Cytomegalovirus Glycoprotein B Genotype in Patients with Anterior Segment Infection
Abstract
:1. Introduction
2. Results
2.1. Distribution of CMV gB Genotype
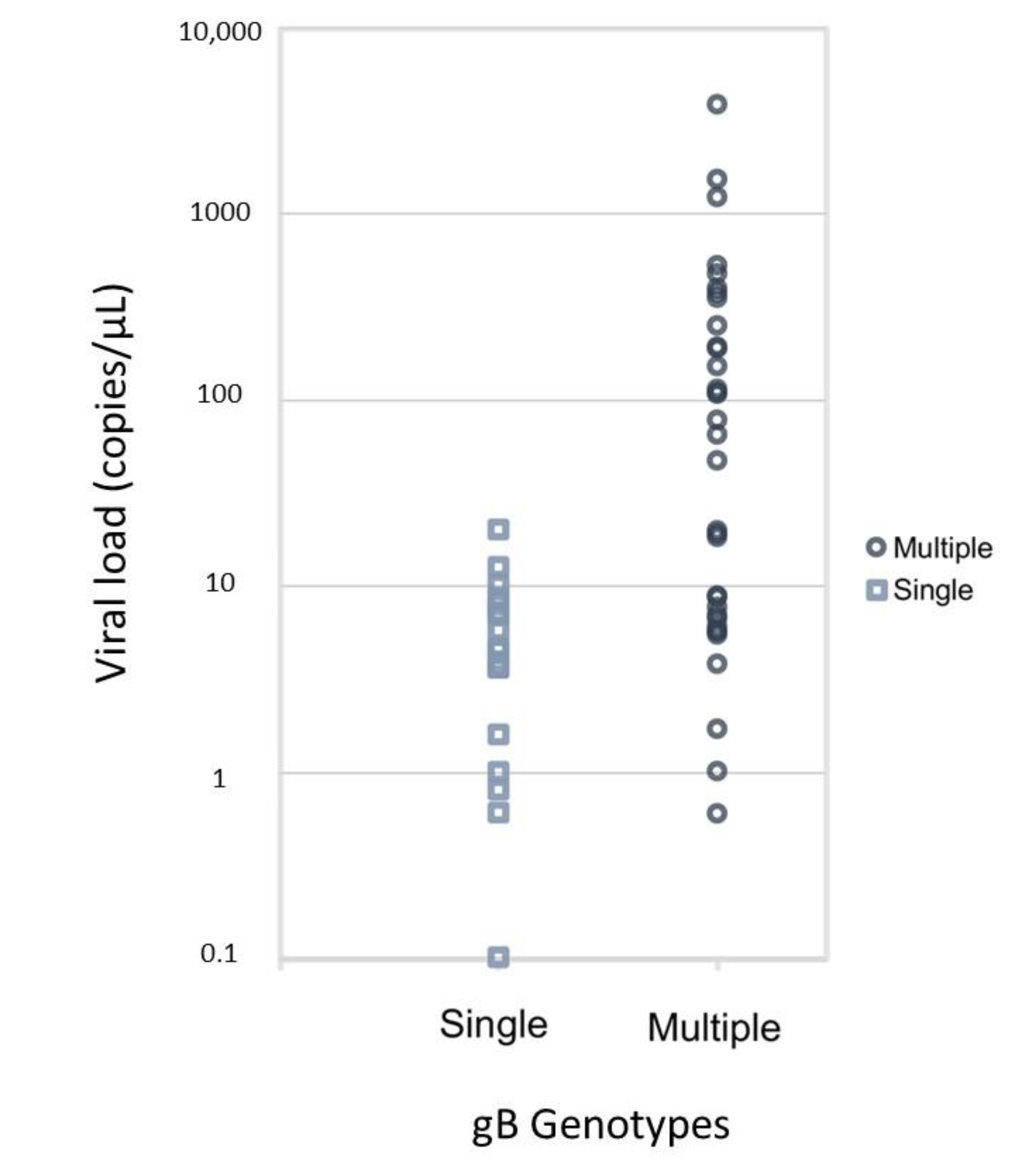
2.2. Single Genotype Infection and Multiple Genotype Infection
2.3. Characteristics and Prognosis with Patients Involving Different Genotypes
2.4. Linear Regression Model
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. CMV gB Genotyping
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hahn, G.; Jores, R.; Mocarski, E.S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3937–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response. Front. Cell Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Suzuki, T.; Uno, T.; Chihara, H.; Shiraishi, A.; Hara, Y.; Inatomi, T.; Sotozono, C.; Kawasaki, S.; Yamasaki, K.; et al. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology 2008, 115, 292–297.e293. [Google Scholar] [CrossRef]
- Chee, S.-P.; Bacsal, K.; Jap, A.; Se-Thoe, S.-Y.; Cheng, C.L.; Tan, B.H. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am. J. Ophthalmol. 2008, 145, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Touhami, S.; Qu, L.; Angi, M.; Bojanova, M.; Touitou, V.; Lehoang, P.; Rozenberg, F.; Bodaghi, B. Cytomegalovirus Anterior Uveitis: Clinical Characteristics and Long-term Outcomes in a French Series. Am. J. Ophthalmol. 2018, 194, 134–142. [Google Scholar] [CrossRef]
- Yu, T.; Peng, R.M.; Xiao, G.G.; Feng, L.N.; Hong, J. Clinical Evaluation of Intravitreal Injection of Ganciclovir in Refractory Corneal Endotheliitis. Ocul. Immunol. Inflamm. 2020, 28, 270–280. [Google Scholar] [CrossRef]
- Chee, S.P.; Jap, A. Cytomegalovirus anterior uveitis: Outcome of treatment. Br. J. Ophthalmol. 2010, 94, 1648–1652. [Google Scholar] [CrossRef]
- La Distia Nora, R.; Putera, I.; Mayasari, Y.D.; Hikmahwati, W.; Pertiwi, A.M.; Ridwan, A.S.; Sitompul, R.; Westcott, M.; Chee, S.P.; Pavesio, C.; et al. Clinical characteristics and treatment outcomes of cytomegalovirus anterior uveitis and endotheliitis: A systematic review and meta-analysis. Surv. Ophthalmol. 2022, 67, 1014–1030. [Google Scholar] [CrossRef]
- Navarro, D.; Paz, P.; Tugizov, S.; Topp, K.; La Vail, J.; Pereira, L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 1993, 197, 143–158. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Stanton, A.; McCarrell, E.; Smith, C.; Osman, M.; Harber, M.; Davenport, A.; Jones, G.; Wheeler, D.C.; O’Beirne, J.; et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 2011, 377, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.W.; Dennison, K.M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 1991, 163, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Shepp, D.H.; Match, M.E.; Lipson, S.M.; Pergolizzi, R.G. A fifth human cytomegalovirus glycoprotein B genotype. Res. Virol. 1998, 149, 109–114. [Google Scholar] [CrossRef]
- Shepp, D.H.; Match, M.E.; Ashraf, A.B.; Lipson, S.M.; Millan, C.; Pergolizzi, R. Cytomegalovirus glycoprotein B groups associated with retinitis in AIDS. J. Infect. Dis. 1996, 174, 184–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Wang, Y.; Xu, Y.; Wu, D.; Sun, A.; Zhu, Z.; Han, Y.; Qiu, H.; Tang, X.; Fu, Z.; et al. Cytomegalovirus glycoprotein B genotype in hematopoietic stem cell transplant patients from China. Biol. Blood. Marrow. Transplant. 2010, 16, 647–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, N.; Suzuki, T.; Inoue, T.; Kobayashi, T.; Ohashi, Y. Polymorphisms in cytomegalovirus genotype in immunocompetent patients with corneal endotheliitis or iridocyclitis. J. Med. Virol. 2015, 87, 1441–1445. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Sheng, Q.; Fan, X.; Kong, X.; Sun, X. Polymorphisms of the cytomegalovirus glycoprotein B genotype in patients with Posner-Schlossman syndrome. Br. J. Ophthalmol. 2021, 106, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, D.; Quereda, C.; Tenorio, A. Different cytomegalovirus glycoprotein B genotype distribution in serum and cerebrospinal fluid specimens determined by a novel multiplex nested PCR. J. Clin. Microbiol. 2003, 41, 2872–2877. [Google Scholar] [CrossRef] [Green Version]
- Sarcinella, L.; Mazzulli, T.; Willey, B.; Humar, A. Cytomegalovirus glycoprotein B genotype does not correlate with outcomes in liver transplant patients. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2002, 24, 99–105. [Google Scholar] [CrossRef]
- Atul Humar, D.K.; Gilbert, C.; Boivin, G. Cytomegalovirus (CMV) Glycoprotein B Genotypes and Response to Antiviral Therapy, in Solid-Organ–Transplant Recipients with CMV Disease. J. Infect. Dis. 2003, 188, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Coaquette, A.; Bourgeois, A.; Dirand, C.; Varin, A.; Chen, W.; Herbein, G. Mixed cytomegalovirus glycoprotein B genotypes in immunocompromised patients. Clin. Infect. Dis. 2004, 39, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Ciotti, M.; Cella, E.; Ritta, M.; Ciccozzi, M.; Cavallo, R.; Perno, C.F.; Costa, C. Cytomegalovirus Glycoprotein B Genotype Distribution in Italian Transplant Patients. Intervirology 2017, 60, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Pati, P.; Jensen, T.L.; Goll, J.B.; Gelber, C.E.; Singh, A.; McNeal, M.; Boppana, S.B.; Bernstein, D.I. Cytomegalovirus Genetic Diversity Following Primary Infection. J. Infect. Dis. 2020, 221, 715–720. [Google Scholar] [CrossRef]
- Miyanaga, M.; Sugita, S.; Shimizu, N.; Morio, T.; Miyata, K.; Maruyama, K.; Kinoshita, S.; Mochizuki, M. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br. J. Ophthalmol. 2010, 94, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Torok-Storb, B.; Boeckh, M.; Hoy, C.; Leisenring, W.; Myerson, D.; Gooley, T. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood 1997, 90, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Ozaki, K.S.; Tomiyama, H.; Camara, N.O.; Granato, C.F. Clinical correlations of human cytomegalovirus strains and viral load in kidney transplant recipients. Int. Immunopharmacol. 2009, 9, 26–31. [Google Scholar] [CrossRef]
- Aquino, V.H.; Figueiredo, L.T. High prevalence of renal transplant recipients infected with more than one cytomegalovirus glycoprotein B genotype. J. Med. Virol. 2000, 61, 138–142. [Google Scholar] [CrossRef]
- Barbi, M.; Binda, S.; Caroppo, S.; Primache, V.; Dido, P.; Guidotti, P.; Corbetta, C.; Melotti, D. CMV gB genotypes and outcome of vertical transmission: Study on dried blood spots of congenitally infected babies. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2001, 21, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.U.; Otte, J.; Koch, F.; Gumbel, H.; Doerr, H.W.; Cinatl, J., Jr. Role of human cytomegalovirus genotype polymorphisms in AIDS patients with cytomegalovirus retinitis. Med. Microbiol. Immunol. 2013, 202, 37–47. [Google Scholar] [CrossRef]
- Pignatelli, S.; Dal Monte, P.; Rossini, G.; Lazzarotto, T.; Gatto, M.R.; Landini, M.P. Intrauterine cytomegalovirus infection and glycoprotein N (gN) genotypes. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2003, 28, 38–43. [Google Scholar] [CrossRef]
- Paradowska, E.; Studzinska, M.; Suski, P.; Kasztelewicz, B.; Wisniewska-Ligier, M.; Zawilinska, B.; Gaj, Z.; Nowakowska, D. Human cytomegalovirus UL55, UL144, and US28 genotype distribution in infants infected congenitally or postnatally. J. Med. Virol. 2015, 87, 1737–1748. [Google Scholar] [CrossRef]
- Paradowska, E.; Jablonska, A.; Studzinska, M.; Kasztelewicz, B.; Wisniewska-Ligier, M.; Dzierzanowska-Fangrat, K.; Wozniakowska-Gesicka, T.; Czech-Kowalska, J. Distribution of the CMV glycoprotein gH/gL/gO and gH/gL/pUL128/pUL130/pUL131A complex variants and associated clinical manifestations in infants infected congenitally or postnatally. Sci. Rep. 2019, 9, 16352. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C.H.; Hwang, Y.S.; Chuang, W.Y.; Ma, D.H.K.; Yeh, L.K.; Chen, S.Y.; Shu, J.C. Prevalence and clinical consequences of cytomegalovirus DNA in the aqueous humour and corneal transplants. Br. J. Ophthalmol. 2019, 103, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Gorzer, I.; Kerschner, H.; Jaksch, P.; Bauer, C.; Seebacher, G.; Klepetko, W.; Puchhammer-Stockl, E. Virus load dynamics of individual CMV-genotypes in lung transplant recipients with mixed-genotype infections. J. Med. Virol. 2008, 80, 1405–1414. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Shen, C.R.; Chang, S.H.; Lai, C.C.; Liu, C.L.; Chen, K.J.; Lin, K.K.; Chen, T.L.; Hsiao, C.H. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 103–110. [Google Scholar] [CrossRef]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Shiraishi, A.; Ohashi, Y.; Kandori, M.; Miyazaki, D.; Inoue, Y.; Soma, T.; Nishida, K.; et al. Clinical features and management of cytomegalovirus corneal endotheliitis: Analysis of 106 cases from the Japan corneal endotheliitis study. Br. J. Ophthalmol. 2015, 99, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Standardization of Uveitis Nomenclature (SUN) Working Group. Classification Criteria for Cytomegalovirus Anterior Uveitis. Am. J. Ophthalmol. 2021, 228, 89–95. [Google Scholar] [CrossRef]
- Trusko, B.; Thorne, J.; Jabs, D.; Belfort, R.; Dick, A.; Gangaputra, S.; Nussenblatt, R.; Okada, A.; Rosenbaum, J. The Standardization of Uveitis Nomenclature (SUN) Project: Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf. Med. 2013, 52, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Kang, E.Y.; Hwang, Y.S.; Hsiao, C.H. Treatment of cytomegalovirus anterior segment infection with intravitreal injection of ganciclovir in adjunction with or without oral valganciclovir: A long-term results. Sci. Rep. 2021, 11, 3105. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Hu, F.R.; Wang, T.H.; Huang, J.Y.; Yeh, P.T.; Lin, C.P.; Wang, I.J. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am. J. Ophthalmol. 2014, 158, 1024–1031.e1022. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; pp. 224–225. [Google Scholar]
| All (n = 57) | Infection with Single Genotype (n = 17) | Infection with Multiple Genotypes(n = 40) | STD | p-Value | |
|---|---|---|---|---|---|
| General information | |||||
| Age (year) | 58.6 ± 13.36 | 59.3 ± 13.45 | 58.4 ± 13.48 | 0.07 | 0.669 |
| Past history | |||||
| Previous anti-viral use | 7 (12.3%) | 3 (17.6%) | 6 (15%) | 0.07 | 1 |
| Previous glaucoma surgery | 7 (12.3%) | 3 (17.6%) | 5 (12.5%) | 0.14 | 0.684 |
| Previous corneal transplant | 6 (10.5%) | 0 | 6 (15%) | −0.59 | 0.164 |
| Clinical feature | |||||
| IOP (mm-Hg) | |||||
| Median [25th, 75th percentile] | 22.9 [15.0–32.4] | 23 [17–43] | 23 [14–28] | - | 0.339 |
| Mean ± standard deviation | 24.7 ± 12.2 | 28.3 ± 14.3 | 23.2 ± 11.1 | 0.40 | 0.149 |
| Corneal edema | 31 (54.4%) | 10 (58.8%) | 21 (52.5%) | 0.13 | 0.780 |
| KPs | 49 (86.0%) | 15 (88.2%) | 34 (85%) | 0.10 | 1 |
| Diagnosis | 0.234 | ||||
| Endotheliitis | 35 (61.4%) | 8 (47.1%) | 27 (67.5%) | −0.42 | |
| Iridocyclitis | 22 (38.6%) | 9 (52.9%) | 13 (32.5%) | 0.42 | |
| Virology | |||||
| Viral load (copies/μL) | |||||
| Median [25th, 75th percentile] | 8.6 [4.6, 112.1] | 4.6 [1.6, 8.0] | 56.0 [6.7, 252.0] | - | <0.001 |
| Mean ± standard deviation | 197.5 ± 592.4 | 5.3 ± 4.8 | 290.8 ± 706.4 | −0.57 | 0.104 |
| Mean ± standard deviation (log-transformed) | 2.88 ± 2.29 | 1.1 ± 1.29 | 3.7 ± 2.21 | −1.42 | <0.001 |
| Prognosis | |||||
| Recurrence in 1year (n = 46) | 15(26.3%) | 6 (35.3%) | 9 (22.5%) | 0.29 | 0.299 |
| Anti-glaucomatic medication use | 35 (61.4%) | 13 (76.5%) | 22 (55%) | 0.46 | 0.207 |
| Further filtering surgery | 10 (17.5%) | 4 (23.6%) | 6 (15%) | 0.21 | 0.471 |
| Corneal decompensation | 8 (14.0%) | 3 (17.6%) | 5 (12.5%) | 0.14 | 0.701 |
| n | % | |
|---|---|---|
| Single genotype | 17 | 29.82% |
| gB1 | 4 | |
| gB3 | 13 | |
| Multiple genotype | 40 | 70.28% |
| 2 genotypes | 21 | 36.84% |
| gB1 + gB3 | 5 | |
| gB1 + gB4 | 10 | |
| gB3 + gB4 | 6 | |
| 3 genotypes | 19 | 33.33% |
| gB1 + gB2 + gB3 | 2 | |
| gB1 + gB2 + gB4 | 4 | |
| gB1 + gB3 + gB4 | 13 | |
| Total | 57 | 100% |
| gB1 | gB3 | gB4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gB1 (n = 38) | non-gB1 (n = 19) | STD | p | gB3 (n = 39) | non-gB3 (n = 18) | STD | p | gB4 (n = 33) | non-gB4 (n = 24) | STD | p | |
| General information | ||||||||||||
| Age | 58 ± 12.93 | 60 ± 14.45 | −0.14 | 0.623 | 59.1 ± 14.35 | 57.7 ± 11.25 | 0.11 | 0.71 | 59.1 ± 13.23 | 58 ± 13.80 | 0.08 | 0.759 |
| Past history | ||||||||||||
| Previous anti-viral use | 5 (13.2%) | 4 (21.1%) | −0.21 | 0.463 | 8 (20.5%) | 1 (5.6%) | 0.46 | 0.247 | 4 (12.1%) | 5 (20.8%) | −0.24 | 0.470 |
| Previous glaucoma surgery | 4 (10.5%) | 4 (21.1%) | −0.29 | 0.420 | 8 (20.5%) | 0 (0%) | 0.72 | 0.046 | 3 (9.1%) | 5 (20.8%) | −0.33 | 0.261 |
| Previous corneal transplant | 6 (15.8%) | 0 (0%) | 0.61 | 0.164 | 3 (7.7%) | 3 (16.7%) | −0.28 | 0.368 | 4 (12.1%) | 2 (8.3%) | 0.13 | 1 |
| Clinical feature | ||||||||||||
| IOP (mm-Hg) | ||||||||||||
| Median [25th, 75th percentile] | 25 [13, 30] | 20 [15, 35] | - | 0.876 | 20 [14, 35] | 26 [17, 28] | - | 0.317 | 21.5 [14, 26] | 26 [16, 39] | - | 0.145 |
| Mean ± standard deviation | 24.7 ± 12.7 | 24.8 ± 11.5 | −0.01 | 0.961 | 23.8 ± 12.4 | 26.6 ± 12.1 | −0.23 | 0.428 | 22.5 ± 10.2 | 27.7 ± 14.2 | −0.42 | 0.113 |
| Corneal edema | 20 (52.6%) | 11 (57.9%) | −0.11 | 1 | 21 (53.8%) | 10 (55.6%) | −0.01 | 0.984 | 17 (51.5%) | 14 (58.3%) | −0.14 | 0.488 |
| KPs | 33 (86.8%) | 16 (84.2%) | 0.07 | 1 | 33 (84.6%) | 16 (88.9%) | 1 | 28 (84.8%) | 21 (87.5%) | −0.08 | 0.638 | |
| Diagnosis | 0.155 | 0.579 | 0.413 | |||||||||
| Endotheliitis | 26 (68.4%) | 9 (47.3%) | 0.44 | 23 (59%) | 12 (66.7%) | −0.16 | 22 (66.7%) | 13 (54.2%) | 0.26 | |||
| Iridocyclitis | 12 (31.6%) | 10 (52.6%) | −0.44 | 16 (41.0%) | 6 (33.3%) | 0.16 | 11 (33.3%) | 11 (45.8%) | −0.26 | |||
| Virology | ||||||||||||
| Viral load (copies/μL) | ||||||||||||
| Median [25th, 75th percentile] | 19.1 [5.6, 192.4] | 7.2 [3.6, 8.1] | - | 0.039 | 8.6 [3.9, 78.3] | 9.8 [4.6, 188.2] | - | 0.519 | 65.3 [6.7, 350.1] | 4.6 [1.6, 8.6] | - | <0.01 |
| Mean ± standard deviation | 294.3 ± 727.5 | 29.2 ± 79.6 | 0.51 | 0.121 | 79.4 ± 145.0 | 420.4 ± 965.2 | −0.49 | 0.047 | 322.6 ± 745.8 | 12.7 ± 24.5 | 0.59 | 0.064 |
| Mean ± standard deviation (log-transformed) | 3.48 ± 2.39 | 1.85 ± 1.74 | 0.78 | <0.01 | 2.59 ± 2.11 | 3.44 ± 2.58 | −0.36 | 0.204 | 3.83 ± 2.24 | 1.48 ± 1.57 | 1.22 | <0.01 |
| Multiple-genotype infection | 34 (89.5%) | 6 (31.6%) | 1.47 | <0.01 | 26 (66.7%) | 14 (77.8%) | −0.25 | 0.394 | 33 (100%) | 7 (29.1%) | 2.20 | <0.01 |
| Prognosis | ||||||||||||
| Recurrence in 1 year (n = 46) | 9 (23.7%) | 6 (31.6%) | −0.18 | 0.495 | 7 (17.9%) | 4 (22.2%) | −0.11 | 0.566 | 7 (21.2%) | 8 (33.3%) | −0.27 | 0.341 |
| Antiglaucomatic medication use | 22 (57.9%) | 13 (68.4%) | −0.16 | 0.758 | 27 (69.2%) | 8 (44.4%) | 0.52 | 0.203 | 17 (51.5%) | 18 (75%) | −0.50 | 0.076 |
| Further filtering surgery | 7 (18.4%) | 3 (15.8%) | 0.08 | 1 | 8 (20.5%) | 2 (11.1%) | 0.26 | 0.705 | 4 (12.1%) | 6 (25%) | −0.34 | 0.298 |
| Corneal decompensation | 5 (13.2%) | 3 (15.8%) | −0.03 | 1 | 7 (17.9%) | 1 (5.6%) | 0.39 | 0.415 | 3 (9.1%) | 5 (25%) | −0.43 | 0.272 |
| Covariates Adjustment | B for Multiple Genotypes (95% CI) | p Value |
|---|---|---|
| None | 2.57 (1.40, 3.74) | <0.001 |
| Endotheliitis | 2.55 (1.35, 3.76) | <0.001 |
| gB1 and endotheliitis | 2.40 (0.94, 3.87) | 0.002 |
| gB3 and endotheliitis | 2.48 (1.26, 3.70) | <0.001 |
| gB4 and endotheliitis | 1.72 (−0.50, 3.94) | 0.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Cheng, Y.-C.; Hwang, Y.-S.; Kang, E.Y.-C.; Hsiao, C.-H. Cytomegalovirus Glycoprotein B Genotype in Patients with Anterior Segment Infection. Int. J. Mol. Sci. 2023, 24, 6304. https://doi.org/10.3390/ijms24076304
Huang C-Y, Cheng Y-C, Hwang Y-S, Kang EY-C, Hsiao C-H. Cytomegalovirus Glycoprotein B Genotype in Patients with Anterior Segment Infection. International Journal of Molecular Sciences. 2023; 24(7):6304. https://doi.org/10.3390/ijms24076304
Chicago/Turabian StyleHuang, Chu-Yen, Yu-Chun Cheng, Yih-Shiou Hwang, Eugene Yu-Chuan Kang, and Ching-Hsi Hsiao. 2023. "Cytomegalovirus Glycoprotein B Genotype in Patients with Anterior Segment Infection" International Journal of Molecular Sciences 24, no. 7: 6304. https://doi.org/10.3390/ijms24076304
APA StyleHuang, C.-Y., Cheng, Y.-C., Hwang, Y.-S., Kang, E. Y.-C., & Hsiao, C.-H. (2023). Cytomegalovirus Glycoprotein B Genotype in Patients with Anterior Segment Infection. International Journal of Molecular Sciences, 24(7), 6304. https://doi.org/10.3390/ijms24076304







