Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations
Abstract
:1. Introduction
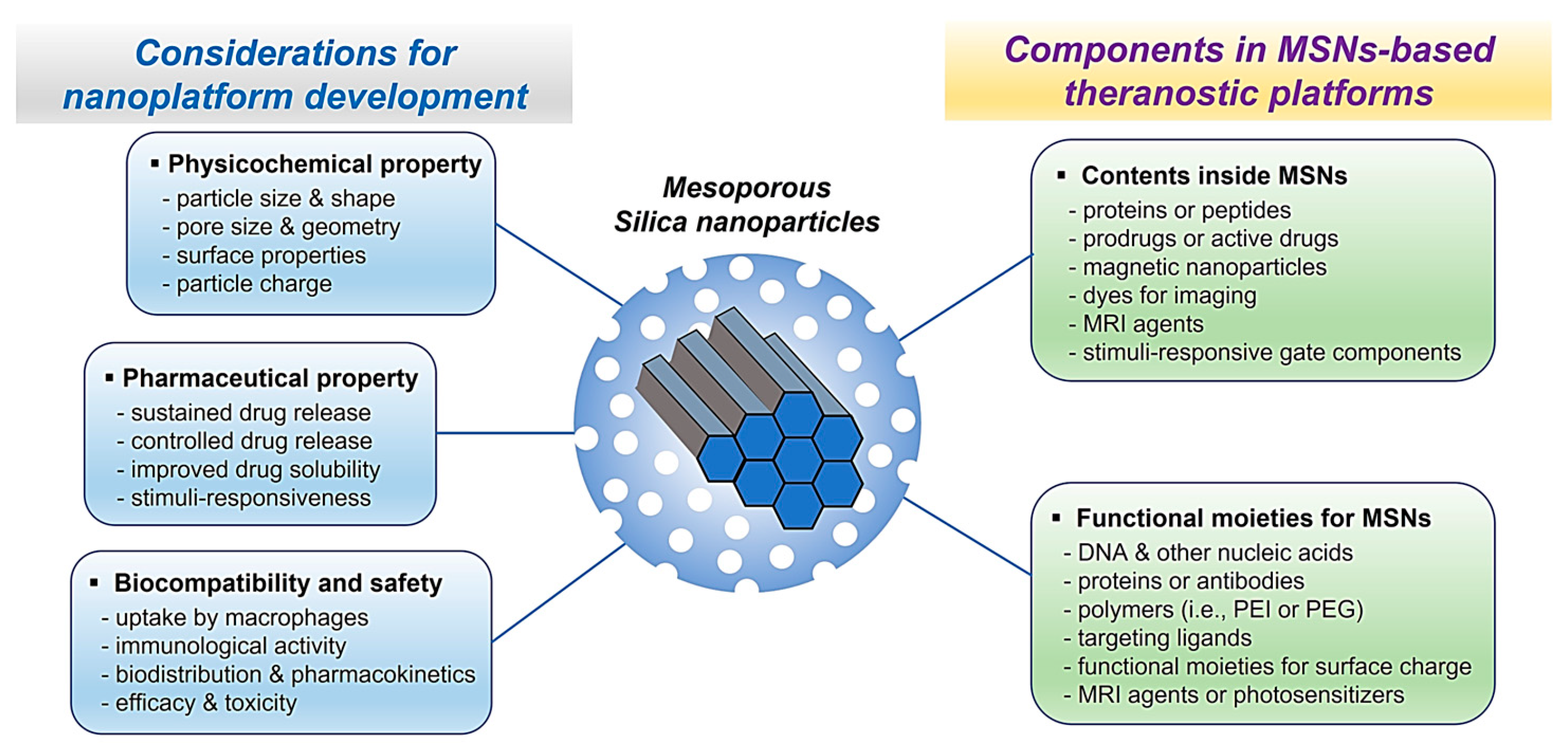
2. Physicochemical Properties of Mesoporous Silica Nanoparticles
3. Synthesis of Mesoporous Silica Nanoparticles
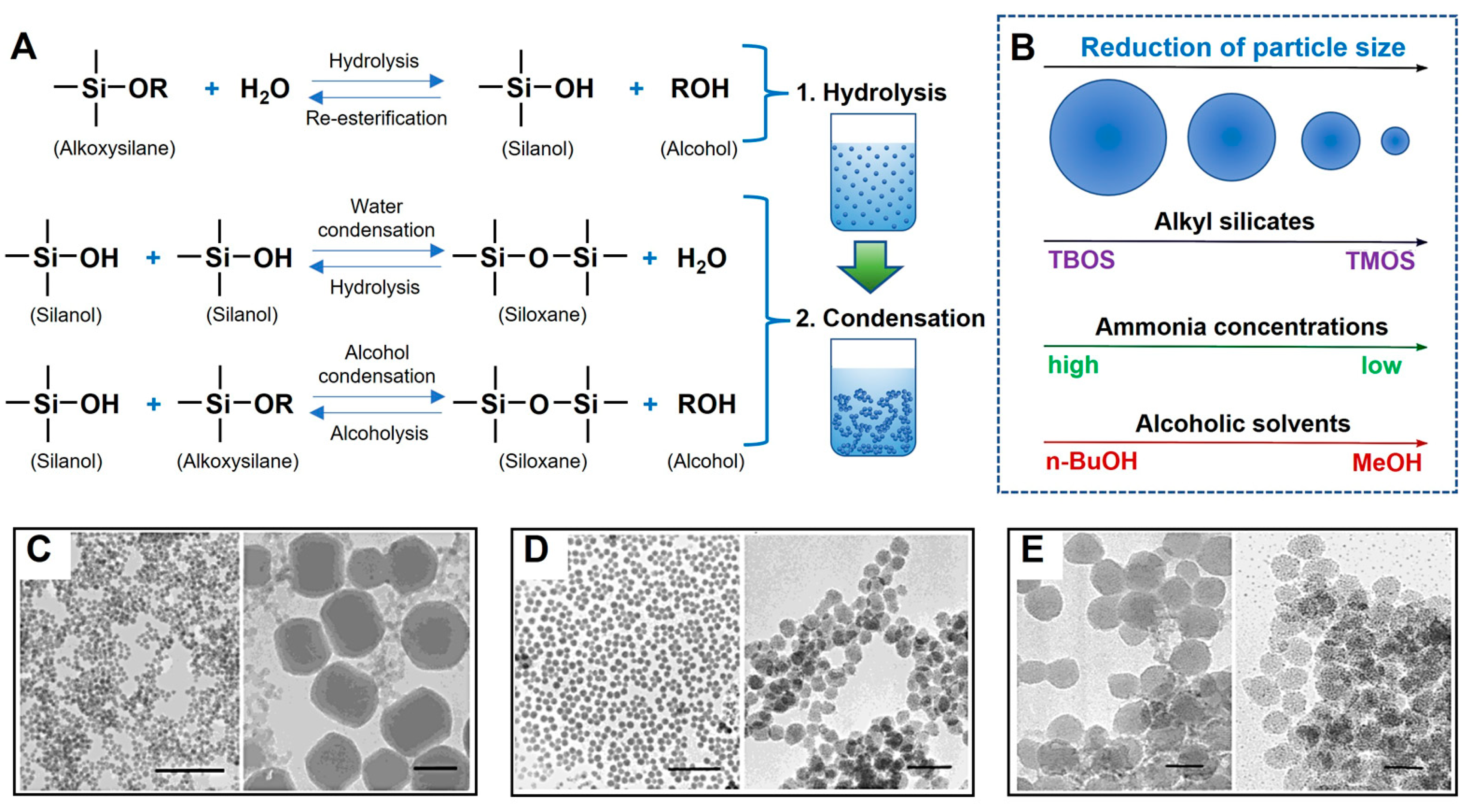
3.1. Sol–Gel Processing of Mesoporous Silica Nanoparticles
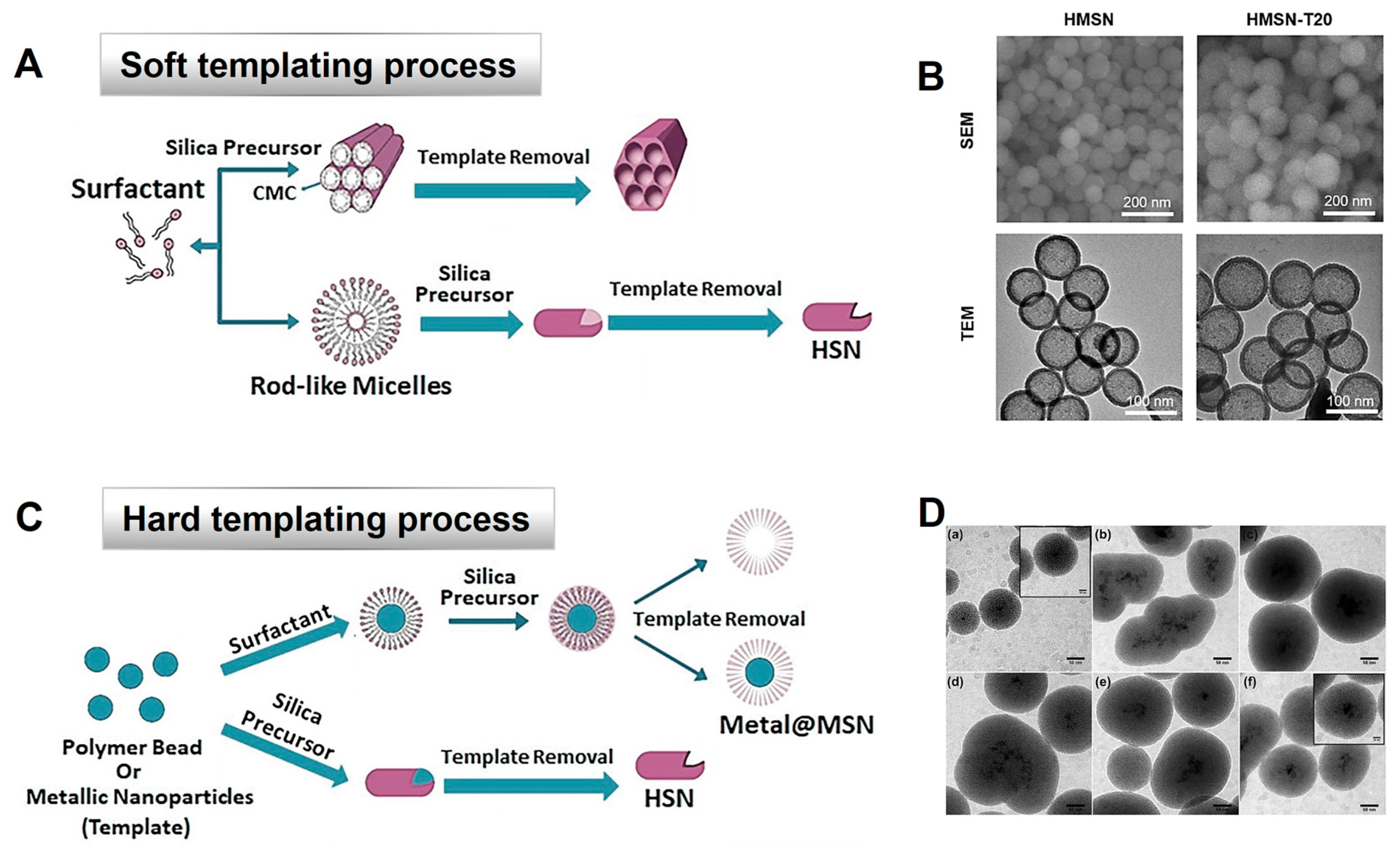
3.2. Synthesis of Hollow Mesoporous Silica Nanoparticles by ‘Soft’ or ‘Hard’ Templating
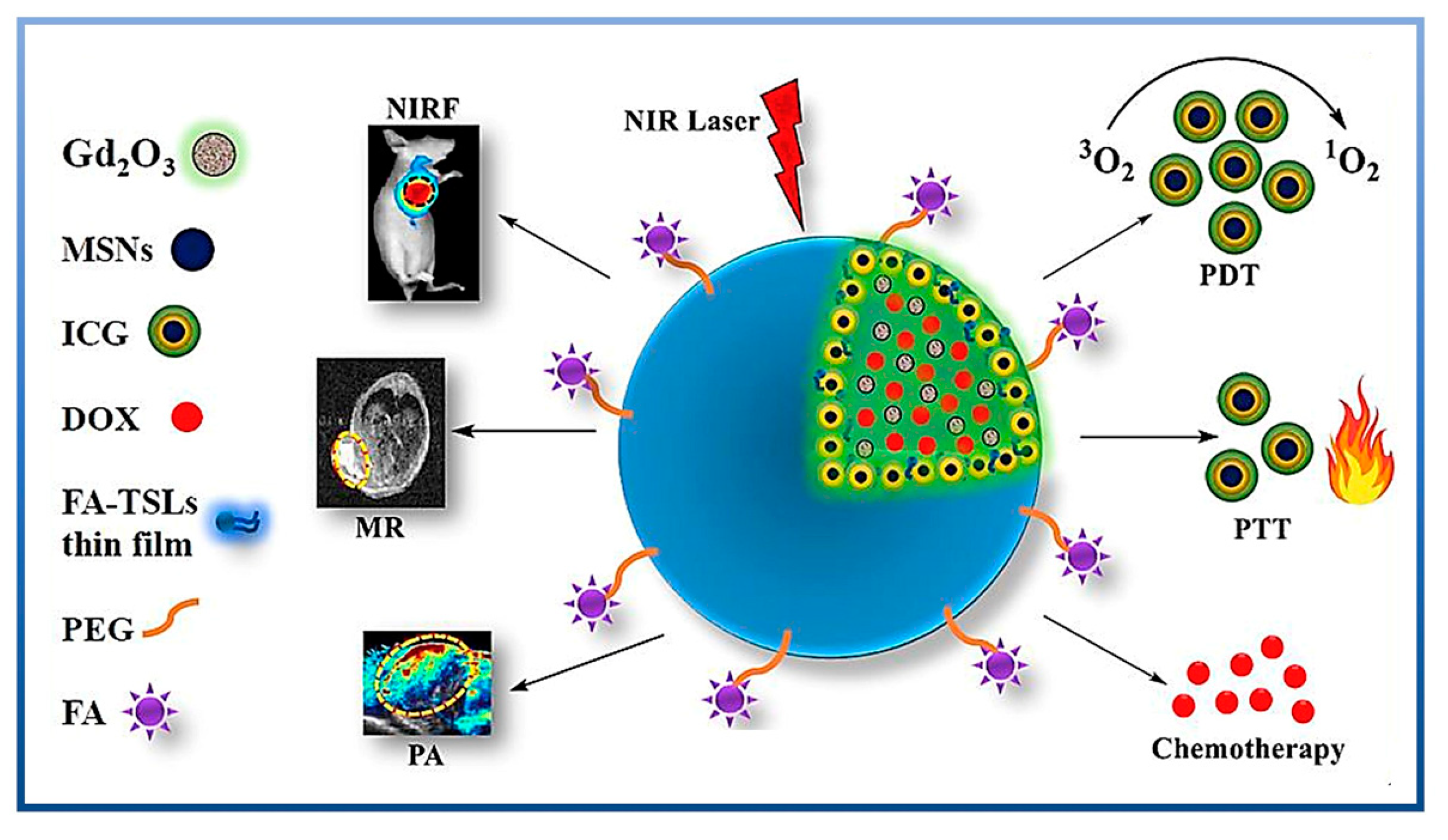
4. Multifunctional MSNs for Theranostics
5. Therapeutic Application of MSNs
5.1. Application of MSNs in Cancer Therapy
5.1.1. Surface-Functionalized MSNs in Cancer Therapy
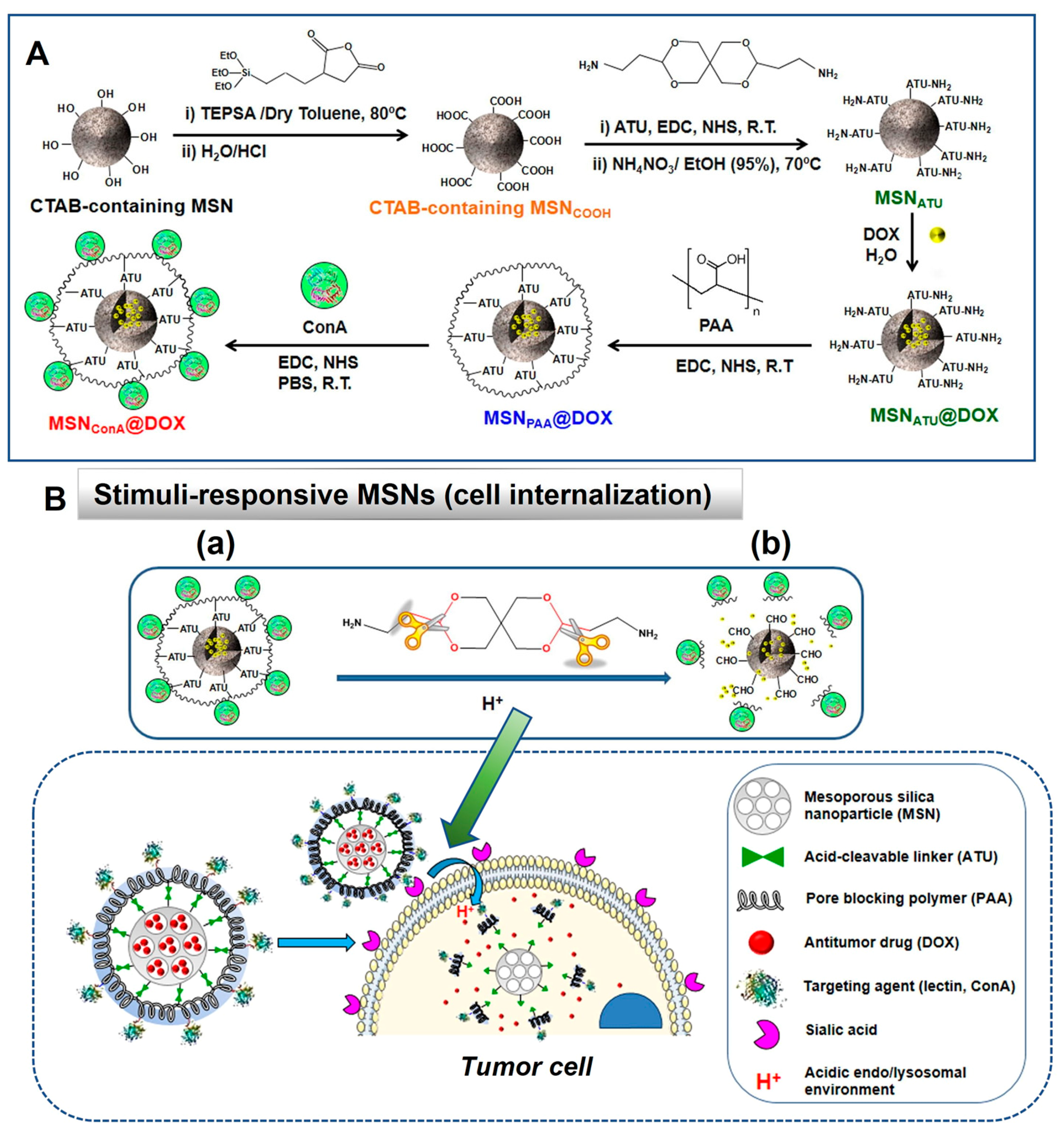
5.1.2. Stimuli-Responsive DDS Using MSNs in Cancer Therapy
5.2. Application of MSNs for Other Diseases
5.2.1. Surface-Functionalized MSNs in Other Diseases
5.2.2. Stimuli-Responsive DDS Using MSNs in Other Diseases
5.3. Application of MSNs in Tissue Engineering
| Therapeutic Agents with Matrix or Scaffold | MSNs Properties | Examined Cell/ Animal Model | Target Disease | Research Outcomes | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) | Diameter (nm) | |||||
| HMSNs-CS-DOX@CuS | - | 1.84 | - | 150 ± 13 | MDA-MB-231 cells; Mice | Breast cancer | Both in vitro and in vivo showed excellent apoptosis effects on cancer cells and provided extended lifetimes in animal models, which can be a promising theranostic (PTT) method in cancer therapy. | Niu, 2021 [59] |
| CAP-MSN capped with CHS-GCA (CAP: capecitabine) | 419.36 ± 6.98 | 8.12 ± 0.43 | 0.73 ± 0.21 | 245.24 ± 5.75 | HCT 116 cells; Rats | Colorectal cancer | Drug was released in a controlled manner from (CAP-MSN)CHS-GCA for up to 72 h, providing a cytotoxic effect at low doses and reducing toxic effects on non-target organs. | Narayan, 2021 [62] |
| DOX@MSN-pTA (DOX: doxorubicin) | 607 | 1.7 | - | ca. 200 | 4T1 cells; Mice | Cancer | DOX@MSN-pTA displayed the highest cytotoxic effect in vitro and as a combined chemo-photothermal (PTT) therapy in vivo. | Shi, 2021 [100] |
| PTX@DMSN@PMAsh-Tf (PTX: paclitaxel) | 376 | 13 | 1.36 | ca. 100 | A549 cells; Mice | Cancer | Particles were efficacious in inhibiting tumor growth in in vivo trials with high drug loading, good colloidal stability, and low cytotoxicity. | Deng, 2021 [101] |
| MTX-loaded MSN–APTES–chitosan (MTX: methotrexate) | 789 ± 4 | - | 0.83 | 97.7 ± 8.8 | MCF7 cells | Breast cancer | Breast cancer cell viability could be significantly affected by MTX-loaded MSN–APTES–chitosan at a relatively low dose | Shakeran, 2021 [102] |
| Curcumin-loaded MSN-HA-C | - | - | - | 75–110 | MCF-7 cells; MDA-MB 231 cells; Mice | Breast cancer | The nanohybrid design effectively reduced tumor volume and increased efficacy against cancer through induction of ROS, cell cycle arrest, and apoptosis rather than free curcumin. | Ghosh, 2021 [103] |
| Myr-loaded MSN (Myr: myricetin) | 109.8 | A594 cells; NCI-H1299 cells; Mice | Non-small-cell lung cancer (NSCLC) | The treatment of NSCLC by Myr-loaded MSN combined with MRP-1 siRNA can be effective given the fact that significant apoptosis occurs in cancer cells with the least amount of side effects. | Song 2020 [104] | |||
| DOX-loaded MSN@MPN | 526.26 | - | 0.973 | 95–110 | A549 cells | Lung cancer | Dox-loaded MSN coated with MPN provided a significant PTT effect and pH-triggered drug release to kill cancer cells effectively. | Yang, 2020 [105] |
| MNPSiO2-FA/Cis-Pt (FA: folic acid, Cis-Pt: cisplatin) | 1011 | 3–5 | 1.10 | 100 | LN18 cells | Glioblastoma cancer | The formulation showed a high cytotoxicity effect and excellent biocompatibility with the controlled drug release in a sustained manner. | Ortiz-Islas, 2021 [106] |
| NMS-MSNs-COOH; IMC-MSNs-COOH (NMS: nimesulide, IMC: indomethacin) | 421; 392 | 1.8; 1.6 | 0.40; 0.38 | 232.5; 238.6 | Rats | Inflammatory diseases | In vivo results demonstrated that NMS/IMC-loaded MSN-COOH could exert a strong anti-inflammatory effect by achieving higher bioavailability for NMS and IMC with increased dissolution. | Gou, 2021 [64] |
| MSN-A-Pre-Eu; MSN-A-Bud-Eu (Pre: prednisolone, Bud: budesonide) | 26 ± 12; 20 ± 14 | - | 0.4 ± 0.02; 0.5 ± 0.02 | 238 ± 12.7; 242 ± 18.6 | Mice | Inflammatory bowel disease | Compared to free drugs, the pH-responsive formulation based on MSNs reduced inflammation by preventing premature drug release and improving drug efficacy. | Qu, 2020 [94] |
| Ibuprofen | 736.5 ± 15.29 | 2.4 | - | 150 | Human Embryo Kidney cells | Inflammatory diseases | Using MSNs-based DDS, solubility, bioavailability, and stability issues of ibuprofen could be improved. Sustained drug release was enabled by MSNs, which reduces the frequency of dosing, thus reducing side effects related to NSAIDs. | Ortega, 2020 [107] |
| N-EDMSNs/pFGF21/Lira (Lira: liraglutide) | 567 | 10.7 | 1.27 | ca. 230 | Hepa1–6 cells; Mice | Type 2 diabetes mellitus (T2DM) | MSNs-based DDS (delivering GLP-1AR (Lira) and FGF-21 plasmids) was more effective than non-MSN types in improving glucose tolerance and inhibiting PEPCK and G-6-Pase activity without causing toxicity or side effects. | Geng, 2021 [91] |
| VLG-SiNPs (VLG: vildagliptin) | 962.5 | 0.95 | 310.9–383.68 | - | Diabetes | A new DDS based on MSNs enabled sustained release of the antidiabetic drug, which might contribute to reducing the frequency of drug administration and ultimately enhancing patients’ compliance. | Shirsath, 2021 [108] | |
| CBC-MCC@hMSN(SM) (CBC: conbercept, MCC: MCC950) | 18.4 | 7.1 | 0.07 | 338.2 | HRVECs; Mice | Ocular vascular disease | The formulation increased anti-angiogenic and anti-inflammatory efficacy, resulting in sustained suppression of inflammatory responses in the ocular tissues. | Sun, 2023 [92] |
| Van-mPEG-TK-MSNs (Van: vancomycin) | 341.4 | - | 0.59 | 100 | MC3T3-E1 cells; S. aureus; Rats | Bacterial infectious diseases | Van-mPEG-TK-MSNs exerted controlled release of antibacterial drug molecules via reactive oxygen species (ROS)-responsive delivery. | Li, 2020 [93] |
| SBA15@NH2/LVX/PLA-NF (LVX: levofloxacin) | 163.96 | 5.4 | 0.011 | - | HFB4 cells; S. aureus; E. coli; C. albicans; A. niger | Infectious diseases | MSNs-based formulation loaded with LVX improved antimicrobial efficacy and cytocompatibility, which could help to reduce side effects. | Abdelbar, 2020 [109] |
| HG@MSN-CCM (HG: hydrogel, CCM: curcumin-loaded mesoporous) | 556 | 6.4 | 1.29 | 158.1 ± 9.64 | L929 cells; Mice | Alzheimer’s disease (AD) | Animal groups treated with MSN-CCM or HG@MSN-CCM showed higher memory retention than those with CCM or HG@MSN. These MSN-based formulations regressed cognitive deficits in mice, suggesting their potential to treat AD. | Riberio, 2022 [95] |
| MSN-Ca-RV-PS (RV: rivastigmine) | 36.20 | 4.20 | 0.25 | >100 | PC12 cells; Rats | Alzheimer’s disease (AD) | Brain uptake clearance, the plasma half-life of the drug, and the brain-to-plasma concentration ratio were improved by using MSNs compared with the free drug. | Basharzad, 2022 [110] |
| MS/LA/RGD/UK (LA: L-arginine, UK: urokinase) | 192 | - | 0.6 | 255 | HUVECs; Rats | Venous thrombosis | MSNs-based DDS reduced ROS levels, improved endothelialization processes with appropriate blood compatibility, and finally increased thrombolytic activity in vivo. | Tao, 2022 [96] |
| Q-MSNs (Q: quercetin) | - | - | - | 100–150 | Rats | Myocardial Ischemia-Reperfusion Injury | MSNs-based formula (Q-MSNs) helped to increase the pharmacological activity of quercetin by inhibiting cell apoptosis and oxidative stress and improving cardiac blood flow recovery. | Liu, 2021 [111] |
| MSN-NGR1-CD11b antibody (NGR1: notoginsenoside R1) | - | - | - | 83 | H9C2 cells; Mice | Myocardial infarction | The combined formulation of MSNs with NGR1 and CD11b antibodies enhanced drug delivery to the target site, showing increased cardiac function and reduced local inflammation in vivo. MSNs protected H9Cs cells from oxidative stress damage. | Li, 2022 [112] |
| SA-PSiO2-SeNDs-PEG (SA: synaptic acid) | - | - | - | 188 | HUVECs; Mice | Cardiovascular disease | The SA-PSiO2-SeNDs-PEG nanocomposite promoted more stable and sustainable drug release and no side effects. The formulation provided some health benefits, i.e., a reduction in ROS stress and a decrease in LDL-C level. | Bi, 2020 [113] |
| SIM@HA-MSN (SIM: simvastatin, HA: hyaluronic acid) | 27.64 | - | 0.187 | 189.1 ± 5.8 | Raw264.7 cells; HUVECs | Atherosclerosis | The results showed a new treatment for atherosclerosis with robust targeting, anti-inflammatory activity, and low toxicity by using MSNs. The formulation exhibited properties of lesion-targeting and long-circulation in blood. | Song, 2022 [114] |
| TNFR-Dex-MSNs (Dex: dexamethasone) | 1167 | 2.5 | 0.96 | 222 ± 17 | Mice | Acute lung injury | By reducing cytokine levels and side effects, MSNs-based DDS capped with a peptide targeting the TNFR1 receptor provided a more significant therapeutic effect than the free drug. | García-Fernández, 2021 [115] |
| Dex-loaded RMSNs | - | - | - | 513.6 ± 63.1 | Rats | Rheumatoid arthritis | Comparatively to the control group, Dex-loaded RMSNs showed significant anti-inflammatory effects and cartilage regeneration as well as high drug-loading efficiency. | Kim, 2022 [116] |
| CSL@HMSNs-Cs (CSL: celastrol; Cs: chitosan) | 52.82 | 2.4 | 0.121 | 260.8–290.2 | Chondrocytes; Rats | Knee osteoarthritis | A pH-responsive MSNs formulation loaded with CSL showed high solubility for intra-articular injection and good therapeutic effect for osteoarthritis by downregulating protein levels in the NF-κB signaling pathway in chondrocytes. | Jin, 2020 [117] |
| MSNs-PA@PEI; MSNs-PEG@PEI | - | 2 | - | 150 | MC3T3-E1 cells; Mice | Osteoporosis | As carriers of SOST siRNAs, MSNs showed superior results at increasing osteogenic expression and delivering active substances to the target site compared to PTH administration. | Mora-Raimundo, 2021 [118] |
| Ca-, Mg- and Sr- co-doped MSNs | 668–1279 | 2.4–3.1 | 0.753–1.994 | 151.9–534.7 | Human periodontal ligament fibroblasts (hPDLFs) | Tissue regeneration | A dope MSNs with Ca, Mg, and Sr was formulated for delivery of moxifloxacin and increased cell proliferation, hemolysis activity, and differentiation of osteoblasts in periodontal ligament cells for tissue regeneration. | Pouroutzidou, 2021 [119] |
| CHX-loaded/MSN-PGA (CHX: chlorhexidine) | - | - | - | 84–98 | S. mutans; Dental pulp stem cells (DPSCs) | Restorative dentistry | Having high antibacterial activity and penetration ability inside dentin and lowering MMP-8 and cathepsin K levels in dentin, the formulation was found to be suitable for adhesive and restorative dentistry. | Akram, 2021 [120] |
| PCL + Cur + SBA-15; PCL + Cur + NH2-SBA-15 (Cur: curcumin) | 116.10 | 4.8 | 0.3 | - | B. Subtilis; E. coli; Swiss 3T6 cells; Rats | Skin wounds | The nanofiber formulation based on SBA-15 or NH2-SBA-15 design exhibited high biocompatibility, cell adhesion, cell viability, antibacterial activity, and significant wound healing effects in vitro and in vivo. | Rathinavel, 2021 [57] |
| Ce@MSNs (Ce: ceria) | 435.45 ± 10 | 3 ± 0.85 | 0.58 ± 0.01 | >70 and <200 | MC3T3-E1 cells; Raw264.7 cells | Osteoporosis | Ce@MSNs showed stable therapeutic effects and antioxidant activity, providing osteogenesis with low side effects. | Pinna, 2021 [121] |
| MSN@PEG/PEI–OGP (OGP: osteogenic growth peptide) | 593 | 3.38 | - | 107.8 ± 1.0 | MC3T3-E1 cells; Rabbits | Bone repair/regeneration | The MSNs-based formulation increased ALP activity and calcium deposition and also showed an excellent osteointegration effect for bone remodeling. | Chu, 2022 [122] |
| Alginate/Chitosan/MSN30 | - | - | - | 100 | BMSCs cells | Implant for craniofacial bone defects | Compared to the control, treatment with Alginate/Chitosan/MSN30 had a significant effect on cell viability and a positive effect on osteogenesis. | Yousefasl, 2021 [123] |
| DMSNs/M-CAG (dexamethasone-loaded MSNs) | - | - | - | 210.6 | BMSCs cells; Rats | Bone tissue regeneration | A composite scaffold was shown to increase cell proliferation and stimulate osteogenesis in rats with calvaria bone defects. | Zhou, 2020 [124] |
| HA-DMSN (HA: hydroxyapatite | - | 6.4 | - | 220 | BMSCs cells; Rats | Bone tissue regeneration | HA-DMSN increased ALP activity, bone regeneration, and calcium deposits in vitro and in vivo, resulting in enhancing bone regeneration in the cranial bone defect model. | Lei, 2020 [125] |
| MSN_miR-26a@PEI–KALA | - | - | - | ~109 | SD rBMSCs | Bone loss | MSNs were able to guarantee the stability of RNA by protecting miR-26a from degradation. The formulation significantly increased osteogenesis with relatively small doses. | Yan, 2020 [126] |
| Ciprofloxacin-loaded Chitosan-MSNs | - | 2.61 | 0.923 | 100 ± 13 | - | Bone regeneration | MSNs provided slow release of the antibacterial drug for 9 h compared to 2 h without MSNs, thus suggesting better treatment for bone regeneration by delivering antibacterial drugs. | Hezma, 2020 [127] |
6. Application of MSNs in Disease Diagnosis
7. Clinical Studies of Silica-Nanoparticle-Based Systems
8. Biocompatibility, Toxicity, and Safety Issues of MSNs for Biomedical Applications
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology applications in drug controlled release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–116. [Google Scholar]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Sabio, R.M.; Meneguin, A.B.; Ribeiro, T.C.; Silva, R.R.; Chorilli, M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Yadav, T.P.; Pandey, B.; Gupta, V.; Singh, S.P. Engineering nanomaterials for smart drug release: Recent advances and challenges. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 411–449. [Google Scholar]
- Rajpoot, K. Solid lipid nanoparticles: A promising nanomaterial in drug delivery. Curr. Pharm. Des. 2019, 25, 3943–3959. [Google Scholar] [CrossRef]
- Sakr, T.M.; Khowessah, O.; Motaleb, M.; Abd El-Bary, A.; El-Kolaly, M.; Swidan, M.M. I-131 doping of silver nanoparticles platform for tumor theranosis guided drug delivery. Eur. J. Pharm. Sci. 2018, 122, 239–245. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Tonga, G.Y.; Solfiell, D.; Rotello, V.M. Inorganic nanosystems for therapeutic delivery: Status and prospects. Adv. Drug Deliv. Rev. 2013, 65, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Meng, H.-M.; Li, Z. Near-infrared inorganic nanomaterial-based nanosystems for photothermal therapy. Nanoscale 2021, 13, 8751–8772. [Google Scholar] [CrossRef] [PubMed]
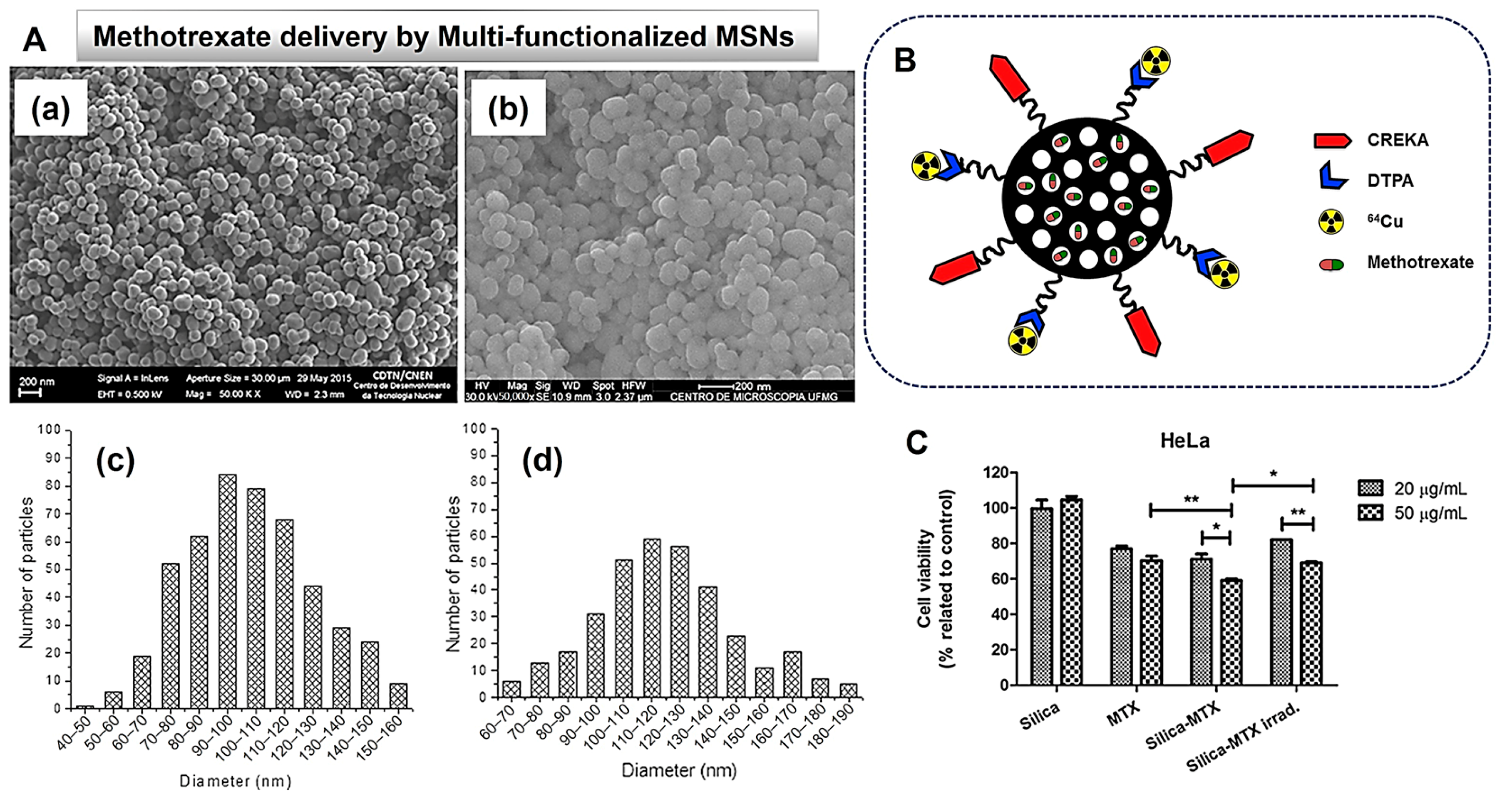
- de Oliveira Freitas, L.B.; de Melo Corgosinho, L.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; de Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanomaterial-based biotechnological and biomedical delivery systems. Nanomedicine 2007, 2, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Fulaz, S.; Hiebner, D.; Barros, C.H.; Devlin, H.; Vitale, S.; Quinn, L.; Casey, E. Ratiometric imaging of the in situ pH distribution of biofilms by use of fluorescent mesoporous silica nanosensors. ACS Appl. Mater. Interfaces 2019, 11, 32679–32688. [Google Scholar] [CrossRef]
- Tsou, C.-J.; Hung, Y.; Mou, C.-Y. Hollow mesoporous silica nanoparticles with tunable shell thickness and pore size distribution for application as broad-ranging pH nanosensor. Microporous Mesoporous Mater. 2014, 190, 181–188. [Google Scholar] [CrossRef]
- Kaczmarek, M. Lanthanide-sensitized luminescence and chemiluminescence in the systems containing most often used medicines; a review. J. Lumin. 2020, 222, 117174. [Google Scholar] [CrossRef]
- Tallury, P.; Payton, K.; Santra, S. Silica-based multimodal/multifunctional nanoparticles for bioimaging and biosensing applications. Nanomedicine 2008, 3, 579–592. [Google Scholar] [CrossRef]
- Murugan, B.; Sagadevan, S.; Lett, A.; Fatimah, I.; Fatema, K.N.; Oh, W.-C.; Mohammad, F.; Johan, M.R. Role of mesoporous silica nanoparticles for the drug delivery applications. Mater. Res. Express 2020, 7, 102002. [Google Scholar] [CrossRef]
- Karges, J.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S.; Gasser, G. Ru (II) Polypyridine Complex-Functionalized Mesoporous Silica Nanoparticles as Photosensitizers for Cancer Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 4394–4405. [Google Scholar] [CrossRef] [PubMed]
- Borawake, D.; Pande, V.; Giri, M. Mesoporous silica nanoparticles as theranostic platform for smart drug delivery: A review. J. Nanomed. Nanosci. 2017, 2017, JNAN-125. [Google Scholar]
- Ma, X.; Zhao, Y.; Liang, X.-J. Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc. Chem. Res. 2011, 44, 1114–1122. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Chomoucka, J.; Adam, V.; Ryvolova, M.; Eckschlager, T.; Hubalek, J.; Kizek, R. Nanocarriers for anticancer drugs-new trends in nanomedicine. Curr. Drug Metab. 2013, 14, 547–564. [Google Scholar] [CrossRef] [Green Version]
- Kresge, A.C.; Leonowicz, M.; Roth, W.J.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Saroj, S.; Rajput, S.J. Etoposide encapsulated functionalized mesoporous silica nanoparticles: Synthesis, characterization and effect of functionalization on dissolution kinetics in simulated and biorelevant media. J. Drug Deliv. Sci. Technol. 2018, 44, 27–40. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Wang, Y.; Caruso, F. Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. Chem. Mater. 2005, 17, 953–961. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Rosenholm, J.M.; Gu, H.-C. Synthesis and characterization of pore size-tunable magnetic mesoporous silica nanoparticles. J. Colloid Interface Sci. 2011, 361, 16–24. [Google Scholar] [CrossRef]
- Kioni, P.N.; Gao, Y.; Tang, Z.; Gatebe, E.; Wanyika, H. Synthesis and characterization of ordered mesoporous silica nanoparticles with tunable physical properties by varying molar composition of reagents. Afr. J. Pharm. Pharmacol. 2011, 5, 2402–2410. [Google Scholar]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef] [Green Version]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Mesoporous silica nanoparticles: A promising multifunctional drug delivery system. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–621. [Google Scholar]
- Li, Y.; Shi, J. Hollow-structured mesoporous materials: Chemical synthesis, functionalization and applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Stober, W.; Fink, A. Preparation of spherical silica nanoparticles: Stober silica. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.-Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khalid, S.H.; Asghar, S.; Irfan, M.; Asif, M.; Khalid, I.; Yousaf, A.M.; Hussain, T. Facile synthesis of mesoporous silica nanoparticles using modified sol-gel method: Optimization and in vitro cytotoxicity studies. Pak. J. Pharm. Sci 2019, 32, 1805–1812. [Google Scholar]
- Keshavarz, M.; Ahmad, N. Characterization and modification of mesoporous silica nanoparticles prepared by sol-gel. J. Nanoparticles 2013, 2013, 102823. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Porrang, S.; Rahemi, N.; Davaran, S.; Mahdavi, M.; Hassanzadeh, B. Preparation and in-vitro evaluation of mesoporous biogenic silica nanoparticles obtained from rice and wheat husk as a biocompatible carrier for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2021, 163, 105866. [Google Scholar] [CrossRef]
- Valtchev, V.; Tosheva, L. Porous nanosized particles: Preparation, properties, and applications. Chem. Rev. 2013, 113, 6734–6760. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.E.; Khushalani, D.; Lebeau, B.; Mann, S. Nanoscale materials with mesostructured interiors. Adv. Mater. 2001, 13, 649–652. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Wu, S.-H.; Tseng, C.-T.; Hung, Y.; Chang, C.; Mou, C.-Y. Synthesis of hollow silica nanospheres with a microemulsion as the template. Chem. Commun. 2009, 24, 3542–3544. [Google Scholar] [CrossRef]
- Li, Y.; Kruk, M. Single-micelle-templated synthesis of hollow silica nanospheres with tunable pore structures. Rsc Adv. 2015, 5, 69870–69877. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W. High storage capacity of curcumin loaded onto hollow mesoporous silica nanoparticles prepared via improved hard-templating method optimized by Taguchi DoE. Eng. Sci. Technol. Int. J. 2022, 33, 101070. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Truong-Thi, N.-H.; Nguyen, D.T.D.; Ching, Y.C.; Huynh, N.T.; Nguyen, D.H. Non-ionic surfactants As co-templates to control the mesopore diameter of hollow mesoporous silica nanoparticles for drug delivery applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130218. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, X.; Gao, Y.-E.; Hou, M.; Xue, P.; Li, C.M.; Kang, Y. Multifunctional silica nanoparticles as a promising theranostic platform for biomedical applications. Mater. Chem. Front. 2017, 1, 1257–1272. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Selvaratnam, B.; Koodali, R.T.; Sun, H. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. J. Control. Release 2018, 279, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-S.; Liu, T.-P.; Chien, F.-C.; Mou, C.-Y.; Wu, S.-H.; Chen, Y.-P. Codelivery of plasmid and curcumin with mesoporous silica nanoparticles for promoting neurite outgrowth. ACS Appl. Mater. Interfaces 2019, 11, 15322–15331. [Google Scholar] [CrossRef]
- Rathinavel, S.; Korrapati, P.S.; Kalaiselvi, P.; Dharmalingam, S. Mesoporous silica incorporated PCL/Curcumin nanofiber for wound healing application. Eur. J. Pharm. Sci. 2021, 167, 106021. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Laraib, U.; Barani, M.; Rahdar, A.; Fatima, I.; Bilal, M.; Pandey, S.; Sharma, R.K.; Kyzas, G.Z. Recent trends in the mesoporous silica nanoparticles with rode-like morphology for cancer theranostics: A review. J. Mol. Struct. 2022, 1261, 132922. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, X.; Williams, G.R.; Wu, J.; Gao, F.; Fu, Z.; Chen, X.; Lu, S.; Zhu, L.-M. Hollow mesoporous silica nanoparticles gated by chitosan-copper sulfide composites as theranostic agents for the treatment of breast cancer. Acta Biomater. 2021, 126, 408–420. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Wang, X.; Zhu, B.; Li, B.; Chen, Z.; Ma, G.; Wan, M. Sustained release of IGF-1 by 3D mesoporous scaffolds promoting cardiac stem cell migration and proliferation. Cell. Physiol. Biochem. 2018, 49, 2358–2370. [Google Scholar] [CrossRef]
- Rathinavel, S.; Ekambaram, S.; Korrapati, P.S.; Sangeetha, D. Design and fabrication of electrospun SBA-15-incorporated PVA with curcumin: A biomimetic nanoscaffold for skin tissue engineering. Biomed. Mater. 2020, 15, 035009. [Google Scholar] [CrossRef]
- Narayan, R.; Gadag, S.; Cheruku, S.P.; Raichur, A.M.; Day, C.M.; Garg, S.; Manandhar, S.; Pai, K.S.R.; Suresh, A.; Mehta, C.H. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: A smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr. Polym. 2021, 261, 117893. [Google Scholar] [CrossRef]
- He, H.; Meng, S.; Li, H.; Yang, Q.; Xu, Z.; Chen, X.; Sun, Z.; Jiang, B.; Li, C. Nanoplatform based on GSH-responsive mesoporous silica nanoparticles for cancer therapy and mitochondrial targeted imaging. Microchim. Acta 2021, 188, 154. [Google Scholar] [CrossRef]
- Gou, K.; Wang, Y.; Guo, X.; Wang, Y.; Bian, Y.; Zhao, H.; Guo, Y.; Pang, Y.; Xie, L.; Li, S. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021, 134, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Hanafi-Bojd, M.Y.; Jaafari, M.R.; Ramezanian, N.; Xue, M.; Amin, M.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur. J. Pharm. Biopharm. 2015, 89, 248–258. [Google Scholar] [CrossRef]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Jin, M.; Lee, H.-K.; Wang, M.-H.; Baek, J.-S.; Cho, C.-W. Effects of lipid nanoparticles on physicochemical properties, cellular uptake, and lymphatic uptake of 6-methoxflavone. J. Pharm. Investig. 2022, 52, 233–241. [Google Scholar] [CrossRef]
- Hegde, M.M.; Prabhu, S.; Mutalik, S.; Chatterjee, A.; Goda, J.S.; Satish Rao, B. Multifunctional lipidic nanocarriers for effective therapy of glioblastoma: Recent advances in stimuli-responsive, receptor and subcellular targeted approaches. J. Pharm. Investig. 2022, 52, 49–74. [Google Scholar] [CrossRef]
- Rhew, K.; Chae, Y.-J.; Chang, J.-E. Progress and recent trends in photodynamic therapy with nanoparticles. J. Pharm. Investig. 2022, 52, 587–599. [Google Scholar] [CrossRef]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of nanomaterials in vivo: Blood circulation and organ clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef]
- Unger, K.; Rupprecht, H.; Valentin, B.; Kircher, W. The use of porous and surface modified silicas as drug delivery and stabilizing agents. Drug Dev. Ind. Pharm. 1983, 9, 69–91. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, B.; Zhang, L.; Zhang, J.; Ma, T.; Zhang, J.; Fu, X.; Tian, W. Folic acid-functionalized mesoporous silica nanospheres hybridized with AIE luminogens for targeted cancer cell imaging. Nanoscale 2013, 5, 2065–2072. [Google Scholar] [CrossRef]
- Chen, N.-T.; Souris, J.S.; Cheng, S.-H.; Chu, C.-H.; Wang, Y.-C.; Konda, V.; Dougherty, U.; Bissonnette, M.; Mou, C.-Y.; Chen, C.-T. Lectin-functionalized mesoporous silica nanoparticles for endoscopic detection of premalignant colonic lesions. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1941–1952. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Sia, C.S.; Lim, H.P.; Tey, B.T.; Goh, B.-H.; Low, L.E. Stimuli-responsive nanoassemblies for targeted delivery against tumor and its microenvironment. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188779. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, F.; Wang, S.; Xia, F.; Ling, D. Biological Stimulus-Driven Assembly/Disassembly of Functional Nanoparticles for Targeted Delivery, Controlled Activation, and Bioelimination. Adv. Healthc. Mater. 2018, 7, 1800359. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-sensitive polymeric micelles based on poly (N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Zou, Z.; He, X.; He, D.; Wang, K.; Qing, Z.; Yang, X.; Wen, L.; Xiong, J.; Li, L.; Cai, L. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials 2015, 58, 35–45. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Ruiz-Hernández, E.; Hennink, W.E.; Vallet-Regí, M. Magnetic mesoporous silica-based core/shell nanoparticles for biomedical applications. Rsc Adv. 2013, 3, 9584–9593. [Google Scholar] [CrossRef]
- Paris, J.L.; Manzano, M.; Cabañas, M.V.; Vallet-Regí, M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale 2018, 10, 6402–6408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Duo, Y.; Bi, J.; Zeng, X.; Mei, L.; Bao, S.; He, L.; Shan, A.; Zhang, Y.; Yu, X. Targeted delivery of anti-miR-155 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Int. J. Nanomed. 2018, 13, 1241. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, C.; Liu, Y.; Chen, F.; Zheng, Z.; Wang, X. Enzyme-/redox-responsive mesoporous silica nanoparticles based on functionalized dopamine as nanocarriers for cancer therapy. ACS Omega 2019, 4, 6097–6105. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhao, Q.; Wang, X.; Mao, Y.; Chen, C.; Gao, Y.; Sun, C.; Wang, S. Multi-stimuli responsive mesoporous silica-coated carbon nanoparticles for chemo-photothermal therapy of tumor. Colloids Surf. B Biointerfaces 2020, 190, 110941. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Kettiger, H.; Einfalt, T.; Huwyler, J.r.; Witzigmann, D. Controlled tyrosine kinase inhibitor delivery to liver cancer cells by gate-capped mesoporous silica nanoparticles. ACS Appl. Bio Mater. 2019, 3, 239–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-T.; Lee, C.-H.; Chen, S.-T.; Lai, J.-Y.; Wu, K.C.-W. Gelatin-functionalized mesoporous silica nanoparticles with sustained release properties for intracameral pharmacotherapy of glaucoma. J. Mater. Chem. B 2017, 5, 7008–7013. [Google Scholar] [CrossRef] [PubMed]
- Nhavene, E.P.F.; da Silva, W.M.; Junior, R.R.T.; Gastelois, P.L.; Venâncio, T.; Nascimento, R.; Batista, R.J.C.; Machado, C.R.; de Almeida Macedo, W.A.; de Sousa, E.M.B. Chitosan grafted into mesoporous silica nanoparticles as benznidazol carrier for Chagas diseases treatment. Microporous Mesoporous Mater. 2018, 272, 265–275. [Google Scholar] [CrossRef]
- Mohamed, A.L.; Elmotasem, H.; Salama, A.A. Colchicine mesoporous silica nanoparticles/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int. J. Biol. Macromol. 2020, 164, 1149–1163. [Google Scholar] [CrossRef]
- Geng, S.; Qin, L.; He, Y.; Li, X.; Yang, M.; Li, L.; Liu, D.; Li, Y.; Niu, D.; Yang, G. Effective and safe delivery of GLP-1AR and FGF-21 plasmids using amino-functionalized dual-mesoporous silica nanoparticles in vitro and in vivo. Biomaterials 2021, 271, 120763. [Google Scholar] [CrossRef]
- Sun, J.; Nie, H.; Pan, P.; Jiang, Q.; Liu, C.; Wang, M.; Deng, Y.; Yan, B. Combined Anti-Angiogenic and Anti-Inflammatory Nanoformulation for Effective Treatment of Ocular Vascular Diseases. Int. J. Nanomed. 2023, 18, 437–453. [Google Scholar] [CrossRef]
- Li, J.; Ding, Z.; Li, Y.; Miao, J.; Wang, W.; Nundlall, K.; Chen, S. Reactive oxygen species-sensitive thioketal-linked mesoporous silica nanoparticles as drug carrier for effective antibacterial activity. Mater. Des. 2020, 195, 109021. [Google Scholar] [CrossRef]
- Qu, Z.; Wong, K.Y.; Moniruzzaman, M.; Begun, J.; Santos, H.A.; Hasnain, S.Z.; Kumeria, T.; McGuckin, M.A.; Popat, A. One-Pot Synthesis of pH-Responsive Eudragit-Mesoporous Silica Nanocomposites Enable Colonic Delivery of Glucocorticoids for the Treatment of Inflammatory Bowel Disease. Adv. Ther. 2021, 4, 2000165. [Google Scholar] [CrossRef]
- Ribeiro, T.d.C.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.d.A.; Planeta, C.d.S.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, X.; Wu, Z.; Chen, C.; Tan, K.; Wan, M.; Zhou, M.; Mao, C. Nitric oxide-driven nanomotors with bowl-shaped mesoporous silica for targeted thrombolysis. J. Colloid Interface Sci. 2022, 611, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-T.; Wu, K.C.-W.; Lee, C.-Y. Development of glycyrrhizin-conjugated, chitosan-coated, lysine-embedded mesoporous silica nanoparticles for hepatocyte-targeted liver tissue regeneration. Materialia 2020, 9, 100568. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.-H.; Eltohamy, M.; Perez, R.; Lee, E.-J.; Kim, H.-W. Collagen gel combined with mesoporous nanoparticles loading nerve growth factor as a feasible therapeutic three-dimensional depot for neural tissue engineering. RSC Adv. 2013, 3, 24202–24214. [Google Scholar] [CrossRef]
- Kim, M.S.; El-Fiqi, A.; Kim, J.-W.; Ahn, H.-S.; Kim, H.; Son, Y.-J.; Kim, H.-W.; Hyun, J.K. Nanotherapeutics of PTEN inhibitor with mesoporous silica nanocarrier effective for axonal outgrowth of adult neurons. ACS Appl. Mater. Interfaces 2016, 8, 18741–18753. [Google Scholar] [CrossRef]
- Shi, Q.; Wu, K.; Huang, X.; Xu, R.; Zhang, W.; Bai, J.; Du, S.; Han, N. Tannic acid/Fe3+ complex coated mesoporous silica nanoparticles for controlled drug release and combined chemo-photothermal therapy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126475. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Y.; Zhou, F.; Wu, M.; Zhang, Q.; Yi, D.; Yuan, W.; Wang, Y. Engineering of dendritic mesoporous silica nanoparticles for efficient delivery of water-insoluble paclitaxel in cancer therapy. J. Colloid Interface Sci. 2021, 593, 424–433. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces 2021, 197, 111404. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, S.; Zeng, J.; Zhang, L.; Zhang, R.; Liang, K.; Xie, L.; Shao, B.; Song, S.; Huang, G. Super-assembled core-shell mesoporous silica-metal-phenolic network nanoparticles for combinatorial photothermal therapy and chemotherapy. Nano Res. 2020, 13, 1013–1019. [Google Scholar] [CrossRef]
- Ortiz-Islas, E.; Sosa-Arróniz, A.; Manríquez-Ramírez, M.E.; Rodríguez-Pérez, C.E.; Tzompantzi, F.; Padilla, J.M. Mesoporous silica nanoparticles functionalized with folic acid for targeted release Cis-Pt to glioblastoma cells. Rev. Adv. Mater. Sci. 2021, 60, 25–37. [Google Scholar] [CrossRef]
- Ortega, E.; Ruiz, M.A.; Peralta, S.; Russo, G.; Morales, M.E. Improvement of mesoporous silica nanoparticles: A new approach in the administration of NSAIDS. J. Drug Deliv. Sci. Technol. 2020, 58, 101833. [Google Scholar] [CrossRef]
- Shirsath, N.R.; Goswami, A.K. Design and development of sustained release vildagliptin-loaded silica nanoparticles for enhancing oral bioavailability. BioNanoScience 2021, 11, 324–335. [Google Scholar] [CrossRef]
- Abdelbar, M.F.; Shams, R.S.; Morsy, O.M.; Hady, M.A.; Shoueir, K.; Abdelmonem, R. Highly ordered functionalized mesoporous silicate nanoparticles reinforced poly (lactic acid) gatekeeper surface for infection treatment. Int. J. Biol. Macromol. 2020, 156, 858–868. [Google Scholar] [CrossRef]
- Basharzad, S.F.; Hamidi, M.; Maleki, A.; Karami, Z.; Mohamadpour, H.; Zanjani, M.R.S. Polysorbate-coated mesoporous silica nanoparticles as an efficient carrier for improved rivastigmine brain delivery. Brain Res. 2022, 1781, 147786. [Google Scholar] [CrossRef]
- Liu, C.-J.; Yao, L.; Hu, Y.-M.; Zhao, B.-T. Effect of quercetin-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury in rats and its mechanism. Int. J. Nanomed. 2021, 16, 741. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Xu, Y.-W.; Mou, F.-F.; Shan, X.-L.; Wang, Q.-L.; Liu, B.-N.; Ning, K.; Liu, J.-J.; Wang, Y.-C. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 2022, 54, 102384. [Google Scholar] [CrossRef]
- Bi, X.; Bian, P.; Li, Z. Synaptic acid encapsulated with selenium-mesoporous silica nanocomposite: A potential drug in treating cardiovascular disease. J. Clust. Sci. 2021, 32, 287–295. [Google Scholar] [CrossRef]
- Song, K.; Tang, Z.; Song, Z.; Meng, S.; Yang, X.; Guo, H.; Zhu, Y.; Wang, X. Hyaluronic Acid-Functionalized Mesoporous Silica Nanoparticles Loading Simvastatin for Targeted Therapy of Atherosclerosis. Pharmaceutics 2022, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Sancho, M.; Bisbal, V.; Amorós, P.; Marcos, M.D.; Orzáez, M.; Sancenón, F.; Martínez-Máñez, R. Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles. A new therapeutic approach for acute lung injury treatment. J. Control. Release 2021, 337, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, Y.; Min, K.T.; Hong, S. Dexamethasone-Loaded Radially Mesoporous Silica Nanoparticles for Sustained Anti-Inflammatory Effects in Rheumatoid Arthritis. Pharmaceutics 2022, 14, 985. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wu, D.; Liu, X.-M.; Xu, J.-T.; Ma, B.-J.; Ji, Y.; Jin, Y.-Y.; Wu, S.-Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnol. 2020, 18, 94. [Google Scholar] [CrossRef]
- Mora-Raimundo, P.; Lozano, D.; Benito, M.; Mulero, F.; Manzano, M.; Vallet-Regí, M. Osteoporosis remission and new bone formation with mesoporous silica nanoparticles. Adv. Sci. 2021, 8, 2101107. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Liverani, L.; Theocharidou, A.; Tsamesidis, I.; Lazaridou, M.; Christodoulou, E.; Beketova, A.; Pappa, C.; Triantafyllidis, K.S.; Anastasiou, A.D. Synthesis and characterization of mesoporous mg-and sr-doped nanoparticles for moxifloxacin drug delivery in promising tissue engineering applications. Int. J. Mol. Sci. 2021, 22, 577. [Google Scholar] [CrossRef]
- Akram, Z.; Aati, S.; Ngo, H.; Fawzy, A. pH-dependent delivery of chlorhexidine from PGA grafted mesoporous silica nanoparticles at resin-dentin interface. J. Nanobiotechnol. 2021, 19, 43. [Google Scholar] [CrossRef]
- Pinna, A.; Baghbaderani, M.T.; Hernández, V.V.; Naruphontjirakul, P.; Li, S.; McFarlane, T.; Hachim, D.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Nanoceria provides antioxidant and osteogenic properties to mesoporous silica nanoparticles for osteoporosis treatment. Acta Biomater. 2021, 122, 365–376. [Google Scholar] [CrossRef]
- Chu, Y.S.; Wong, P.-C.; Jang, J.S.-C.; Chen, C.-H.; Wu, S.-H. Combining Mg–Zn–Ca Bulk Metallic Glass with a Mesoporous Silica Nanocomposite for Bone Tissue Engineering. Pharmaceutics 2022, 14, 1078. [Google Scholar] [CrossRef]
- Yousefiasl, S.; Manoochehri, H.; Makvandi, P.; Afshar, S.; Salahinejad, E.; Khosraviyan, P.; Saidijam, M.; Soleimani Asl, S.; Sharifi, E. Chitosan/alginate bionanocomposites adorned with mesoporous silica nanoparticles for bone tissue engineering. J. Nanostructure Chem. 2022, 1–15. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, P.; Nie, W.; Peng, C.; Li, T.; Qiang, L.; He, C.; Wang, J. Incorporation of dexamethasone-loaded mesoporous silica nanoparticles into mineralized porous biocomposite scaffolds for improving osteogenic activity. Int. J. Biol. Macromol. 2020, 149, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Cao, Y.; Hosseinpour, S.; Gao, F.; Liu, J.; Fu, J.; Staples, R.; Ivanovski, S.; Xu, C. Hierarchical dual-porous hydroxyapatite doped dendritic mesoporous silica nanoparticles based scaffolds promote osteogenesis in vitro and in vivo. Nano Res. 2021, 14, 770–777. [Google Scholar] [CrossRef]
- Yan, J.; Lu, X.; Zhu, X.; Hu, X.; Wang, L.; Qian, J.; Zhang, F.; Liu, M. Effects of miR-26a on osteogenic differentiation of bone marrow mesenchymal stem cells by a mesoporous silica nanoparticle-PEI-peptide system. Int. J. Nanomed. 2020, 15, 497–511. [Google Scholar] [CrossRef] [Green Version]
- Hezma, A.; Elkhooly, T.A.; El-Bahy, G.S. Fabrication and characterization of bioactive chitosan microspheres incorporated with mesoporous silica nanoparticles for biomedical applications. J. Porous Mater. 2020, 27, 555–562. [Google Scholar] [CrossRef]
- Abbasi, M.; Ghoran, S.H.; Niakan, M.H.; Jamali, K.; Moeini, Z.; Jangjou, A.; Izadpanah, P.; Amani, A.M. Mesoporous silica nanoparticle: Heralding a brighter future in cancer nanomedicine. Microporous Mesoporous Mater. 2021, 319, 110967. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Ding, S.-N. Simultaneous detection of two ovarian cancer biomarkers in human serums with biotin-enriched dendritic mesoporous silica nanoparticles-labeled multiplex lateral flow immunoassay. Sens. Actuators B Chem. 2022, 371, 132597. [Google Scholar] [CrossRef]
- Todea, M.; Simon, V.; Muresan-Pop, M.; Vulpoi, A.; Rusu, M.; Simion, A.; Vasilescu, M.; Damian, G.; Petrisor, D.; Simon, S. Silica-based microspheres with aluminum-iron oxide shell for diagnosis and cancer treatment. J. Mol. Struct. 2021, 1246, 131149. [Google Scholar] [CrossRef]
- Tuna, B.G.; Durdabak, D.B.; Ercan, M.K.; Dogan, S.; Kavruk, M.; Dursun, A.D.; Tekol, S.D.; Celik, C.; Ozalp, V.C. Detection of viruses by probe-gated silica nanoparticles directly from swab samples. Talanta 2022, 246, 123429. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Feinstein, J.A.; Cramer, J.A.; Boothroyd, J.A.; Shishkina, E.V.; Shur, V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: Long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017, 13, 345–363. [Google Scholar] [CrossRef]
- Meola, T.R.; Abuhelwa, A.Y.; Joyce, P.; Clifton, P.; Prestidge, C.A. A safety, tolerability, and pharmacokinetic study of a novel simvastatin silica-lipid hybrid formulation in healthy male participants. Drug Deliv. Transl. Res. 2021, 11, 1261–1272. [Google Scholar] [CrossRef]
- Tan, A.; Eskandar, N.G.; Rao, S.; Prestidge, C.A. First in man bioavailability and tolerability studies of a silica-lipid hybrid (Lipoceramic) formulation: A Phase I study with ibuprofen. Drug Deliv. Transl. Res. 2014, 4, 212–221. [Google Scholar] [CrossRef]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, D.K.; Stambuk, H.E.; Madajewski, B.; Montero, P.H.; Matsuura, D.; Busam, K.J.; Ma, K.; Turker, M.Z.; Sequeira, S.; Gonen, M.; et al. Use of Ultrasmall Core-Shell Fluorescent Silica Nanoparticles for Image-Guided Sentinel Lymph Node Biopsy in Head and Neck Melanoma: A Nonrandomized Clinical Trial. JAMA Netw. Open 2021, 4, e211936. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [Green Version]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern views on desilicification: Biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef]
- Kumar, R.; Roy, I.; Ohulchanskky, T.Y.; Vathy, L.A.; Bergey, E.J.; Sajjad, M.; Prasad, P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Kim, I.Y.; Choi, J.W.; Kwon, I.H.; Hwangbo, S.; Bae, S.-H.; Kwak, M.; Kim, J.; Lee, T.G.; Heo, M.B. Variations in in vitro toxicity of silica nanoparticles according to scaffold type in a 3D culture system using a micropillar/microwell chip platform. Sens. Actuators B: Chem. 2022, 369, 132328. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Youn, J.K.; Na, H.B.; Yu, T.; Kim, H.; Lee, S.M.; Koo, Y.M.; Kwak, J.H.; Park, H.G. Simple synthesis of functionalized superparamagnetic magnetite/silica core/shell nanoparticles and their application as magnetically separable high-performance biocatalysts. Small 2008, 4, 143–152. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Deng, Y.-D.; Zhang, X.-D.; Yang, X.-S.; Huang, Z.-L.; Wei, X.; Yang, X.-F.; Liao, W.-Z. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Desouky, E.M.; Hozayen, W.G.; Bin-Jumah, M.; El-Nahass, E.-S.; Soliman, H.A.; Farghali, A.A. Mesoporous silica nanoparticles trigger liver and kidney injury and fibrosis via altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 signaling in rats. Biomolecules 2019, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, R.; Xu, H.; Wang, G. Integrative Metabolomics, Proteomics and Transcriptomics Analysis Reveals Liver Toxicity of Mesoporous Silica Nanoparticles. Front. Pharmacol. 2022, 13, 73. [Google Scholar] [CrossRef]
- Garrido-Cano, I.; Candela-Noguera, V.; Herrera, G.; Cejalvo, J.M.; Lluch, A.; Marcos, M.D.; Sancenon, F.; Eroles, P.; Martínez-Máñez, R. Biocompatibility and internalization assessment of bare and functionalised mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2021, 310, 110593. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.; Li, J.; Li, S.; Li, H. Superiority of L-tartaric acid modified chiral mesoporous silica nanoparticle as a drug carrier: Structure, wettability, degradation, bio-adhesion and biocompatibility. Int. J. Nanomed. 2020, 15, 601–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, A.; Sokunbi, M.; Patel, T.; Chang, M.-W.; Ahmad, Z.; Singh, N. Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles. Nanomaterials 2022, 12, 2016. [Google Scholar] [CrossRef] [PubMed]


| Stimuli | Mechanism for Cancer Treatment | Research Reference |
|---|---|---|
| pH | Extracellular pH of malignant tissues and inflammatory tissues are in slightly acidic condition, i.e., around 6.0–7.0, whereas pH in normal healthy tissues is 7.4. Therefore, once reaching the target sites, MSNs are triggered, ensuring the release of the drug at the therapeutic concentration. | Sia et al., 2022 [76] |
| Redox state | Reduced Glutathione (GSH) is overexpressed in neoplastic tissue compared to healthy tissues. Therefore, under the different microenvironments of GSH levels in neoplastic and healthy tissues, disulfide cleavage of GSH-sensitive MSNs can be triggered to promote drug or gene delivery in tumor sites. | Hu et al., 2018 [77] |
| Temperature | Temperature is slightly increased by 4–5 °C in cancer. Based on this mechanism, a temperature-responsive controlled release system can be developed by grafting a temperature-sensitive nano-switch on the surface of MSNs and MSNs can release drugs with increased penetrability, specifically in cancer tissues. As for the temperature-sensitive component, polymers based on poly-N-isopropylacrylamide (PNIPAM) and its derivatives can be used. | Wei et al., 2009 [78] |
| Enzyme | Employing upregulated enzymes in any pathological condition can be used as a triggering point for designing stimuli-responsive MSNs that catalyze any chemical reactions in cancer tissues. For instance, matrix metalloproteinase in the cancer microenvironment or phospholipases in pancreatic cancer, etc., can be used in tailoring MSNs by changing linkers and capping agents on their functionalized surface. | Zou et al., 2015 [79] |
| Light | By incorporating a light-triggering system in MSNs, photodynamic or photothermal (PTT) therapy can be feasible by releasing drugs in tumor sites. For example, fluorophores such as near-infrared (NIR) dyes can be used for preparing MSNs for bioimaging or PTT. | Yang et al., 2012 [80] |
| Magnetic stimuli | Magnetic nanoparticles embedded in MSNs can be efficiently used in cancer therapy by using the activity of magnetic hyperthermia. The heat generated by magnetic nanoparticles under application of alternating magnetic field can facilitate efficiency of cancer treatment. | Knežević et al., 2013 [81] |
| Ultrasound | MSNs-based nanocomposites can be used for ultrasound-triggered drug delivery to cancer sites. Ultrasound and MSNs showed synergistic effects for cancer treatment and also could be applied for photoacoustic-imaging-guided chemotherapy. | Paris et al., 2018 [82] |
| Year (Actual Study Start) | Condition | Recruitment Status | Location | Identifier (Website Accessed on 2 February 2023) |
|---|---|---|---|---|
| 2007 | Stable angina Heart failure Atherosclerosis Multivessel coronary artery disease | Completed | Netherlands Russian Federation | NCT01270139 (NANOM-FIM) https://clinicaltrials.gov/ct2/show/NCT01270139 |
| 2008 | Head and neck cancer | Completed | United States, Arizona United States, Texas | NCT00848042 (Auroshell) https://clinicaltrials.gov/ct2/show/NCT00848042 |
| 2010 | Coronary artery disease Atherosclerosis | Terminated | Netherlands Russian Federation | NCT01436123 (NANOM-PCI) https://clinicaltrials.gov/ct2/show/NCT01436123 |
| 2011 | Newly diagnosed or recurrent metastatic melanoma patients Malignant brain tumors | Active, not recruiting | United States, New York | NCT01266096 https://clinicaltrials.gov/ct2/show/NCT01266096 |
| 2014 | Head and neck melanoma | Recruiting | United States, New York | NCT02106598 https://clinicaltrials.gov/ct2/show/NCT02106598 |
| 2016 | Neoplasms of the prostate | Completed | United States, Maryland United States, Michigan United States, New York United States, Texas | NCT02680535 https://clinicaltrials.gov/ct2/show/NCT02680535 |
| 2018 | Brain cancer Pituitary adenoma | Active, not recruiting | United States, New York | NCT03465618 https://clinicaltrials.gov/ct2/show/NCT03465618 |
| 2021 | Prostate cancer | Recruiting | United States, New York | NCT04167969 https://clinicaltrials.gov/ct2/show/NCT04167969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. https://doi.org/10.3390/ijms24076349
Djayanti K, Maharjan P, Cho KH, Jeong S, Kim MS, Shin MC, Min KA. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. International Journal of Molecular Sciences. 2023; 24(7):6349. https://doi.org/10.3390/ijms24076349
Chicago/Turabian StyleDjayanti, Krismala, Pooja Maharjan, Kwan Hyung Cho, Sehoon Jeong, Man Su Kim, Meong Cheol Shin, and Kyoung Ah Min. 2023. "Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations" International Journal of Molecular Sciences 24, no. 7: 6349. https://doi.org/10.3390/ijms24076349
APA StyleDjayanti, K., Maharjan, P., Cho, K. H., Jeong, S., Kim, M. S., Shin, M. C., & Min, K. A. (2023). Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. International Journal of Molecular Sciences, 24(7), 6349. https://doi.org/10.3390/ijms24076349






