Reduced High-Density Lipoprotein Cholesterol Is an Independent Determinant of Altered Bone Quality in Women with Type 2 Diabetes
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. DXA Results
2.3. Metabolic Correlates of Impaired TBS in T2DM Patients
3. Discussion
4. Materials and Methods
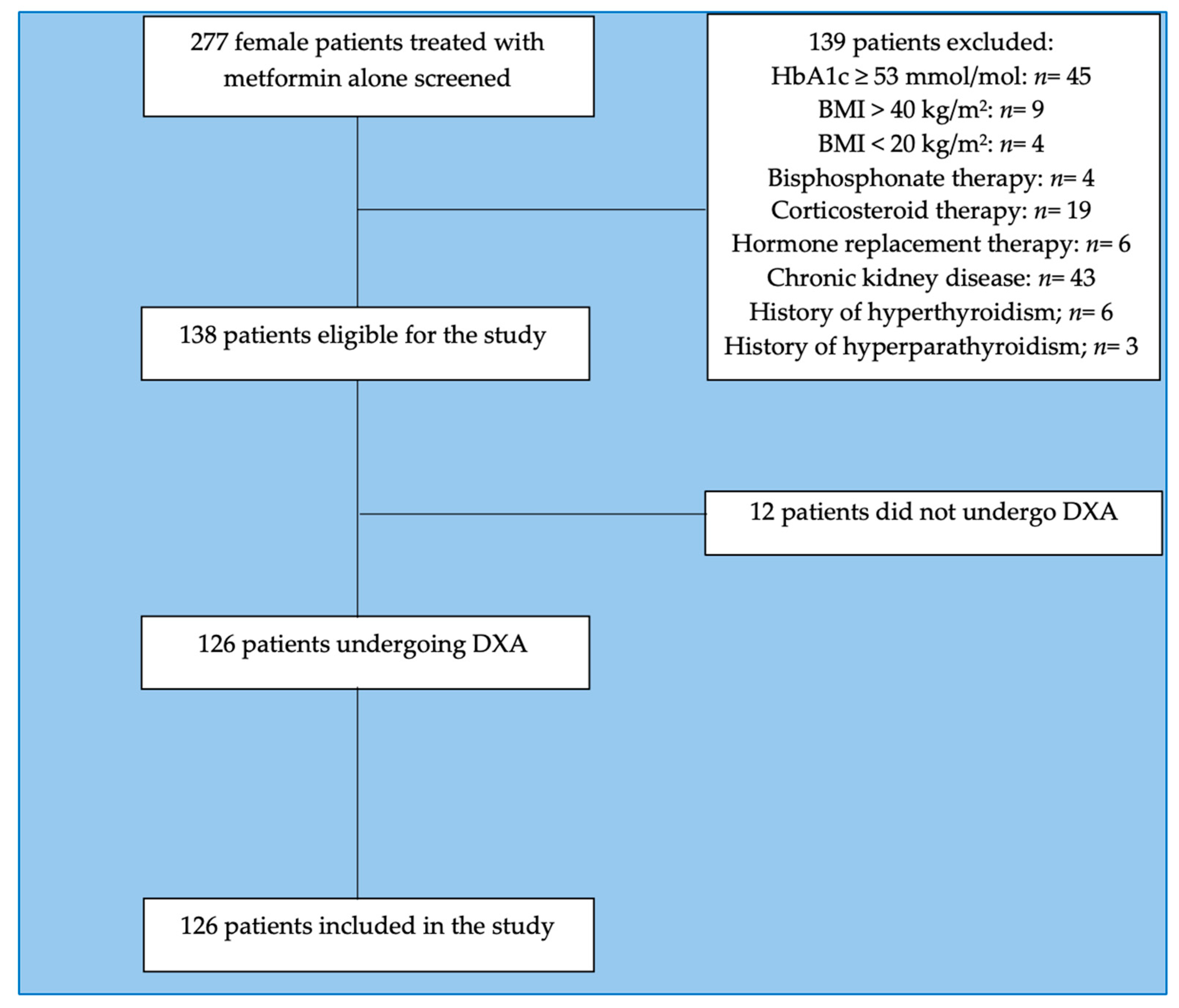
4.1. Study Population
4.2. Metabolic Evaluations
4.3. DXA and TBS Assessment
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pr. 2021, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.H.; Stedman, M.; Davies, M.; Livingston, M.; Alshames, R.; Lunt, M.; Rayman, G.; Gadsby, R. Estimating life years lost to diabetes: Outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc. Endocrinol. Metab. 2020, 9, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Ministero Della Salute, Salute Della Donna, Osteoporosi. Available online: https://www.salute.gov.it/portale/donna/dettaglioContenutiDonna.jsp?area=Salute+donna&id=4491&menu=patologie (accessed on 11 March 2023).
- Murray, C.E.; Coleman, C.M. Impact of Diabetes Mellitus on Bone Health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.-C.; Chuang, P.-Y.; Yang, T.-Y.; Huang, T.-W.; Chang, S.-F. Hyperglycemia inhibits osteoblastogenesis of rat bone marrow stromal cells via activation of the Notch2 signaling pathway. Int. J. Med. Sci. 2019, 16, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.S.; Ferreira, V.M.; Maquigussa, E.; Naves, M.A.; Boim, M.A. Effects of high glucose and high insulin concentrations on osteoblast function in vitro. Cell Tissue Res. 2014, 358, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Marumo, K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif. Tissue Int. 2015, 97, 242–261. [Google Scholar] [CrossRef]
- Shahen, V.; Gerbaix, M.; Koeppenkastrop, S.; Lim, S.; McFarlane, K.; Nguyen, A.N.; Peng, X.; Weiss, N.; Brennan-Speranza, T. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2020, 55, 109–118. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Zhang, Q. Differences in the roles of types 1 and 2 diabetes in the susceptibility to the risk of fracture: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 84. [Google Scholar] [CrossRef]
- Thong, E.P.; Milat, F.; Enticott, J.C.; Joham, A.E.; Ebeling, P.R.; Mishra, G.D.; Teede, H.J. The diabetes-fracture association in women with type 1 and type 2 diabetes is partially mediated by falls: A 15-year longitudinal study. Osteoporos. Int. 2021, 32, 1175–1184. [Google Scholar] [CrossRef]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K. Risk of Fracture in Women with Type 2 Diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Zeng, W.-N.; Rong, X.; Liang, Z.; Zhou, Z. Do patients with diabetes have an increased risk of impaired fracture healing? A systematic review and meta-analysis. ANZ J. Surg. 2020, 90, 1259–1264. [Google Scholar] [CrossRef]
- Tebé, C.; Martínez-Laguna, D.; Carbonell-Abella, C.; Reyes, C.; Moreno, V.; Diez-Perez, A.; Collins, G.; Prieto-Alhambra, D. The association between type 2 diabetes mellitus, hip fracture, and post-hip fracture mortality: A multi-state cohort analysis. Osteoporos. Int. 2019, 30, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocr. Pr. 2020, 26, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xu, X.; Jin, F.; Zhu, Z. The Correlation of Type 2 Diabetes Status with Bone Mineral Density in Middle-Aged Adults. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 3269–3276. [Google Scholar] [CrossRef]
- Samelson, E.J.; Demissie, S.; Cupples, L.A.; Zhang, X.; Xu, H.; Liu, C.T. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size: Framingham HR-pQCT Study. J. Bone Miner. Res. 2018, 33, 54–62. [Google Scholar] [CrossRef]
- Ma, R.; Wang, L.; Zhao, B.; Liu, C.; Liu, H.; Zhu, R.; Chen, B.; Li, L.; Zhao, D.; Mo, F.; et al. Diabetes Perturbs Bone Microarchitecture and Bone Strength through Regulation of Sema3A/IGF-1/β-Catenin in Rats. Cell. Physiol. Biochem. 2017, 41, 55–66. [Google Scholar] [CrossRef]
- Sihota, P.; Yadav, R.N.; Dhaliwal, R.; Bose, J.C.; Dhiman, V.; Neradi, D.; Karn, S.; Sharma, S.; Aggarwal, S.; Goni, V.G.; et al. Investigation of Mechanical, Material, and Compositional Determinants of Human Trabecular Bone Quality in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e2271–e2289. [Google Scholar] [CrossRef]
- Walle, M.; Whittier, D.E.; Frost, M.; Müller, R.; Collins, C.J. Meta-analysis of Diabetes Mellitus-Associated Differences in Bone Structure Assessed by High-Resolution Peripheral Quantitative Computed Tomography. Curr. Osteoporos. Rep. 2022, 20, 398–409. [Google Scholar] [CrossRef]
- Leslie, W.D.; Aubry-Rozier, B.; Lamy, O.; Hans, D.; Program, F.T.M.B.D. TBS (Trabecular Bone Score) and Diabetes-Related Fracture Risk. J. Clin. Endocrinol. Metab. 2013, 98, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Xia, W. Assessment of bone quality in patients with diabetes mellitus. Osteoporos. Int. 2018, 29, 1721–1736. [Google Scholar] [CrossRef]
- Ji, X.; Hong, J.; Qu, Z.; Yang, W.; Wang, Y.; Lin, J.; Li, C.; Wang, J.; Mou, H.; Shi, M.; et al. HemoglobinA1c Is a Risk Factor for Changes of Bone Mineral Density: A Mendelian Randomization Study. Front. Endocrinol. 2022, 13, 942878. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhong, S.; Ma, D.; Li, C.; Hao, Y.; Gao, Y.; Zhang, L.; Shen, L. A cross-sectional study: An assessment of low muscle mass and osteoporosis in type 2 diabetes mellitus patients with a high glycated hemoglobin level. Ther. Adv. Chronic Dis. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.M.; Dempster, D.W.; Zhou, H.; Roschger, P.; Fratzl-Zelman, N.; Fratzl, P.; Silverberg, S.J.; Shane, E.; Cohen, A.; Stein, E.; et al. Relationship of bone mineralization density distribution (BMDD) in cortical and cancellous bone within the iliac crest of healthy premenopausal women. Calcif. Tissue Int. 2014, 95, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Cao, Y.; Tao, Y.; Meng, E.; Tang, J.; Liu, Y.; Li, F. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pr. 2019, 159, 107990. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Q.; Liang, Y.; Dong, Y.; Mo, X.; Zhang, L.; Zhang, B. Insulin use and fracture risk in patients with type 2 diabetes: A meta-analysis of 138,690 patients. Exp. Ther. Med. 2019, 17, 3957–3964. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.-N.; Jiang, Y.-F.; Ding, T. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials. Bone 2014, 68, 115–123. [Google Scholar] [CrossRef]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The Interplay Between Bone and Glucose Metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, E.V.; Oden, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A meta-analysis oftrabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Yu, X. Lipid metabolism disorders and bone dysfunction-interrelated and mutually regulated (Review). Mol. Med. Rep. 2015, 12, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Panahi, N.; Soltani, A.; Ghasem-Zadeh, A.; Shafiee, G.; Heshmat, R.; Razi, F.; Mehrdad, N.; Nabipour, I.; Larijani, B.; Ostovar, A. Associations between the lipid profile and the lumbar spine bone mineral density and trabecular bone score in elderly Iranian individuals participating in the Bushehr Elderly Health Program: A population-based study. Arch. Osteoporos. 2019, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.L.; Olmos, J.M.; Pariente, E.; Ramos, C.; Martínez, J.; Nan, D. The atherogenic index of plasma is related to a degraded bone microarchitecture assessed by the trabecular bone score in postmenopausal women: The Camargo Cohort Study. Maturitas 2021, 148, 1–6. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathinavelu, S.; Guidry-Elizondo, C.; Banu, J. Molecular Modulation of Osteoblasts and Osteoclasts in Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, C.; Martin-Falstrault, L.; Brissette, L.; Moreau, R. The atherogenic Scarb1 null mouse model shows a high bone mass phenotype. Am. J. Physiol. Metab. 2014, 306, E48–E57. [Google Scholar] [CrossRef] [Green Version]
- van der Vorst, E.P.C. High-Density Lipoproteins and Apolipoprotein A1. Vertebr. Invertebr. Respir. Proteins Lipoproteins Other Body Fluid Proteins 2020, 94, 399–420. [Google Scholar] [CrossRef]
- Blair, H.C.; Kalyvioti, E.; Papachristou, N.I.; Tourkova, I.L.; Syggelos, S.A.; Deligianni, D.; Orkoula, M.G.; Kontoyannis, C.G.; Karavia, E.A.; Kypreos, K.E.; et al. Apolipoprotein A-1 regulates osteoblast and lipoblast precursor cells in mice. Lab. Investig. 2016, 96, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Cline-Smith, A.; Shashkova, E.; Perla, A.; Katyal, A.; Aurora, R. T-Cell Mediated Inflammation in Postmenopausal Osteoporosis. Front. Immunol. 2021, 12, 687551. [Google Scholar] [CrossRef] [PubMed]
- Hyka, N.; Hayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K., 3rd; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef]
- Suzuki, M.; Pritchard, D.K.; Becker, L.; Hoofnagle, A.N.; Tanimura, N.; Bammler, T.K.; Beyer, R.P.; Bumgarner, R.; Vaisar, T.; de Beer, M.C.; et al. High-Density Lipoprotein Suppresses the Type I Interferon Response, a Family of Potent Antiviral Immunoregulators, in Macrophages Challenged With Lipopolysaccharide. Circulation 2010, 122, 1919–1927. [Google Scholar] [CrossRef] [Green Version]
- Papachristou, N.I.; Blair, H.C.; Kypreos, K.; Papachristou, D.J. High-density lipoprotein (HDL) metabolism and bone mass. J. Endocrinol. 2017, 233, R95–R107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-S.; Zheng, Y.-D.; Yuan, Y.; Chen, S.-C.; Xie, B.-C. Effects of Anti-Diabetic Drugs on Fracture Risk: A Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2021, 12, 735824. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Shi, B.M. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in real-world use: Systematic review and meta-analysis of observational studies. Osteoporos. Int. 2019, 30, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ye, Z.; Lin, R.; Sun, A.R.; Liu, L.; Wei, J.; Deng, F.; Zhong, X.; Cui, L.; et al. Association of metformin use with fracture risk in type 2 diabetes: A systematic review and meta-analysis of observational studies. Front. Endocrinol. 2023, 13, 1038603. [Google Scholar] [CrossRef]
- Iorga, R.A.; Bacalbasa, N.; Carsote, M.; Bratu, O.G.; Stanescu, A.M.A.; Bungau, S.; Pantis, C.; Diaconu, C.C. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (Review). Exp. Ther. Med. 2020, 20, 2396–2400. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cradock, K.A.; Ólaighin, G.; Finucane, F.M.; Gainforth, H.L.; Quinlan, L.R.; Ginis, K.A.M. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, Y.; Chen, Y.; Jiang, Y. Effects of dairy products on bone mineral density in healthy postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Arch. Osteoporos. 2020, 15, 48. [Google Scholar] [CrossRef]
- Downloadable Questionnaires—International Physical Activity Questionnaire n.d. Available online: https://sites.google.com/site/theipaq/questionnaire_links (accessed on 7 September 2022).
- Cherukuri, L.; Kinninger, A.; Birudaraju, D.; Lakshmanan, S.; Li, D.; Flores, F.; Mao, S.S.; Budoff, M.J. Effect of body mass index on bone mineral density is age-specific. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1767–1773. [Google Scholar] [CrossRef]
- Palomo, T.; Dreyer, P.; Muszkat, P.; Weiler, F.G.; Bonansea, T.C.; Domingues, F.C.; Vieira, J.G.; Silva, B.C.; Brandão, C.M. Effect of soft tissue noise on trabecular bone score in postmenopausal women with diabetes: A cross sectional study. Bone 2022, 157, 116339. [Google Scholar] [CrossRef] [PubMed]
- Knapp, K.M.; Welsman, J.R.; Hopkins, S.J.; Fogelman, I.; Blake, G.M. Obesity Increases Precision Errors in Dual-Energy X-Ray Absorptiometry Measurements. J. Clin. Densitom. 2012, 15, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Hayón-Ponce, M.; García-Fontana, B.; Avilés-Pérez, M.D.; González-Salvatierra, S.; Andújar-Vera, F.; Moratalla-Aranda, E.; Muñoz-Torres, M. Lower trabecular bone score in type 2 diabetes mellitus: A role for fat mass and insulin resistance beyond hyperglycaemia. Diabetes Metab. 2021, 47, 101276. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Wu, J.; Kuo, S.-F.; Cheung, Y.-C.; Sung, C.-M.; Fan, C.-M.; Chen, F.-P.; Mhuircheartaigh, J.N. Vertebral Fractures in Type 2 Diabetes Patients: Utility of Trabecular Bone Score and Relationship With Serum Bone Turnover Biomarkers. J. Clin. Densitom. 2020, 23, 37–43. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Prevention and Management of Osteoporosis: Report of a WHO Scientific Group. n.d. Available online: https://apps.who.int/iris/handle/10665/42841 (accessed on 15 February 2023).
- IBM Corp. IBM SPSS Statistics for Macintosh, Version 27.0; Released 2020; IBM Corp: Armonk, NY, USA, 2020.
- Ho-Pham, L.T.; Nguyen, T.V. Association between trabecular bone score and type 2 diabetes: A quantitative update of evidence. Osteoporos. Int. 2019, 30, 2079–2085. [Google Scholar] [CrossRef]

| Clinical Parameters | T2DM (n = 126) | Controls (n = 117) | p Value |
|---|---|---|---|
| Age (years) | 63 ± 6.7 | 61.9 ± 5.8 | 0.20 |
| BMI (kg/m2) | 31.6 ± 7.1 | 32.6 ± 7.1 | 0.27 |
| Waist circumference (cm) | 106.2 ± 15.7 | 111.6 ± 14.6 | 0.015 |
| Menopause onset age (years) | 49.2 ± 4.6 | 49.1 ± 4.7 | 0.81 |
| SBP (mmHg) | 131.4 ± 20.8 | 133.1 ± 15.4 | 0.49 |
| DBP (mmHg) | 82.5 ± 9.1 | 81.9 ± 11.3 | 0.66 |
| FBG (mg/dL) | 117.5± 19.6 | 88.3 ± 8.2 | <0.001 |
| HbA1c (mmol/mol) | 45.5 ± 0.1 | 35.3 ± 0.1 | <0.001 |
| Total cholesterol (mg/dL) | 182.8 ± 35.5 | 209.7 ± 35.8 | <0.001 |
| HDL-C-c (mg/dL) | 53.4 ± 13.3 | 59.7 ± 14.4 | <0.001 |
| LDL-c (mg/dL) | 104.6 ± 33.8 | 128.7 ± 32.8 | <0.001 |
| Triglycerides (mg/dL) | 135.4 ± 62.3 | 111.7 ± 47.3 | 0.001 |
| AST (IU/L) | 22.8 ± 13.5 | 20.6 ± 9.4 | 0.18 |
| ALT (IU/L) | 25.1 ± 15.6 | 22.4 ± 11.7 | 0.16 |
| GGT (IU/L) | 31.9 ± 30 | 20.7 ± 18 | 0.005 |
| Serum Creatinine (mg/dL) | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.83 |
| TSH (mU/L) | 2.1 ± 1.3 | 2 ± 2 | 0.87 |
| PTH (pg/mL) | 57 ± 39.8 | 46 ± 22 | 0.018 |
| Vitamin D (ng/mL) | 24.7 ± 16.1 | 23.4 ± 13.6 | 0.54 |
| Calcium (mg/dL) | 9.5 ± 0.4 | 9.4 ± 0.5 | 0.13 |
| Phosphate (mg/mL) | 3.6 ± 0.5 | 3.6 ± 0.5 | 0.47 |
| Presence of menopausal state | 92.1% | 97.4% | 0.063 |
| Physical activity | |||
| None-low intensity | 46% | 46.1% | 0.53 |
| Moderate intensity | 46% | 43.6% | |
| High intensity | 8% | 10.3% | |
| Presence of statin treatment | 42.2% | 21.3% | 0.002 |
| Atorvastatin | 37.7% | 40% | |
| Simvastatin | 34% | 28% | 0.56 |
| Rosuvastatin | 28.3% | 32% | |
| Presence of Vitamin D supplementation | 25% | 22.2% | 0.64 |
| DXA-Derived Parameters | T2DM (n = 126) | Controls (n = 117) | p Value |
|---|---|---|---|
| Lumbar spine BMD (g/cm2) | 1.010 ± 0.165 | 0.937 ± 0.161 | 0.031 |
| Lumbar spine T-score | −0.620 ± 1.258 | −0.787 ± 1.524 | 0.359 |
| Total hip BMD (g/cm2) | 0.936 ± 0.131 | 0.891 ± 0.145 | 0.080 |
| Total hip T-score | −0.384 ± 0.961 | −0.491 ± 1.132 | 0.801 |
| Femoral neck BMD (g/cm2) | 0.744 ± 0.125 | 0.725 ± 0.119 | 0.391 |
| Femoral neck T-score | −1.002 ± 1.137 | −1.213 ± 0.903 | 0.373 |
| TBS | 1.180 ± 0.112 | 1.209 ± 0.120 | 0.060 |
| Individuals with degraded bone architecture (TBS ≤ 1.23) | 62.7% | 59.5% | 0.61 |
| Individuals with partially degraded bone architecture (1.23 < TBS ≤ 1.31) | 30.2% | 22.4% | 0.17 |
| Individuals with normal bone architecture (TBS > 1.31) | 7.1% | 18.1% | 0.01 |
| Variables | Unstandardized Coefficient | Standardized Coefficient | p Value | |
|---|---|---|---|---|
| β | Standard Error | β | ||
| Age | −0.004 | 0.005 | −0.162 | 0.464 |
| Menopausal state | 0.008 | 0.005 | 0.303 | 0.175 |
| BMI | −0.007 | 0.008 | −0.304 | 0.411 |
| Waist circumference | 0.002 | 0.004 | 0.199 | 0.634 |
| HDL-c | 0.006 | 0.002 | 0.603 | 0.028 |
| Physical activity | −0.056 | 0.069 | −0.214 | 0.432 |
| Statin treatment | 0.016 | 0.061 | 0.066 | 0.793 |
| Vitamin D | 0.002 | 0.061 | 0.066 | 0.793 |
| Constant | 0.686 | 0.514 | - | 0.205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dule, S.; Barchetta, I.; Cimini, F.A.; Passarella, G.; Dellanno, A.; Filardi, T.; Venditti, V.; Bleve, E.; Bailetti, D.; Romagnoli, E.; et al. Reduced High-Density Lipoprotein Cholesterol Is an Independent Determinant of Altered Bone Quality in Women with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 6474. https://doi.org/10.3390/ijms24076474
Dule S, Barchetta I, Cimini FA, Passarella G, Dellanno A, Filardi T, Venditti V, Bleve E, Bailetti D, Romagnoli E, et al. Reduced High-Density Lipoprotein Cholesterol Is an Independent Determinant of Altered Bone Quality in Women with Type 2 Diabetes. International Journal of Molecular Sciences. 2023; 24(7):6474. https://doi.org/10.3390/ijms24076474
Chicago/Turabian StyleDule, Sara, Ilaria Barchetta, Flavia Agata Cimini, Giulia Passarella, Arianna Dellanno, Tiziana Filardi, Vittorio Venditti, Enrico Bleve, Diego Bailetti, Elisabetta Romagnoli, and et al. 2023. "Reduced High-Density Lipoprotein Cholesterol Is an Independent Determinant of Altered Bone Quality in Women with Type 2 Diabetes" International Journal of Molecular Sciences 24, no. 7: 6474. https://doi.org/10.3390/ijms24076474






