Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts
Abstract
1. Introduction
2. Results
2.1. Ovarian Mast Cell Population in Patients without Pathological Changes
2.2. Ovarian Mast Cells in Patients with Endometrioid Cysts
2.3. Histotopographic Features
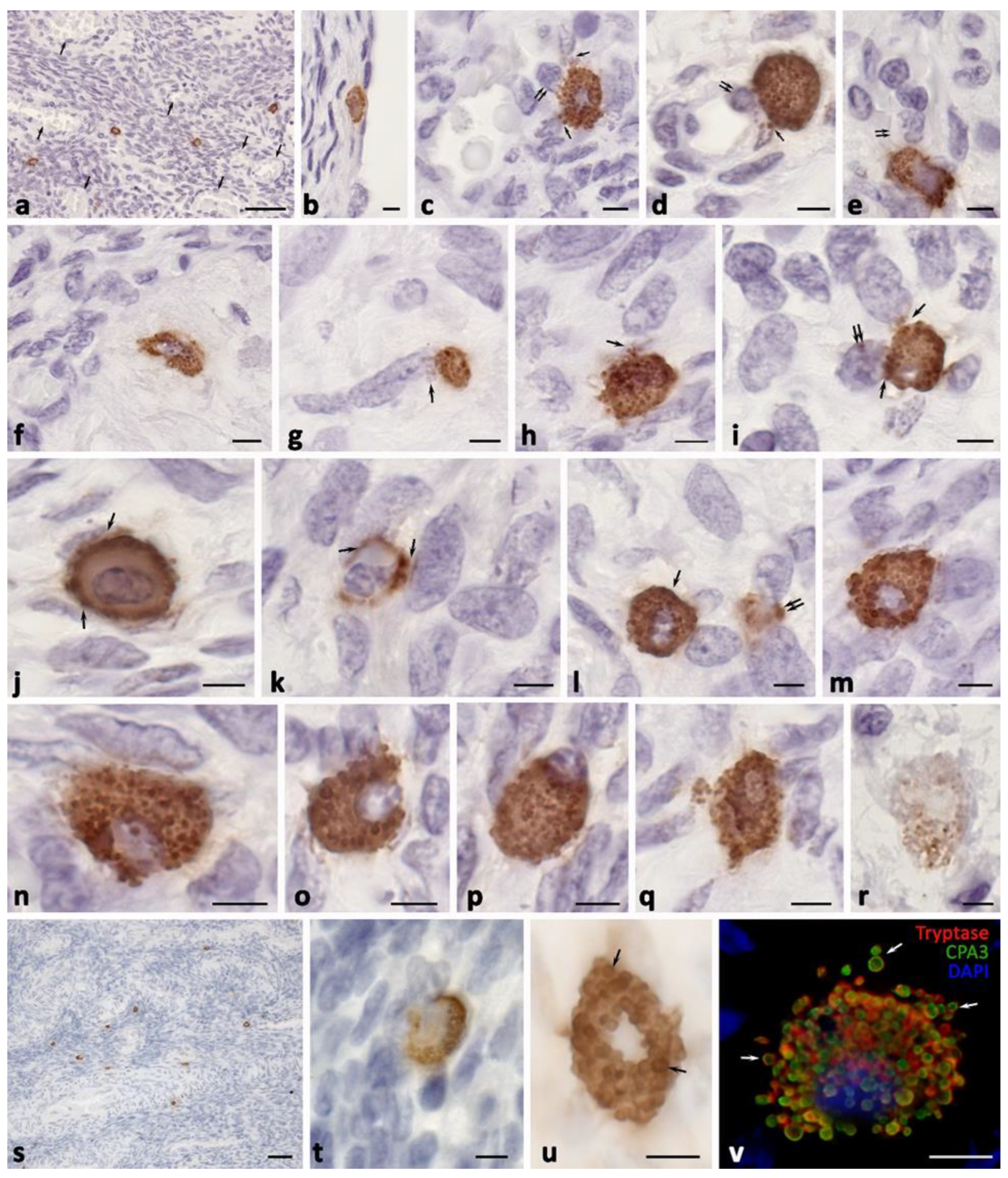
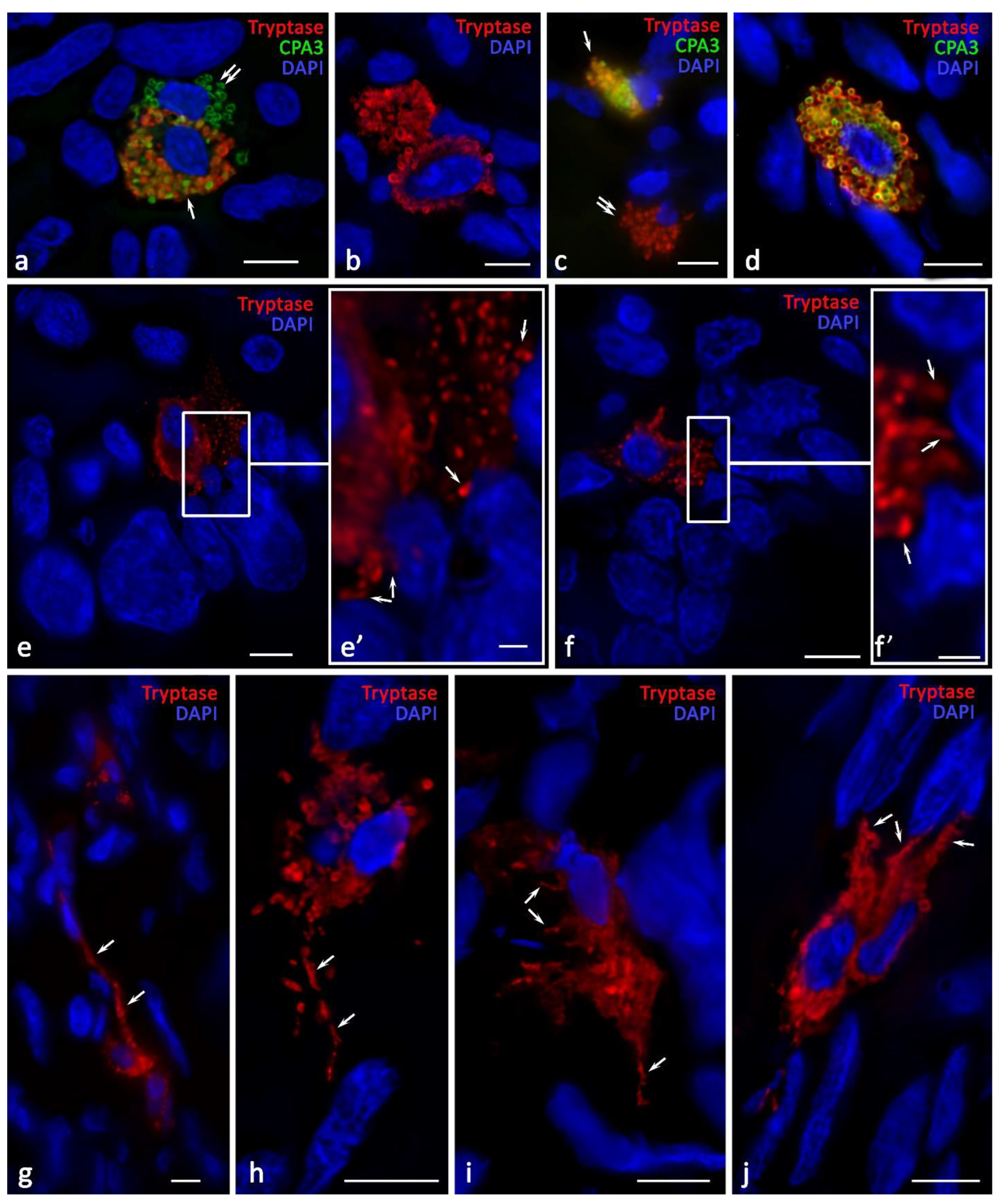
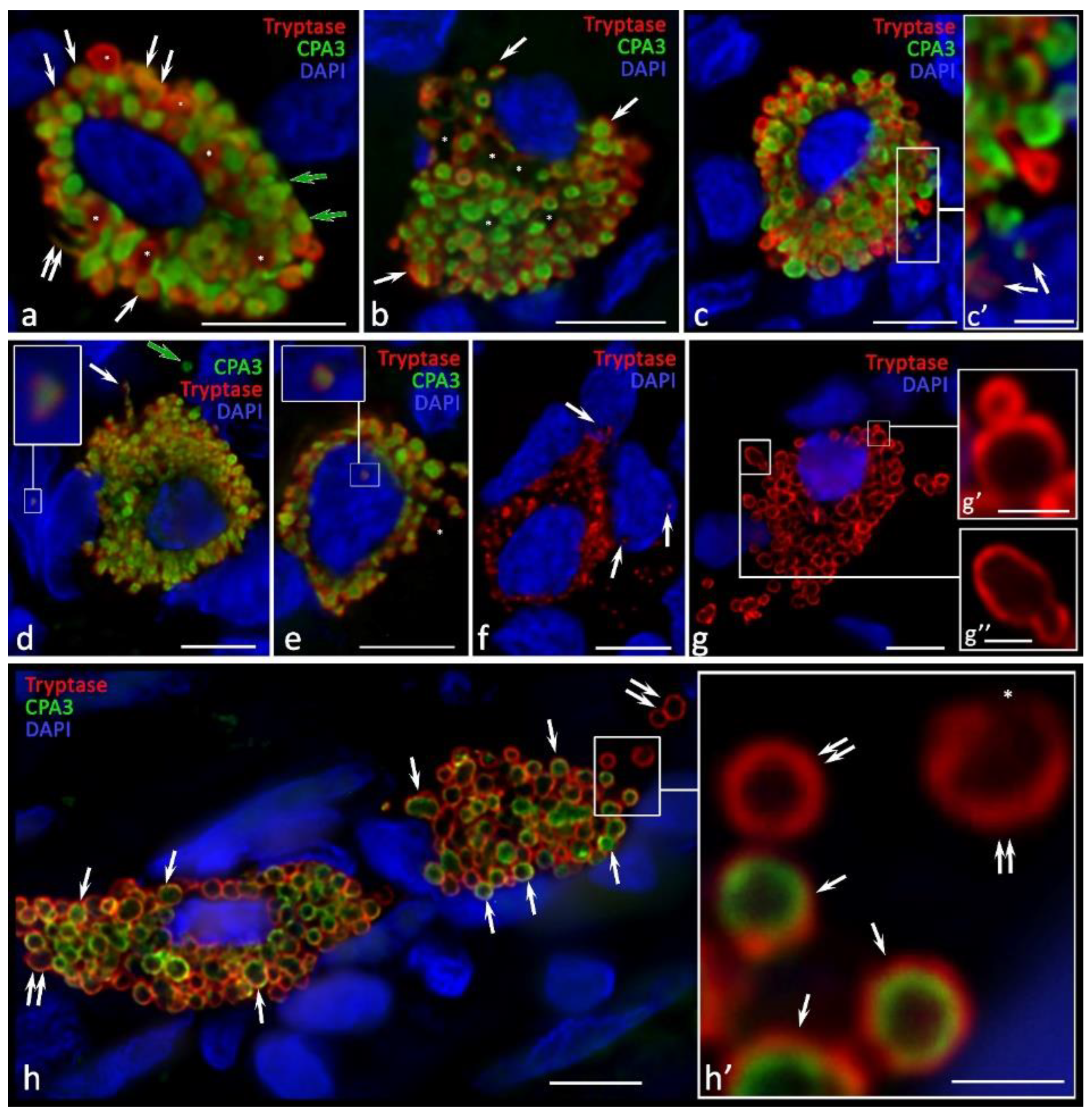
2.4. Cytotopographic Features
3. Discussion
4. Materials and Methods
4.1. Case Selection
4.2. Tissue Probe Staining
4.3. Immunohistochemistry
4.4. Controls
4.5. Image Acquisition
4.6. Statistical Analysis
4.7. Data Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Morassutto, C.; Monasta, L.; Ricci, G.; Barbone, F.; Ronfani, L. Incidence and Estimated Prevalence of Endometriosis and Adenomyosis in Northeast Italy: A Data Linkage Study. PLoS ONE 2016, 11, e0154227. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Mikhaleva, L.M.; Davydov, A.I.; Patsap, O.I.; Mikhaylenko, E.V.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Malignant Transformation and Associated Biomarkers of Ovarian Endometriosis: A Narrative Review. Adv. Ther. 2020, 37, 2580–2603. [Google Scholar] [CrossRef] [PubMed]
- Mikhaleva, L.M.; Solomatina, A.A.; Milovanov, A.P.; Beeraka, N.M.; Khovanskaya, T.N.; Chabieva, L.B.; Mikhalev, S.A.; Gracheva, N.A.; Chigray, L.V.; Beylerli, O.; et al. Histomorphological and Functional Features of the Eutopic Endometrium in Patients with Ovarian Endometriosis After Surgery-a Clinical Study. Reprod. Sci. 2021, 28, 2350–2358. [Google Scholar] [CrossRef]
- Devlin, M.J.; Miller, R.; Laforets, F.; Kotantaki, P.; Garsed, D.W.; Kristeleit, R.; Bowtell, D.D.; McDermott, J.; Maniati, E.; Balkwill, F.R. The Tumor Microenvironment of Clear-Cell Ovarian Cancer. Cancer Immunol. Res. 2022, 10, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Osuga, Y. Current concepts of the pathogenesis of endometriosis. Reprod. Med. Biol. 2010, 9, 1–7. [Google Scholar] [CrossRef]
- Osuchowska-Grochowska, I.; Blicharska, E.; Gogacz, M.; Nogalska, A.; Winkler, I.; Szopa, A.; Ekiert, H.; Tymczyna-Borowicz, B.; Rahnama-Hezavah, M.; Grochowski, C. Brief Review of Endometriosis and the Role of Trace Elements. Int. J. Mol. Sci. 2021, 22, 11098. [Google Scholar] [CrossRef]
- Pascolo, L.; Pachetti, M.; Camillo, A.; Cernogoraz, A.; Rizzardi, C.; Mikus, K.V.; Zanconati, F.; Salome, M.; Suarez, V.T.; Romano, F.; et al. Detention and mapping of iron and toxic environmental elements in human ovarian endometriosis: A suggested combined role. Sci. Total Environ. 2023, 864, 161028. [Google Scholar] [CrossRef]
- Vallve-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Wu, Y.; Zhang, B.; Wei, J.; Li, J.; Huang, Y.; Chen, L.; He, X. Bioinformatics identification and validation of biomarkers and infiltrating immune cells in endometriosis. Front. Immunol. 2022, 13, 944683. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.A.S.; Haro, M.; Wright, K.N.; Lin, X.; Abbasi, F.; Sun, J.; Hernandez, L.; Orr, N.L.; Hong, J.; Choi-Kuaea, Y.; et al. Single-cell transcriptomic analysis of endometriosis. Nat. Genet. 2023, 55, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef] [PubMed]
- McCallion, A.; Nasirzadeh, Y.; Lingegowda, H.; Miller, J.E.; Khalaj, K.; Ahn, S.; Monsanto, S.P.; Bidarimath, M.; Sisnett, D.J.; Craig, A.W.; et al. Estrogen mediates inflammatory role of mast cells in endometriosis pathophysiology. Front. Immunol. 2022, 13, 961599. [Google Scholar] [CrossRef]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef]
- Shi, S.; Ye, L.; Yu, X.; Jin, K.; Wu, W. Focus on mast cells in the tumor microenvironment: Current knowledge and future directions. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188845. [Google Scholar] [CrossRef]
- Vitte, J.; Vibhushan, S.; Bratti, M.; Montero-Hernandez, J.E.; Blank, U. Allergy, Anaphylaxis, and Nonallergic Hypersensitivity: IgE, Mast Cells, and Beyond. Med. Princ. Pract. 2022, 31, 501–515. [Google Scholar] [CrossRef]
- West, P.W.; Bulfone-Paus, S. Mast cell tissue heterogeneity and specificity of immune cell recruitment. Front. Immunol. 2022, 13, 932090. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.S.; Ginhoux, F. New perspectives on the origins and heterogeneity of mast cells. Nat. Rev. Immunol. 2023, 23, 55–68. [Google Scholar] [CrossRef]
- Buchwalow, I.; Boecker, W.; Tiemann, M. The contribution of Paul Ehrlich to histochemistry: A tribute on the occasion of the centenary of his death. Virchows Arch. 2015, 466, 111–116. [Google Scholar] [CrossRef]
- Crivellato, E.; Beltrami, C.; Mallardi, F.; Ribatti, D. Paul Ehrlich’s doctoral thesis: A milestone in the study of mast cells. Br. J. Haematol. 2003, 123, 19–21. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Theoharides, T.C. Neuroendocrinology of mast cells: Challenges and controversies. Exp. Dermatol. 2017, 26, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Valent, P.; Galli, S.J. KIT as a master regulator of the mast cell lineage. J. Allergy Clin. Immunol. 2022, 149, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Sobiepanek, A.; Kuryk, L.; Garofalo, M.; Kumar, S.; Baran, J.; Musolf, P.; Siebenhaar, F.; Fluhr, J.W.; Kobiela, T.; Plasenzotti, R.; et al. The Multifaceted Roles of Mast Cells in Immune Homeostasis, Infections and Cancers. Int. J. Mol. Sci. 2022, 23, 2249. [Google Scholar] [CrossRef]
- Pejler, G.; Abrink, M.; Ringvall, M.; Wernersson, S. Mast cell proteases. Adv. Immunol. 2007, 95, 167–255. [Google Scholar] [CrossRef]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol 2018, 149, 461–477. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Trotsenko, I.; Samoilova, V.; Buchwalow, I.; Tiemann, M. Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells. Cells 2022, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases—In Vivo Targets and Function. Front. Immunol. 2022, 13, 918305. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef]
- Solimando, A.G.; Desantis, V.; Ribatti, D. Mast Cells and Interleukins. Int. J. Mol. Sci. 2022, 23, 14004. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Nakamura, Y. Time will tell about mast cells: Circadian control of mast cell activation. Allergol. Int. 2022, 71, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef]
- Fujiwara, H.; Konno, R.; Netsu, S.; Sugamata, M.; Shibahara, H.; Ohwada, M.; Suzuki, M. Localization of mast cells in endometrial cysts. Am. J. Reprod. Immunol. 2004, 51, 341–344. [Google Scholar] [CrossRef]
- Ribatti, D.; Finato, N.; Crivellato, E.; Marzullo, A.; Mangieri, D.; Nico, B.; Vacca, A.; Beltrami, C.A. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am. J. Obstet. Gynecol. 2005, 193, 1961–1965. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Guo, X.; Yu, Q.; Ding, S.; Xu, X.; Peng, Y.; Zhu, L.; Zou, G.; Zhang, X. Possible involvement of crosstalk between endometrial cells and mast cells in the development of endometriosis via CCL8/CCR1. Biomed. Pharmacother. 2020, 129, 110476. [Google Scholar] [CrossRef]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noel, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Heider, U.; Pedal, I.; Spanel-Borowski, K. Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertil. Steril. 2001, 75, 1141–1147. [Google Scholar] [CrossRef]
- Gauche Cazalis, C.; Koskas, M.; Martin, B.; Palazzo, L.; Madelenat, P.; Yazbeck, C. Preoperative imaging of deeply infiltrating endometriosis in: Transvaginal sonography, rectal endoscopic sonography and magnetic resonance imaging. Gynecol. Obstet. Fertil. 2012, 40, 634–641. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Luongo, L.; Cipriano, M.; Palazzo, E.; Cinelli, M.P.; de Novellis, V.; Maione, S.; Iuvone, T. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol. Pain 2011, 7, 3. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol. Neurobiol. 2013, 48, 340–352. [Google Scholar] [CrossRef]
- Iuvone, T.; Affaitati, G.; De Filippis, D.; Lopopolo, M.; Grassia, G.; Lapenna, D.; Negro, L.; Costantini, R.; Vaia, M.; Cipollone, F.; et al. Ultramicronized palmitoylethanolamide reduces viscerovisceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis: Role of mast cells. Pain 2016, 157, 80–91. [Google Scholar] [CrossRef]
- Dalton, D.K.; Noelle, R.J. The roles of mast cells in anticancer immunity. Cancer Immunol. Immunother. 2012, 61, 1511–1520. [Google Scholar] [CrossRef]
- Oldford, S.A.; Marshall, J.S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 2015, 63, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yi, T. Mast cell tryptases in allergic inflammation and immediate hypersensitivity. Curr. Opin. Immunol. 2021, 72, 94–106. [Google Scholar] [CrossRef]
- Rabelo Melo, F.; Santosh Martin, S.; Sommerhoff, C.P.; Pejler, G. Exosome-mediated uptake of mast cell tryptase into the nucleus of melanoma cells: A novel axis for regulating tumor cell proliferation and gene expression. Cell Death Dis. 2019, 10, 659. [Google Scholar] [CrossRef]
- Melo, F.R.; Vita, F.; Berent-Maoz, B.; Levi-Schaffer, F.; Zabucchi, G.; Pejler, G. Proteolytic histone modification by mast cell tryptase, a serglycin proteoglycan-dependent secretory granule protease. J. Biol. Chem. 2014, 289, 7682–7690. [Google Scholar] [CrossRef]
- Alanazi, S.; Rabelo Melo, F.; Pejler, G. Tryptase Regulates the Epigenetic Modification of Core Histones in Mast Cell Leukemia Cells. Front. Immunol. 2021, 12, 804408. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.; Grujic, M.; Lampinen, M.; Rollman, O.; Sommerhoff, C.P.; Pejler, G.; Melo, F.R. Mast Cell beta-Tryptase Is Enzymatically Stabilized by DNA. Int. J. Mol. Sci. 2020, 21, 5065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.H.; Ding, S.J.; Li, T.T.; Zhu, L.B.; Huang, X.F.; Zhang, X.M. Estrogen is an important mediator of mast cell activation in ovarian endometriomas. Reproduction 2018, 155, 73–83. [Google Scholar] [CrossRef]
- Lin, K.Q.; Zhu, L.B.; Zhang, X.M.; Lin, J. Role of mast cells in estrogen-mediated experimental endometriosis in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015, 44, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Redhu, D.; Franke, K.; Aparicio-Soto, M.; Kumari, V.; Pazur, K.; Illerhaus, A.; Hartmann, K.; Worm, M.; Babina, M. Mast cells instruct keratinocytes to produce thymic stromal lymphopoietin: Relevance of the tryptase/protease-activated receptor 2 axis. J. Allergy Clin. Immunol. 2022, 149, 2053–2061.e6. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol. Immunol. 2015, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast cell proteases as protective and inflammatory mediators. Adv. Exp. Med. Biol. 2011, 716, 212–234. [Google Scholar] [CrossRef]
- Yu, M.; Tsai, M.; Tam, S.Y.; Jones, C.; Zehnder, J.; Galli, S.J. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Investig. 2006, 116, 1633–1641. [Google Scholar] [CrossRef]
- Dai, H.; Korthuis, R.J. Mast Cell Proteases and Inflammation. Drug Discov. Today Dis. Models 2011, 8, 47–55. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Aponte-Lopez, A.; Munoz-Cruz, S. Mast Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 159–173. [Google Scholar] [CrossRef]
- Ribatti, D. Mast cells in lymphomas. Crit. Rev. Oncol. Hematol. 2016, 101, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Qian, N.; Li, X.; Wang, X.; Wu, C.; Yin, L.; Zhi, X. Tryptase promotes breast cancer angiogenesis through PAR-2 mediated endothelial progenitor cell activation. Oncol. Lett. 2018, 16, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Costa Neto, H.; Andrade, A.; Carmo, A.F.D.; Freitas, R.A.; Galvao, H.C. Involvement of tryptase-positive mast cells and angiogenesis in the growth of inflammatory odontogenic cysts. Braz. Oral. Res. 2021, 35, e061. [Google Scholar] [CrossRef]
- Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 1997, 61, 233–245. [Google Scholar] [CrossRef]
- Welle, M.M.; Audige, L.; Belz, J.P. The equine endometrial mast cell during the puerperal period: Evaluation of mast cell numbers and types in comparison to other inflammatory changes. Vet. Pathol. 1997, 34, 23–30. [Google Scholar] [CrossRef]
- Hallgren, J.; Pejler, G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006, 273, 1871–1895. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Piliponsky, A.M. Tryptase, a novel link between allergic inflammation and fibrosis. Trends Immunol. 2003, 24, 158–161. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Lucena, F.; McDougall, J.J. Protease Activated Receptors and Arthritis. Int. J. Mol. Sci. 2021, 22, 9352. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Buddenkotte, J.; Shpacovitch, V.; Rattenholl, A.; Moormann, C.; Vergnolle, N.; Luger, T.A.; Hollenberg, M.D. Proteinase-activated receptors: Transducers of proteinase-mediated signaling in inflammation and immune response. Endocr. Rev. 2005, 26, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Ui, H.; Andoh, T.; Lee, J.B.; Nojima, H.; Kuraishi, Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur. J. Pharmacol. 2006, 530, 172–178. [Google Scholar] [CrossRef]
- Molinari, J.F.; Scuri, M.; Moore, W.R.; Clark, J.; Tanaka, R.; Abraham, W.M. Inhaled tryptase causes bronchoconstriction in sheep via histamine release. Am. J. Respir. Crit. Care Med. 1996, 154, 649–653. [Google Scholar] [CrossRef]
- Zheng, Y.; Khan, Z.; Zanfagnin, V.; Correa, L.F.; Delaney, A.A.; Daftary, G.S. Epigenetic Modulation of Collagen 1A1: Therapeutic Implications in Fibrosis and Endometriosis. Biol. Reprod. 2016, 94, 87. [Google Scholar] [CrossRef]
- Arafah, M.; Rashid, S.; Akhtar, M. Endometriosis: A Comprehensive Review. Adv. Anat. Pathol. 2021, 28, 30–43. [Google Scholar] [CrossRef]
- Wang, P.H.; Yang, S.T.; Chang, W.H.; Liu, C.H.; Lee, F.K.; Lee, W.L. Endometriosis: Part I. Basic concept. Taiwan J. Obstet. Gynecol. 2022, 61, 927–934. [Google Scholar] [CrossRef]
- Ke, J.; Ye, J.; Li, M.; Zhu, Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules 2021, 11, 1739. [Google Scholar] [CrossRef]
- Garcia Garcia, J.M.; Vannuzzi, V.; Donati, C.; Bernacchioni, C.; Bruni, P.; Petraglia, F. Endometriosis: Cellular and Molecular Mechanisms Leading to Fibrosis. Reprod. Sci. 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Abe, M.; Kurosawa, M.; Ishikawa, O.; Miyachi, Y. Effect of mast cell-derived mediators and mast cell-related neutral proteases on human dermal fibroblast proliferation and type I collagen production. J. Allergy Clin. Immunol. 2000, 106, S78–S84. [Google Scholar] [CrossRef]
- Dell’Italia, L.J.; Collawn, J.F.; Ferrario, C.M. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ. Res. 2018, 122, 319–336. [Google Scholar] [CrossRef]
- Ramirez-GarciaLuna, J.L.; Chan, D.; Samberg, R.; Abou-Rjeili, M.; Wong, T.H.; Li, A.; Feyerabend, T.B.; Rodewald, H.R.; Henderson, J.E.; Martineau, P.A. Defective bone repair in mast cell-deficient Cpa3Cre/+ mice. PLoS ONE 2017, 12, e0174396. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Tsilioni, I.; Theoharides, T.C. Nanotube Formation: A Rapid Form of “Alarm Signaling”? Clin. Ther. 2016, 38, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Franke, K.; Bal, G. How “Neuronal” Are Human Skin Mast Cells? Int. J. Mol. Sci. 2022, 23, 10871. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Pizzi, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Soriano, A.; Bisagni, A.; Martino, G.; Caprera, C.; Moretti, M.; et al. Gastrointestinal Manifestations in Systemic Mastocytosis: The Need of a Multidisciplinary Approach. Cancers 2021, 13, 3316. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Boecker, W. Immunohistochemistry: Basics and Methods, 1st ed.; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2010. [Google Scholar]
- Buchwalow, I.; Samoilova, V.; Boecker, W.; Tiemann, M. Non-specific binding of antibodies in immunohistochemistry: Fallacies and facts. Sci. Rep. 2011, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Buchwalow, I.; Samoilova, V.; Boecker, W.; Tiemann, M. Multiple immunolabeling with antibodies from the same host species in combination with tyramide signal amplification. Acta Histochem. 2018, 120, 405–411. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]



| Patient Groups | MC Histotopography | MC Specific Proteases | |
|---|---|---|---|
| Tryptase-Positive MCs (M ± m) | Carboxypeptidase A3-Positive MCs (M ± m) | ||
| Patients with OEC (ovarian endometrioid cysts) (n = 27) | Medulla of the ovary | 33.24 ± 4.57 * | 41.21 ± 9.7 * |
| The cortex of the ovary | 3.38 ± 1.87 * | 9.7 ± 2.1 * | |
| OEC wall | 42.48 ± 5.67 * | 47.5 ± 10.7 * | |
| Control group (n = 6) | Medulla of the ovary | 10 ± 1.72 | 8.0 ± 3.5 |
| Cortex of the ovary | 2.75 ± 0.75 | 1.25 ± 0.9 | |
| OEC wall | - | - | |
| Antibodies | Host | Catalogue Nr. | Dilution | Sourse |
|---|---|---|---|---|
| Tryptase | Mouse monoclonal Ab | #ab2378 | 1:3000 | AbCam, United Kingdom |
| Carboxypeptidase A3 (CPA3) | Rabbit polyclonal Ab | #ab251696 | 1:2000 | AbCam, United Kingdom |
| Antibodies and Other Reagents | Source | Dilution | Label |
|---|---|---|---|
| Goat anti-mouse IgG Ab (#ab97035) | AbCam, United Kingdom | 1/500 | Cy3 |
| Goat anti-rabbit IgG Ab (#ab150077): | AbCam, United Kingdom | 1/500 | Alexa Fluor 488 |
| AmpliStain™ anti-Mouse 1-Step HRP (#AS-M1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| AmpliStain™ anti-Rabbit 1-Step HRP (#AS-R1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| 4′,6-diamidino-2-phenylindole (DAPI, #D9542-5MG) | Sigma, Hamburg, Germany | 5 µg/mL | w/o |
| VECTASHIELD® Mounting Medium (#H-1000) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
| DAB Peroxidase Substrat Kit (#SK-4100) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | DAB |
| Mayer’s hematoxylin (#MHS128) | Sigma-Aldrich | ready-to-use | w/o |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiakshin, D.; Patsap, O.; Kostin, A.; Mikhalyova, L.; Buchwalow, I.; Tiemann, M. Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts. Int. J. Mol. Sci. 2023, 24, 6498. https://doi.org/10.3390/ijms24076498
Atiakshin D, Patsap O, Kostin A, Mikhalyova L, Buchwalow I, Tiemann M. Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts. International Journal of Molecular Sciences. 2023; 24(7):6498. https://doi.org/10.3390/ijms24076498
Chicago/Turabian StyleAtiakshin, Dmitri, Olga Patsap, Andrey Kostin, Lyudmila Mikhalyova, Igor Buchwalow, and Markus Tiemann. 2023. "Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts" International Journal of Molecular Sciences 24, no. 7: 6498. https://doi.org/10.3390/ijms24076498
APA StyleAtiakshin, D., Patsap, O., Kostin, A., Mikhalyova, L., Buchwalow, I., & Tiemann, M. (2023). Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts. International Journal of Molecular Sciences, 24(7), 6498. https://doi.org/10.3390/ijms24076498






