Ye, M.-Y.; Wang, C.; Ma, X.-H.; Wu, N.-N.; Zhong, C.-L.; Zhang, Y.; Cheng, N.; Nakata, P.A.; Zeng, L.; et al. Soybean GmSAUL1, a Bona Fide U-Box E3 Ligase, Negatively Regulates Immunity Likely through Repressing the Activation of GmMPK3. Int. J. Mol. Sci. 2023, 24, 6240. https://doi.org/10.3390/ijms24076240
Li J-M, Ye M-Y, Wang C, Ma X-H, Wu N-N, Zhong C-L, Zhang Y, Cheng N, Nakata PA, Zeng L, et al. Soybean GmSAUL1, a Bona Fide U-Box E3 Ligase, Negatively Regulates Immunity Likely through Repressing the Activation of GmMPK3. International Journal of Molecular Sciences. 2023; 24(7):6240. https://doi.org/10.3390/ijms24076240
Chicago/Turabian StyleLi, Jun-Mei, Mei-Yan Ye, Chaofeng Wang, Xiao-Han Ma, Ni-Ni Wu, Chen-Li Zhong, Yanjun Zhang, Ninghui Cheng, Paul A. Nakata, Lirong Zeng, and et al. 2023. "Soybean GmSAUL1, a Bona Fide U-Box E3 Ligase, Negatively Regulates Immunity Likely through Repressing the Activation of GmMPK3" International Journal of Molecular Sciences 24, no. 7: 6240. https://doi.org/10.3390/ijms24076240
APA StyleLi, J.-M., Ye, M.-Y., Wang, C., Ma, X.-H., Wu, N.-N., Zhong, C.-L., Zhang, Y., Cheng, N., Nakata, P. A., Zeng, L., & Liu, J.-Z. (2023). Soybean GmSAUL1, a Bona Fide U-Box E3 Ligase, Negatively Regulates Immunity Likely through Repressing the Activation of GmMPK3. International Journal of Molecular Sciences, 24(7), 6240. https://doi.org/10.3390/ijms24076240






