Exploring Oxidoreductases from Extremophiles for Biosynthesis in a Non-Aqueous System
Abstract
:1. Introduction
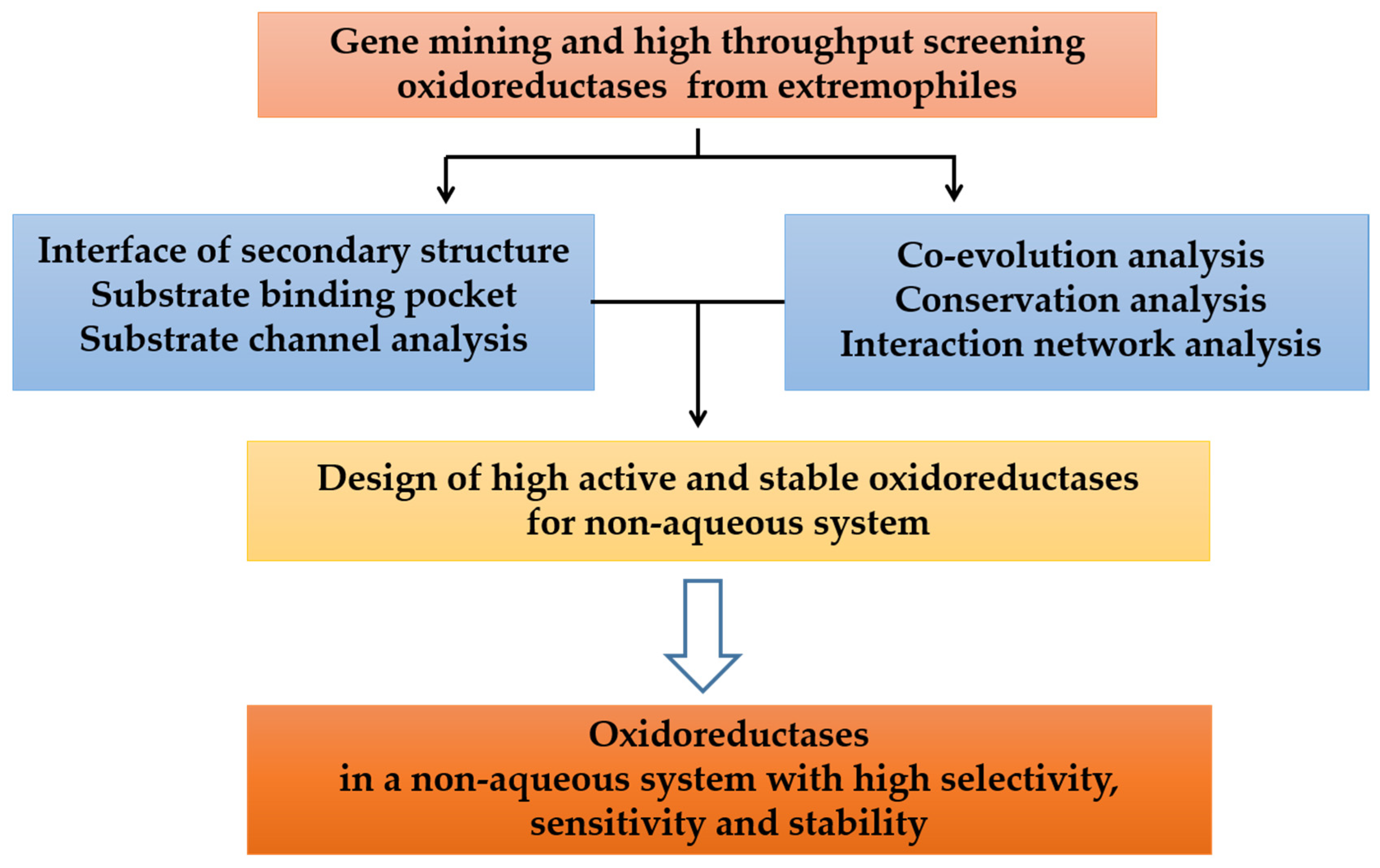
2. Mining Oxidoreductases from Extremophiles
3. Reaction of Oxidoreductases in Organic Media
4. Mechanism Study of Extreme Oxidoreductases in Organic Solvents
4.1. Structural and Dynamic Analysis
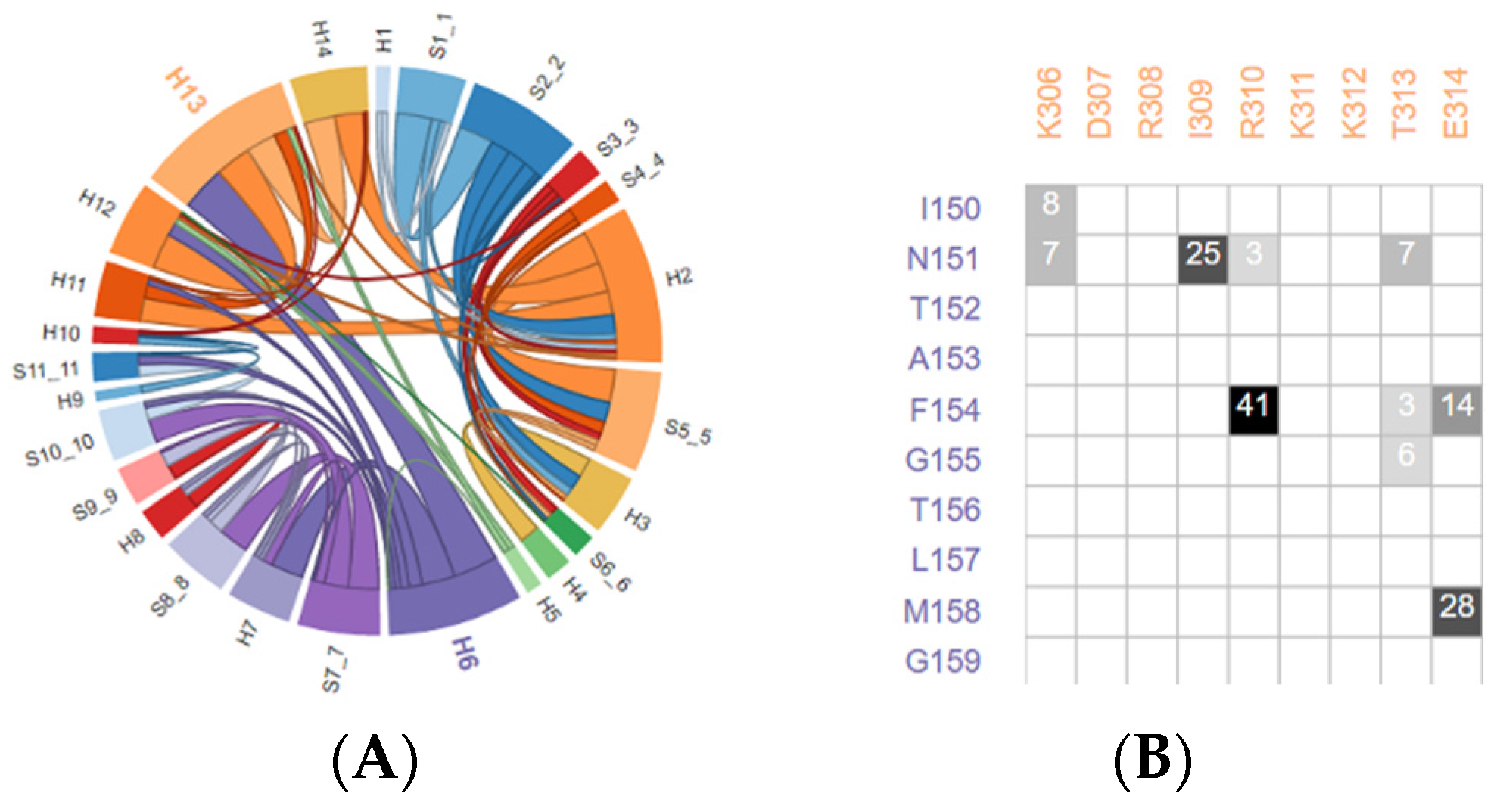
4.2. Amino Acid Residues Interaction Network
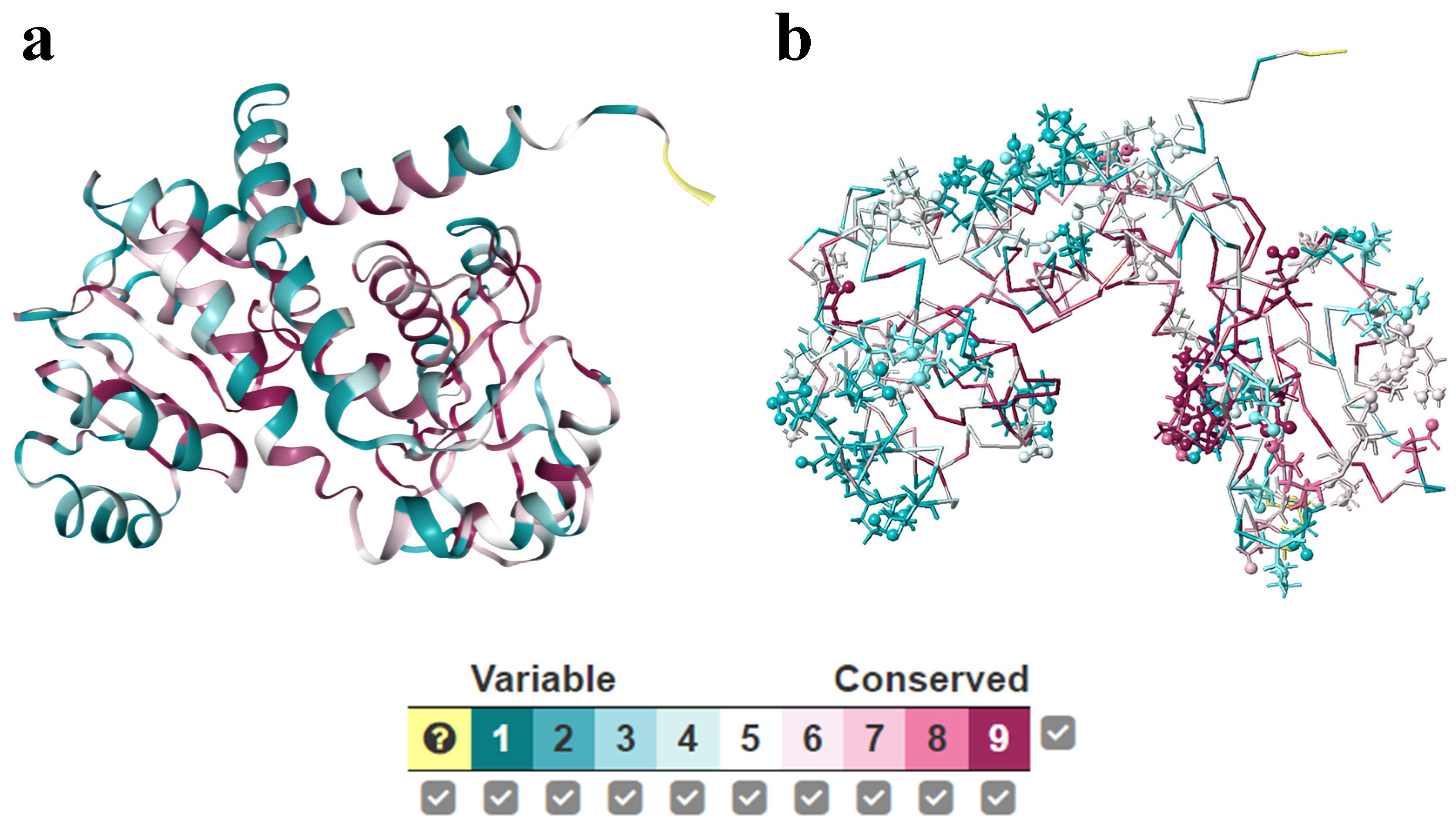
4.3. Conservation and Co-Evolution Analysis
5. Design of Oxidoreductases with High Organic Solvents Tolerance
6. Conclusions and Outlook
- A current challenge is how metagenomic mining can improve the discovery of polyextremophilic enzymes from multiple sources. Biotechnological exploitation of oxidoreductases from sequence derived statistics to the detailed atomic-level modeling of enzymes can provide cutting-edge technology in sequence annotation pipelines and bioinformatics resources;
- Using machine learning to effectively analyze big data on a higher-dimensional levels to establish an exemplary discipline assist experiment and reveal the interrelationships between the gene data, structure, and function [62];
- Key research challenges focus on high throughput screening and for the expression of extreme enzymes through dedicated hosts and purification systems and upscaling;
- Using extreme enzymes for electrochemical reaction offers enhanced stability and applications in extreme environments where common oxidoreductase would not survive [63]. The development of organic phase enzyme electrodes (OPEEs) is essential for the detection of various hydrophobic substrates in organic media for a variety of health and industrial applications [64,65];
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPME | Cyclopentyl methyl ether |
| MOF | Metal-organic frameworks |
| MDS | Molecular dynamic simulation |
| DMSO | Dimethyl sulfoxide |
| MTBE | Methyl tert-butyl ether |
References
- DasSarma, S.; DasSarma, P. Halophiles and their enzymes: Negativity put to good use. Curr. Opin. Microbiol. 2015, 25, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Khare, S.K. Enzymes from solvent-tolerant microbes: Useful biocatalysts for non-aqueous enzymology. Crit. Rev. Biotechnol. 2009, 29, 44–54. [Google Scholar] [CrossRef]
- Liszka, M.J.; Clark, M.E.; Schneider, E.; Clark, D.S. Nature versus nurture: Developing enzymes that function under extreme conditions. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 77–102. [Google Scholar] [CrossRef] [Green Version]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A potential source for industrial applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef]
- Qiu, J.J.; Han, R.; Wang, C. Microbial halophilic lipases: A review. J. Basic Microb. 2021, 61, 594–602. [Google Scholar] [CrossRef]
- Ando, N.; Barquera, B.; Bartlett, D.H.; Boyd, E.; Burnim, A.A.; Byer, A.S.; Colman, D.; Gillilan, R.E.; Gruebele, M.; Makhatadze, G.; et al. The molecular basis for life in extreme environments. Annu. Rev. Biophys. 2021, 50, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Yamaguchi, R.; Tokunaga, H.; Tokunaga, M. Unique Features of Halophilic Proteins. Curr. Protein Pept. Sci. 2016, 18, 65–71. [Google Scholar] [CrossRef]
- Morgan, J.A.; Clark, D.S. Salt-activation of nonhydrolase enzymes for use in organic solvents. Biotechnol. Bioeng. 2004, 85, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.; Paradisi, F. Haloquadratum walsbyi yields a versatile, NAD(+)/NADP(+) dual affinity, thermostable, alcohol dehydrogenase (HwADH). Mol. Biotechnol. 2018, 60, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Alsafadi, D.; Paradisi, F. Effect of organic solvents on the activity and stability of halophilic alcohol dehydrogenase (ADH2) from Haloferax volcanii. Extremophiles 2013, 17, 115–122. [Google Scholar] [CrossRef]
- Ding, H.-T.; Du, Y.-Q.; Liu, D.-F.; Li, Z.-L.; Chen, X.-J.; Zhao, Y.-H. Cloning and expression in E. coli of an organic solvent-tolerant and alkali-resistant glucose 1-dehydrogenase from Lysinibacillus sphaericus G10. Bioresour. Technol. 2010, 102, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, C.; Orita, I.; Imanaka, T.; Fukui, T.; Xing, X.-H. Thermostable Alcohol Dehydrogenase from Thermococcus kodakarensis KOD1 for Enantioselective Bioconversion of Aromatic Secondary Alcohols. Appl. Environ. Microbiol. 2013, 79, 2209–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, D.; Bayer, T.; Badenhorst, C.P.S.; Wu, S.; Doerr, M.; Höhne, M.; Bornscheuer, U.T. Recent trends in biocatalysis. Chem. Soc. Rev. 2021, 50, 8003–8049. [Google Scholar] [CrossRef]
- Jin, M.; Gai, Y.; Guo, X.; Hou, Y.; Zeng, R. Properties and applications of extremozymes from deep-sea extremophilic microorganisms: A mini review. Mar. Drugs 2019, 17, 656. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.; Méndez-García, C.; Bargiela, R.; Chow, J.; Alonso, S.; García-Moyano, A.; Bjerga, G.E.K.; Steen, I.H.; Schwabe, T.; Blom, C.; et al. Decoding the ocean's microbiological secrets for marine enzyme biodiscovery. Fems Microbiol. Lett. 2019, 366, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.X.; Yao, G.X.; Jiang, L.; Wang, S. Genome mining of organic solvent tolerant amino acid dehydrogenase for biosynthesis of unnatural amino acids in non-aqueous system. J. Chem. Ind. Eng. 2021, 72, 3757–3767. [Google Scholar]
- Schmermund, L.; Bierbaumer, S.; Schein, V.K.; Winkler, C.; Kara, S.; Kroutil, W. Extending the library of light-dependent protochlorophyllide oxidoreductases and their solvent tolerance, stability in light and cofactor flexibility. ChemCatChem 2020, 12, 4044–4051. [Google Scholar] [CrossRef]
- Wang, S.Z.; Fang, B.S.; Lin, P. A Kind of Method of High Throughput Testing Bacterial Strain Organic Solvent-Resistant Oxidoreductase. CN106191025B, 14 June 2019. [Google Scholar]
- Wang, S.-Z.; Cruaud, C.; Aury, J.-M.; Vallenet, D.; Poulain, J.; Vacherie, B.; Zaparucha, A.; Vergne-Vaxelaire, C. Complete genome sequences of two pseudomonas species isolated from marine environments of the pacific ocean. Microbiol. Resour. Announc. 2021, 10, e01062-19. [Google Scholar] [CrossRef]
- Li, M.; Wen, J.P. Recent progress in the application of omics technologies in the study of bio-mining microorganisms from extreme environments. Microb. Cell Fact. 2021, 20, 178. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L.; Chen, X.; Lv, K.; Basiony, M.; Zhu, G.; Karthik, L.; Ouyang, L.; Zhang, L.; Liu, X. Recent advances in biotechnology for marine enzymes and molecules. Curr. Opin. Biotechol. 2021, 69, 308–315. [Google Scholar] [CrossRef]
- Contente, M.L.; Fiore, N.; Cannazza, P.; Padrosa, D.R.; Molinari, F.; Gourlay, L.; Paradisi, F. Uncommon overoxidative catalytic activity in a new halo-tolerant alcohol dehydrogenase. Chemcatchem 2020, 12, 5679–5685. [Google Scholar] [CrossRef]
- Akal, A.L.; Karan, R.; Hohl, A.; Alam, I.; Vogler, M.; Grötzinger, S.W.; Eppinger, J.; Rueping, M. A polyextremophilic alcohol dehydrogenase from the Atlantis II Deep Red Sea brine pool. Febs. Open Bio 2019, 9, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpdağtaş, S.; Çelik, A.; Ertan, F.; Binay, B. DMSO tolerant NAD(P)H recycler enzyme from a pathogenic bacterium, burkholderia dolosa PC543: Effect of N-/C-terminal his tag extension on protein solubility and activity. Eng. Life Sci. 2018, 18, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Sunder, A.V.; Singh, P.; Wangikar, P.P. Characterization and application of a robust glucose dehydrogenase from paenibacillus pini for cofactor regeneration in biocatalysis. Indian J. Microbiol. 2020, 60, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Munawar, N.; Engel, P.C. Overexpression in a non-native halophilic host and biotechnological potential of NAD(+)-dependent glutamate dehydrogenase from halobacterium salinarum strain NRC-36014. Extremophiles 2012, 16, 463–476. [Google Scholar] [CrossRef]
- Huang, L.; Aalbers, F.; Tang, W.; Röllig, R.; Fraaije, M.W.; Kara, S. Convergent cascade catalyzed by monooxygenase-alcohol dehydrogenase fusion applied in organic media. Chembiochem 2019, 20, 1653–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohtashami, M.; Fooladi, J.; Haddad-Mashadrizeh, A.; Housaindokht, M.R.; Monhemi, H. Molecular mechanism of enzyme tolerance against organic solvents: Insights from molecular dynamics simulation. Int. J. Biol. Macromol. 2019, 122, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Milčić, N.; Švaco, P.; Sudar, M.; Tang, L.; Blažević, Z.F.; Elenkov, M.M. Impact of organic solvents on the catalytic performance of halohydrin dehalogenase. Appl. Microbiol. Biotechnol. 2023, 107, 2351–2361. [Google Scholar] [CrossRef]
- Zhang, N.; Bittner, J.P.; Fiedler, M.; Beretta, T.; de María, P.D.; Jakobtorweihen, S.; Kara, S. Unraveling alcohol dehydrogenase catalysis in organic-aqueous biphasic systems combining experiments and molecular dynamics simulations. ACS Catal. 2022, 12, 9171–9180. [Google Scholar] [CrossRef]
- Lenton, S.; Walsh, D.L.; Rhys, N.H.; Soper, A.K.; Dougan, L. Structural evidence for solvent-stabilisation by aspartic acid as a mechanism for halophilic protein stability in high salt concentrations. Phys. Chem. Chem. Phys. 2016, 18, 18054–18062. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Zhang, R.Z.; Xu, Y. Structure-based mechanisms: On the way to apply alcohol dehydrogenases/reductases to organic-aqueous systems. Int. J. Biol. Macromol. 2021, 168, 412–427. [Google Scholar] [CrossRef]
- Dudkaitė, V.; Kairys, V.; Bagdžiūnas, G. Understanding the activity of glucose oxidase after exposure to organic solvents. J. Mater. Chem. B 2023, 11, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Ortega, G.; Diercks, T.; Millet, O. Halophilic protein adaptation results from synergistic residue-ion interactions in the folded and unfolded states. Chem. Biol. 2015, 22, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Wedberg, R.; Abildskov, J.; Peters, G.H. Protein dynamics in organic media at varying water activity studied by molecular dynamics simulation. J. Phys. Chem. B 2012, 116, 2575–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.K.; Bernard, D.N.; Bafna, K.; Doucet, N. Enzyme dynamics: Looking beyond a single structure. Chemcatchem 2020, 12, 4704–4720. [Google Scholar] [CrossRef]
- Vijayabaskar, M.S.; Vishveshwara, S. Interaction energy based protein structure networks. Biophys. J. 2010, 99, 3704–3715. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Fang, B.-S. Coenzyme binding site analysis of an isopropanol dehydrogenase with wide substrate spectrum and excellent organic solvent tolerance. Appl. Biochem. Biotechnol. 2020, 190, 18–29. [Google Scholar] [CrossRef]
- Kayikci, M.; Venkatakrishnan, A.J.; Scott-Brown, J.; Ravarani, C.N.J.; Flock, T.; Babu, M.M. Visualization and analysis of non-covalent contacts using the protein contacts atlas. Nat. Struct. Mol. Biol. 2018, 25, 185–194. [Google Scholar] [CrossRef]
- Anishchenko, I.; Ovchinnikov, S.; Kamisetty, H.; Baker, D. Origins of coevolution between residues distant in protein 3D structures. Proc. Natl. Acad. Sci. USA 2017, 114, 9122–9127. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, C.; Gagné, D.; Reynolds, K.A.; Doucet, N. Conserved amino acid networks modulate discrete functional properties in an enzyme superfamily. Sci. Rep. 2017, 7, 3207. [Google Scholar] [CrossRef] [Green Version]
- Hockenberry, A.J.; Wilke, C.O. Evolutionary couplings detect side-chain interactions. PeerJ 2019, 7, e7280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.J.M.; Tyzack, J.D.; Borkakoti, N.; Holliday, G.L.; Thornton, J.M. A global analysis of function and conservation of catalytic residues in enzymes. J. Biol. Chem. 2020, 295, 314–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanquart, S.; Groussin, M.; Le Roy, A.; Szöllosi, G.J.; Girard, E.; Franzetti, B.; Gouy, M.; Madern, D. Resurrection of ancestral malate dehydrogenases reveals the evolutionary history of halobacterial proteins: Deciphering gene trajectories and changes in biochemical properties. Mol. Biol. Evol. 2021, 38, 3754–3774. [Google Scholar] [CrossRef]
- Campbell, E.; Kaltenbach, M.; Correy, G.J.; Carr, P.D.; Porebski, B.T.; Livingstone, E.K.; Afriat-Jurnou, L.; Buckle, A.M.; Weik, M.; Hollfelder, F.; et al. The role of protein dynamics in the evolution of new enzyme function. Nat. Chem. Biol. 2016, 12, 944–950. [Google Scholar] [CrossRef]
- Sfriso, P.; Duran-Frigola, M.; Mosca, R.; Emperador, A.; Aloy, P.; Orozco, M. Residues coevolution guides the systematic identification of alternative functional conformations in proteins. Structure 2016, 24, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Pinto, G.P.; Corbella, M.; Demkiv, A.O.; Kamerlin, S.C.L. Exploiting enzyme evolution for computational protein design. Trends Biochem. Sci. 2022, 47, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. Consurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Stadtmüller, T.H.; Jiang, Q.; Jaeger, K.; Schwaneberg, U.; Davari, M.D. How to engineer organic solvent resistant enzymes: Insights from combined molecular dynamics and directed evolution study. Chemcatchem 2020, 12, 4073–4083. [Google Scholar] [CrossRef]
- Singh, A. Bottom-up de novo protein design. Nat. Methods 2021, 18, 233. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, L.; Eltoukhy, L.; Jiang, Q.; Korkunç, S.K.; Jaeger, K.-E.; Schwaneberg, U.; Davari, M.D. Enzyme hydration determines resistance in organic cosolvents. ACS Catal. 2020, 10, 14847–14856. [Google Scholar] [CrossRef]
- Guo, C.; Biewenga, L.; Lubberink, M.; Van Merkerk, R.; Poelarends, G.J. Tuning enzyme activity for nonaqueous solvents: Engineering an enantioselective "michaelase" for catalysis in high concentrations of ethanol. Chembiochem 2020, 21, 1499–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; Li, M.; Wei, D.; Zhang, X.; Jia, D.; Liu, Z.; Zheng, Y. Enabling biocatalysis in high-concentration organic cosolvent by enzyme gate engineering. Biotechnol. Bioeng. 2022, 119, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.M.; Vieille, C.; Phillips, R.S. Secondary alcohol dehydrogenases from thermoanaerobacter pseudoethanolicus and thermoanaerobacter brockii as robust catalysts. Chembiochem 2021, 22, 1884–1893. [Google Scholar] [CrossRef]
- Munawar, N.; Engel, P.C. Prospects for robust biocatalysis: Engineering of novel specificity in a halophilic amino acid dehydrogenase. Extremophiles 2013, 17, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Martin-Diaz, J.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Alcalde, M. Directed evolution of unspecific peroxygenase in organic solvents. Biotechnol. Bioeng. 2021, 118, 3002–3014. [Google Scholar] [CrossRef]
- Hua, L.; Qianqian, B.; Jianfeng, Z.; Yinbiao, X.; Shengyu, Y.; Weishi, X.; Yang, S.; Yupeng, L. Directed evolution engineering to improve activity of glucose dehydrogenase by increasing pocket hydrophobicity. Front. Microbiol. 2022, 13, 1044226. [Google Scholar] [CrossRef]
- Rosli, N.E.; Ali, M.S.M.; Kamarudin, N.H.A.; Masomian, M.; Latip, W.; Saadon, S.; Rahman, R.N.Z.R.A. Structure Prediction and Characterization of Thermostable Aldehyde Dehydrogenase from Newly Isolated Anoxybacillus geothermalis Strain D9. Microorganisms 2022, 10, 1444. [Google Scholar] [CrossRef]
- Planas-Iglesias, J.; Marques, S.M.; Pinto, G.P.; Musil, M.; Stourac, J.; Damborsky, J.; Bednar, D. Computational design of enzymes for biotechnological applications. Biotechol. Adv. 2021, 47, 107696. [Google Scholar] [CrossRef]
- Wang, S.Z.; Huo, H.Y.; Jiang, L. Oxidoreductases and Their Design, Preparation and Applications. CN112626042A, 9 April 2021. [Google Scholar]
- Katsimpouras, C.; Stephanopoulos, G. Enzymes in biotechnology: Critical platform technologies for bioprocess development. Curr. Opin. Biotech. 2021, 69, 91–102. [Google Scholar] [CrossRef]
- Ovchinnikov, S.; Huang, P.S. Structure-based protein design with deep learning. Curr. Opin. Biotech. 2021, 65, 136–144. [Google Scholar] [CrossRef]
- Wang, S.Z.; Liu, K.L.; Zhang, D.P. Advances of bioelectrocatalysis by oxidoreductases. Chin. Sci. Bull. 2021, 66, 1240–1249. [Google Scholar] [CrossRef]
- Raddadi, N.; Cherif, A.; Daffonchio, D.; Neifar, M.; Fava, F. Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl. Microbiol. Biot. 2015, 99, 7907–7913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Xiong, Y.; Sartin, M.M.; Zhan, D. Research advances in regulating the microenviroment of enzyme electrodes in non-aqueous systems: A mini-review. Electroanal 2022, 34, 590–598. [Google Scholar] [CrossRef]
- Greifenstein, R.; Ballweg, T.; Hashem, T.; Gottwald, E.; Achauer, D.; Kirschhöfer, F.; Nusser, M.; Brenner-Weiß, G.; Sedghamiz, E.; Wenzel, W.; et al. MOF-hosted enzymes for continuous flow catalysis in aqueous and organic solvents. Angew Chem. Int. Ed. 2022, 61, e202117144. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, F.; Chapman, R.; Kuchel, R.P.; Coureault, M.; Zetterlund, P.B.; Stenzel, M.H. Polymeric nanocapsules for enzyme stabilization in organic solvents. Macromolecules 2018, 51, 438–446. [Google Scholar] [CrossRef]
- de Maria, P.D. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
| Enzyme | Reaction System | Substrate and Products | Cofactor | Activity | Ref. |
|---|---|---|---|---|---|
| HeADH-II from Halomonas elongata,coupled with a NADH-oxidase from Lactobacillus pentosus (LpNOX) | 10–20% DMSO | Cinnamaldehyde to cinnamyl alcohol | NAD+ | 92% activity in 10% (v/v) DMSO 82% in 20% (v/v) DMSO | [22] |
| Alcohol Dehydrogenase from the Atlantis II Deep Red Sea brine pool | 10% (v/v) acetonitrile, methanol, and DMSO | Cinnamyl-methyl-ketone to aliphatic alcohols; raspberry ketone to aromatic alcohols | NADH | 70–80% activity after 32 h in 10% (v/v) acetonitrile, methanol, and DMSO | [23] |
| Halophilic alcohol dehydrogenase (ADH2) from Haloferax volcanii | DMSO and methanol | Alcohol to acetaldehyde | NAD+ | 47% activity in 30%DMSO 38% activity in 30% MeOH after 72 h | [10] |
| Formate dehydrogenase from Burkholderia dolosa PC543 (BdFDH) | DMSO, acetonitrile, methanol, ethanol, isopropanol | Sodium formate to carbon dioxide | NAD(P)+ | 170% activity in 30% DMSO | [24] |
| Glucose-1-dehydrogenase (GDH) from Paenibacillus pini | 10% v/v or 50% v/v of DMSO | Glucose to glucono-δ-lactone | NAD+ | 90% activity in 10% v/v DMSO; 80% activity in 50% v/v DMSO | [25] |
| Glutamate dehydrogenase from Halobacterium salinarum strain NRC-36014 | Methanol, ethanol, acetone, acetonitrile, 2-propanol, DMSO and DMSF | Glutamate to α-ketoglutarate | NADH/NAD+ | 100% activity 10% DMSO; more than 80% activity in 10% MeOH and 10% acetonitrile | [26] |
| Type II flavin-containing monooxygenase (FMO-E) and horse liver alcohol dehydrogenase (HLADH) as a bifunctional fusion enzyme | MTBE and CPME | Cyclobutanone and butane-1,4-diol to γ-butyrolactone | NAD+/NADH | 95 % (v/v) organic solvent, and 5 % (v/v) aqueous buffer 0.5 mM NAD+ | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Lei, H.; Ji, Z. Exploring Oxidoreductases from Extremophiles for Biosynthesis in a Non-Aqueous System. Int. J. Mol. Sci. 2023, 24, 6396. https://doi.org/10.3390/ijms24076396
Wang S, Lei H, Ji Z. Exploring Oxidoreductases from Extremophiles for Biosynthesis in a Non-Aqueous System. International Journal of Molecular Sciences. 2023; 24(7):6396. https://doi.org/10.3390/ijms24076396
Chicago/Turabian StyleWang, Shizhen, Hangbin Lei, and Zhehui Ji. 2023. "Exploring Oxidoreductases from Extremophiles for Biosynthesis in a Non-Aqueous System" International Journal of Molecular Sciences 24, no. 7: 6396. https://doi.org/10.3390/ijms24076396
APA StyleWang, S., Lei, H., & Ji, Z. (2023). Exploring Oxidoreductases from Extremophiles for Biosynthesis in a Non-Aqueous System. International Journal of Molecular Sciences, 24(7), 6396. https://doi.org/10.3390/ijms24076396






