Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities
Abstract
:1. Introduction
2. Molecular Structural and Biological Function of Galectin-1
3. Galectin-1 Functions as a Context-Dependent Regulator in Infection
3.1. Bacterial Infection
3.2. Viral Infection
3.3. Parasitic Infection
4. Galectin-1: A “Guardian” of Allogeneic Graft
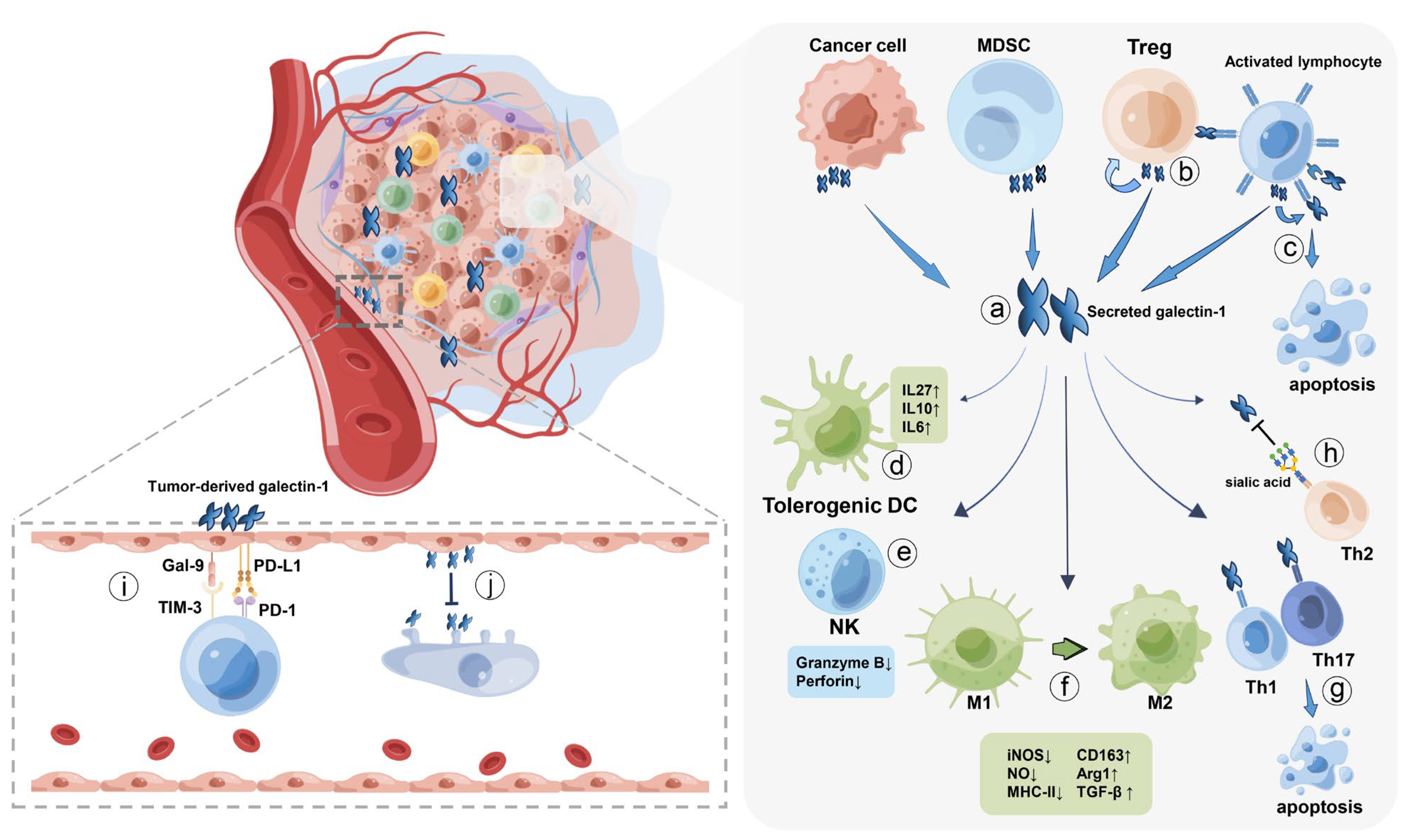
5. Cancer Cells Hijack Galectin-1 to Evade Immune Surveillance
5.1. Macrophages
5.2. Dendritic Cells
5.3. T lymphocyte Cells
5.3.1. Cytotoxic T cells
5.3.2. Helper T Cells
5.3.3. Regulatory T Cells
5.4. Other Immune Cells
6. Galectin-1 Acts as a Two-Edged Sword in Autoimmune Diseases
6.1. Promotive Role of Galectin-1 in Autoimmune Diseases
6.2. Protective Role of Galectin-1 in Autoimmune Diseases
7. Galectin-1: A Key Regulator of Allergic Inflammation
8. Treatment Targeting Galectin-1
8.1. Agents Inhibiting Galectin-1 Binding
8.2. Agents Inhibiting Galectin-1 Expression
8.3. Agents with Unclear Mechanisms
| Agents | Materials | Mechanisms | Models/Trials | Refs |
|---|---|---|---|---|
| Thiodigalactoside | Disaccharides | Competitively inhibit galectin-1 binding | Melanoma, breast cancer | [149,151,152] |
| TD139 | Derivatives of TDG | Competitively inhibit galectin-1 binding | Idiopathic Pulmonary Fibrosis (Phase Ib/IIa) | NCT02257177 [154,155] |
| Anginex | β-peptide | Alter the equilibrium of galectin-ligand binding | Murine ovarian carcinoma model | [156,157,158,159] |
| OTX008 | Calixarene compound | Allosteric inhibitor of galectin-ligand binding | Human advanced solid tumors (Phase I) | NCT01724320 [160,161,162,163,164,165] |
| LLS30 | Small molecule | Allosteric inhibitor of galectin-ligand binding | Prostate cancer, hepatocellular carcinoma | [166,167] |
| 4-F-GlcNAc | Glycan | Dampen the biosynthesis of LacNAcs | Melanoma, lymphoma | [168] |
| AP-74 M-545 | Single-stranded DNA aptamer | Impair galectin-ligand binding | Murine lung cancer model | [169] |
| 8F4F8G7 | Monoclonal antibody | Eliminate galectin-1 in tumor tissue | Kaposi’s sarcoma, prostate cancer | [170,171,172] |
| Gal-1-mAb3 | Monoclonal antibody | Antibody with higher affinity and selectivity | - | [173] |
| APN | Antibody-like polymeric nanoparticle | Eliminate galectin-1 in tumor tissue | - | [174] |
| TRX–mGal1 | Murine galectin-1 vaccine | Induce generation of endogenous antibody | Melanoma | [175] |
| Minigene DNA vaccine | DNA plasmid | Encode peptide fragment of galectin-1 | Neuroblastoma | [176] |
| Intranasal siRNA | siRNA-loaded chitosan nanoparticles | Inhibit galectin-1 expression | Glioblastoma multiforme | [74,179] |
| GM-CT-01 (Davanat) | Galactomannan | Bind to galectin-1 at a site opposite CRD | Metastatic colorectal cancer | NCT00054977 and |
| (Phase I and Phase II) | NCT00110721 [180] | |||
| Increase TCR:CD8 colocalization | Melanoma (Phase I/II) | NCT01723813 [181] | ||
| GR-MD-02 (Belapectin) | Polysaccharide | Remain obscure | NASH, Non-alcoholic fatty liver disease | NCT02421094, |
| NCT02462967 and | ||||
| NCT01899859 [186,187,188] | ||||
| Head and neck cancer, melanoma | NCT02575404 [182] | |||
| combined with pembrolizumab (Phase I) |
9. Conclusions and Perspectives
| Disease/Disease Model | Mechanisms | Effects | Refs | |
|---|---|---|---|---|
| Pro-inflammation | Sepsis | Inhibit the unfavorable role of CD45 in endotoxin shock | Worsen lethal inflammation | [28] |
| Infection of Dengue virus, influenzavirus, and NiV | Bind to certain envelope glycoproteins such as DENV-1, NiV-F, and NiV-G | Anti-infection | [48,49,50,51] | |
| Experimental autoimmune orchitis | Induce apoptosis of germ cells Synergistically enhance TNFα-induced inflammatory cytokine expression | Disease progression | [122,123] | |
| Osteoarthritis | Activate the NF-κB pathway and elevate secretion of matrix metalloproteinases | Disease progression | [124] | |
| Anti-inflammation | Infection of Yersinia enterocolitica and Tropheryma whipplei | Attenuate production of IFN-γ, IL-17, TNF, NO; protect YOPs from trypsin digestion; facilitate bacterial cell entry | Pro-infection | [30,31,32] |
| Murine acute inflammation model | Impair expression of adhesion molecules | Inhibit polymorphonuclear leukocyte migration | [38] | |
| Parasitic infection | Promote adhesion of parasites to host; fuel the immunotolerant circuits | Pro-infection | [40,41,42,43] | |
| Infection of HIV and NiV | Mediate virus adhesion to macrophages, CD4+ T cells, and epithelium | Pro-infection | [44,45,46,47] | |
| Graft versus host disease and graft rejection | Attenuate production of IL-2, IL-17, IFN-γ, and TNFα; suppress proliferation and alloreactivity of T cells | Prolong survival | [52,53,54,55,56,57,58,59,60] | |
| Cancer | Recruit suppressive immune cells; impair functions of cytotoxic leukocytes and alter the differentiation of naïve immune cells | Cancer progression | [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] | |
| Autoimmune diseases | Dampen antigen-specific T cell response; facilitate macrophage polarization towards M2; recruit suppressive immune cells | Disease remission | [125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142] | |
| Allergic inflammation | Dampen activation of mast cells and eosinophils and secretion of inflammatory cytokines | Disease remission | [143,144,145,146,147,148] |
| Immune Cells | Effects | Conditions | Refs | |
|---|---|---|---|---|
| Pro-inflammation | Neutrophil | Promote ROS production | Primed neutrophil | [36] |
| Facilitate migration | Physiological status | [39] | ||
| MDDC | Promote migration and maturation | High concentration (20 μM) | [66] | |
| Anti-inflammation | Neutrophil | Attenuate ROS production | Pretreatment with galectin-1 before activation | [36,37] |
| Inhibit migration | Acute inflammation | [38] | ||
| Promoted phagocytosis by macrophages | Activated neutrophil | [34,35] | ||
| Eosinophil | Inhibit migration; induce apoptosis | - | [144,145,146] | |
| Mast cell | Inhibit activation | Bind to IgE/FcεRI complexes | [148] | |
| Macrophage | Suppress iNOS and NO production; induce polarization towards M2 | - | [31,65,72,73,74,75,137] | |
| Monocyte | Inhibit migration; induce apoptosis | - | [65,76] | |
| DC | Inhibit migration and maturation of immunogenic DC | Low concentration | [79,80,81,82] | |
| CD8+ T, Th1 and Th17 | Induce apoptosis; impair function and infiltration | Activated state | [89,103,104,105,106,107,108,109] | |
| Naïve Th cell | Promote suppressive cytokine secretion | Lower than apoptotic concentration | [111] | |
| Th2 cell | Resist apoptotic effect of galectin-1 | - | [110] | |
| Regulatory T cell | Maintain suppressive function; promote infiltration | - | [112,113,114,115] | |
| NK cell | Impair cytotoxic property | - | [67,118] | |
| MDSC | Promote infiltration | - | [75,116,117] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRD | Carbohydrate recognition domain |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| NO | Nitric oxide |
| NF- kB | Nuclear factor kB |
| Th | T helper |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| fMLP | N-formyl-methionyl-leucyl-phenylalanine |
| PMA | Phorbol myristate acetate |
| LPS | Lipopolysaccharide |
| NiV | Nipah virus |
| DENV-1 | Dengue virus type 1 |
| DC | Dendritic cell |
| Treg | Regulatory T cell |
| GVHD | Graft versus host disease |
| HIF-1α | Hypoxia inducible factor-1α |
| NK | Natural killer |
| TAMs | Tumor associated macrophages |
| iNOS | Inducible nitric oxide synthase |
| VEGFA | Vascular endothelial growth factor A |
| CCL2 | C-C motif chemokine ligand 2 |
| TGF-β | Transforming growth factor-β |
| MDDCs | Monocyte-derived DCs |
| STAT | Signal transducer and activator of transcription |
| TCR | T cell receptor |
| C2GnT | Core 2 beta-1,6-N-acetylglucosaminyltransferase |
| AP-1 | Activation protein-1 |
| JNK | c-Jun N-terminal kinase |
| ERK | Extracellular signal-regulated kinase |
| ST6Gal1 | α2-6 sialyltransferase |
| LAT | Linker for activation of T cells |
| MDSC | Myeloid-derived suppressor cells |
| TLR7 | Toll-like receptor 7 |
| IRF | Interferon regulatory factor |
| EAO | Experimental autoimmune orchitis |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MAPK | Mitogen-activated protein kinase |
| CIA | Collagen-induced arthritis |
| EAE | Experimental autoimmune encephalomyelitis |
| MS | Multiple sclerosis |
| Con A | Concanavalin A |
| SLE | Systemic lupus erythematosus |
| OVA | Ovalbumin |
| SIT | Allergen-specific immunotherapy |
| LacNAc | N-acetyllactosamine |
| TDG | Thiodigalactoside |
| 4-F-GlcNAc | 4-fluoro-glucosamine |
| mAb | Monoclonal antibody |
| APN | Antibody-like polymeric nanoparticle |
| TRX | Thioredoxin |
| mGal1 | Mouse galectin-1 |
| NASH | Non-alcoholic steatohepatitis |
| TIL | Tumor Infiltrating Lymphocytes |
References
- Schnaar, R.L. Glycans and glycan-binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J. Allergy Clin. Immunol. 2015, 135, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremsreiter, S.M.; Kroell, A.H.; Weinberger, K.; Boehm, H. Glycan-Lectin Interactions in Cancer and Viral Infections and How to Disrupt Them. Int. J. Mol. Sci. 2021, 22, 10577. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.C.; Chen, H.Y.; Kuo, C.C.; Sytwu, H.K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef] [Green Version]
- Van Kooyk, Y.; Rabinovich, G.A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nature reviews. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Nickel, W. Unconventional secretory routes: Direct protein export across the plasma membrane of mammalian cells. Traffic 2005, 6, 607–614. [Google Scholar] [CrossRef]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Krishnamoorthy, L.; Bess, J.W., Jr.; Preston, A.B.; Nagashima, K.; Mahal, L.K. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat. Chem. Biol. 2009, 5, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Dagher, S.F.; Wang, J.L.; Patterson, R.J. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc. Natl. Acad. Sci. USA 1995, 92, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Davidson, P.J.; Davis, M.J.; Patterson, R.J.; Ripoche, M.A.; Poirier, F.; Wang, J.L. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology 2002, 12, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin-Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Kadoya, T.; Horie, H. Structural and functional studies of galectin-1: A novel axonal regeneration-promoting activity for oxidized galectin-1. Curr. Drug Targets 2005, 6, 375–383. [Google Scholar] [CrossRef]
- Harrison, F.L. Soluble vertebrate lectins: Ubiquitous but inscrutable proteins. J. Cell Sci. 1991, 100, 9–14. [Google Scholar] [CrossRef]
- Liao, D.I.; Kapadia, G.; Ahmed, H.; Vasta, G.R.; Herzberg, O. Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside-binding protein. Proc. Natl. Acad. Sci. USA 1994, 91, 1428–1432. [Google Scholar] [CrossRef] [Green Version]
- Guardia, C.M.; Caramelo, J.J.; Trujillo, M.; Méndez-Huergo, S.P.; Radi, R.; Estrin, D.A.; Rabinovich, G.A. Structural basis of redox-dependent modulation of galectin-1 dynamics and function. Glycobiology 2014, 24, 428–441. [Google Scholar] [CrossRef] [Green Version]
- Horie, H.; Kadoya, T.; Hikawa, N.; Sango, K.; Inoue, H.; Takeshita, K.; Asawa, R.; Hiroi, T.; Sato, M.; Yoshioka, T.; et al. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 1873–1880. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Wang, W.; Park, J.W.; Wang, J.L.; Patterson, R.J. Immunoprecipitation of spliceosomal RNAs by antisera to galectin-1 and galectin-3. Nucleic Acids Res. 2006, 34, 5166–5174. [Google Scholar] [CrossRef] [Green Version]
- Dhirapong, A.; Lleo, A.; Leung, P.; Gershwin, M.E.; Liu, F.T. The immunological potential of galectin-1 and -3. Autoimmun. Rev. 2009, 8, 360–363. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Croci, D.O. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 2012, 36, 322–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, V.L.; Griffioen, A.W. Galectin-1 and -9 in angiogenesis: A sweet couple. Glycobiology 2014, 24, 915–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Baum, L.G. Presentation of galectin-1 by extracellular matrix triggers T cell death. J. Biol. Chem. 2004, 279, 4705–4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez Hernández, E.; Sánchez-Maldonado, C.; Mayoral Chávez, M.A.; Hernández-Zimbrón, L.F.; Patricio Martínez, A.; Zenteno, E.; Limón Pérez de León, I.D. The therapeutic potential of galectin-1 and galectin-3 in the treatment of neurodegenerative diseases. Expert Rev. Neurother. 2020, 20, 439–448. [Google Scholar] [CrossRef]
- Tirado-González, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, G.; Freitag, N.; Tirado-González, I.; Unverdorben, L.; Jeschke, U.; Thijssen, V.L.; Blois, S.M. Involvement of galectin-1 in reproduction: Past, present and future. Hum. Reprod. Update 2014, 20, 175–193. [Google Scholar] [CrossRef] [Green Version]
- Rabinovich, G.A.; Sotomayor, C.E.; Riera, C.M.; Bianco, I.; Correa, S.G. Evidence of a role for galectin-1 in acute inflammation. Eur. J. Immunol. 2000, 30, 1331–1339. [Google Scholar] [CrossRef]
- Russo, A.J.; Vasudevan, S.O.; Méndez-Huergo, S.P.; Kumari, P.; Menoret, A.; Duduskar, S.; Wang, C.; Pérez Sáez, J.M.; Fettis, M.M.; Li, C.; et al. Intracellular immune sensing promotes inflammation via gasdermin D-driven release of a lectin alarmin. Nat. Immunol. 2021, 22, 154–165. [Google Scholar] [CrossRef]
- Sundblad, V.; Morosi, L.G.; Geffner, J.R.; Rabinovich, G.A. Galectin-1: A Jack-of-All-Trades in the Resolution of Acute and Chronic Inflammation. J. Immunol. 2017, 199, 3721–3730. [Google Scholar] [CrossRef] [Green Version]
- Davicino, R.C.; Méndez-Huergo, S.P.; Eliçabe, R.J.; Stupirski, J.C.; Autenrieth, I.; Di Genaro, M.S.; Rabinovich, G.A. Galectin-1-Driven Tolerogenic Programs Aggravate Yersinia enterocolitica Infection by Repressing Antibacterial Immunity. J. Immunol. 2017, 199, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Jofre, B.L.; Eliçabe, R.J.; Silva, J.E.; Pérez Sáez, J.M.; Paez, M.D.; Callegari, E.; Mariño, K.V.; Di Genaro, M.S.; Rabinovich, G.A.; Davicino, R.C. Galectin-1 Cooperates with Yersinia Outer Protein (Yop) P to Thwart Protective Immunity by Repressing Nitric Oxide Production. Biomolecules 2021, 11, 1636. [Google Scholar] [CrossRef]
- Ayona, D.; Zarza, S.M.; Landemarre, L.; Roubinet, B.; Decloquement, P.; Raoult, D.; Fournier, P.E.; Desnues, B. Human galectin-1 and galectin-3 promote Tropheryma whipplei infection. Gut Microbes 2021, 13, 1884515. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Cao, Z.; Thitiprasert, T.; Zaidi, T.S.; Panjwani, N. Galectin-1-mediated suppression of Pseudomonas aeruginosa-induced corneal immunopathology. J. Immunol. 2013, 190, 6397–6409. [Google Scholar] [CrossRef] [Green Version]
- Stowell, S.R.; Karmakar, S.; Arthur, C.M.; Ju, T.; Rodrigues, L.C.; Riul, T.B.; Dias-Baruffi, M.; Miner, J.; McEver, R.P.; Cummings, R.D. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol. Biol. Cell 2009, 20, 1408–1418. [Google Scholar] [CrossRef] [Green Version]
- Dias-Baruffi, M.; Zhu, H.; Cho, M.; Karmakar, S.; McEver, R.P.; Cummings, R.D. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 2003, 278, 41282–41293. [Google Scholar] [CrossRef] [Green Version]
- Almkvist, J.; Dahlgren, C.; Leffler, H.; Karlsson, A. Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J. Immunol. 2002, 168, 4034–4041. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.C.; Kabeya, L.M.; Azzolini, A.; Cerri, D.G.; Stowell, S.R.; Cummings, R.D.; Lucisano-Valim, Y.M.; Dias-Baruffi, M. Galectin-1 modulation of neutrophil reactive oxygen species production depends on the cell activation state. Mol. Immunol. 2019, 116, 80–89. [Google Scholar] [CrossRef]
- Gil, C.D.; Gullo, C.E.; Oliani, S.M. Effect of exogenous galectin-1 on leukocyte migration: Modulation of cytokine levels and adhesion molecules. Int. J. Clin. Exp. Pathol. 2010, 4, 74–84. [Google Scholar]
- Auvynet, C.; Moreno, S.; Melchy, E.; Coronado-Martínez, I.; Montiel, J.L.; Aguilar-Delfin, I.; Rosenstein, Y. Galectin-1 promotes human neutrophil migration. Glycobiology 2013, 23, 32–42. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, C.; Manya, H.; Ouellet, M.; Clark, G.F.; Endo, T.; Tremblay, M.J.; Sato, S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 2011, 85, 11742–11751. [Google Scholar] [CrossRef] [Green Version]
- Mercier, S.; St-Pierre, C.; Pelletier, I.; Ouellet, M.; Tremblay, M.J.; Sato, S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 2008, 371, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, J.L.; Law, W.C.; Mahajan, S.D.; Aalinkeel, R.; Nair, B.; Sykes, D.E.; Mammen, M.J.; Yong, K.T.; Hui, R.; Prasad, P.N.; et al. Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J. Immunol. 2012, 188, 3757–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, O.B.; Yun, T.; Pernet, O.; Aguilar, H.C.; Park, A.; Bowden, T.A.; Freiberg, A.N.; Lee, B.; Baum, L.G. Timing of galectin-1 exposure differentially modulates Nipah virus entry and syncytium formation in endothelial cells. J. Virol. 2015, 89, 2520–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, K.A.; Fermino, M.L.; Andrade Cdel, C.; Riul, T.B.; Alves, R.T.; Muller, V.D.; Russo, R.R.; Stowell, S.R.; Cummings, R.D.; Aquino, V.H.; et al. Galectin-1 exerts inhibitory effects during DENV-1 infection. PLoS ONE 2014, 9, e112474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.L.; Chen, Y.H.; Wang, S.W.; Huang, Y.J.; Leu, C.H.; Yeh, N.C.; Chu, C.Y.; Lin, C.C.; Shieh, G.S.; Chen, Y.L.; et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J. Virol. 2011, 85, 10010–10020. [Google Scholar] [CrossRef] [Green Version]
- Garner, O.B.; Aguilar, H.C.; Fulcher, J.A.; Levroney, E.L.; Harrison, R.; Wright, L.; Robinson, L.R.; Aspericueta, V.; Panico, M.; Haslam, S.M.; et al. Endothelial galectin-1 binds to specific glycans on nipah virus fusion protein and inhibits maturation, mobility, and function to block syncytia formation. PLoS Pathog. 2010, 6, e1000993. [Google Scholar] [CrossRef]
- Levroney, E.L.; Aguilar, H.C.; Fulcher, J.A.; Kohatsu, L.; Pace, K.E.; Pang, M.; Gurney, K.B.; Baum, L.G.; Lee, B. Novel innate immune functions for galectin-1: Galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J. Immunol. 2005, 175, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Poncini, C.V.; Ilarregui, J.M.; Batalla, E.I.; Engels, S.; Cerliani, J.P.; Cucher, M.A.; van Kooyk, Y.; González-Cappa, S.M.; Rabinovich, G.A. Trypanosoma cruzi Infection Imparts a Regulatory Program in Dendritic Cells and T Cells via Galectin-1-Dependent Mechanisms. J. Immunol. 2015, 195, 3311–3324. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xiao, M.; Xie, Z.; Shi, Q.; Zhang, Y.; Leavenworth, J.W.; Yan, B.; Huang, H. Angiostrongylus cantonensis Galectin-1 interacts with Annexin A2 to impair the viability of macrophages via activating JNK pathway. Parasites Vectors 2020, 13, 183. [Google Scholar] [CrossRef] [Green Version]
- Okumura, C.Y.; Baum, L.G.; Johnson, P.J. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell. Microbiol. 2008, 10, 2078–2090. [Google Scholar] [CrossRef] [Green Version]
- Petropolis, D.B.; Faust, D.M.; Deep Jhingan, G.; Guillen, N. A new human 3D-liver model unravels the role of galectins in liver infection by the parasite Entamoeba histolytica. PLoS Pathog. 2014, 10, e1004381. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Baum, L.G.; Blackall, D.P.; Arias-Magallano, S.; Nanigian, D.; Uh, S.Y.; Browne, J.M.; Hoffmann, D.; Emmanouilides, C.E.; Territo, M.C.; Baldwin, G.C. Amelioration of graft versus host disease by galectin-1. Clin. Immunol. 2003, 109, 295–307. [Google Scholar] [CrossRef]
- Gieseke, F.; Böhringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Müller, I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Ramhorst, R.E.; Rubinstein, N.; Corigliano, A.; Daroqui, M.C.; Kier-Joffé, E.B.; Fainboim, L. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 2002, 9, 661–670. [Google Scholar] [CrossRef]
- Xu, G.; Tu, W.; Xu, C. Immunological tolerance induced by galectin-1 in rat allogeneic renal transplantation. Int. Immunopharmacol. 2010, 10, 643–647. [Google Scholar] [CrossRef]
- Moreau, A.; Noble, A.; Ratnasothy, K.; Chai, J.G.; Deltour, L.; Cuturi, M.C.; Simpson, E.; Lechler, R.; Lombardi, G. Absence of Galectin-1 accelerates CD8+ T cell-mediated graft rejection. Eur. J. Immunol. 2012, 42, 2881–2888. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, S.; Jiang, G.; Zhou, L.; Xie, H.; Xie, X.; Yu, X.; Ding, Y.; Tian, J.; Dai, Y.; et al. Galectin-1 prolongs survival of mouse liver allografts from Flt3L-pretreated donors. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 13, 569–579. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Shen, Q.H.; Chen, H.Y.; Yang, Z.; Shuai, M.Q.; Zheng, S. Galectin-1 Restores Immune Tolerance to Liver Transplantation Through Activation of Hepatic Stellate Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 863–879. [Google Scholar] [CrossRef]
- Wei, S.; Cao, D.; Liu, Z.; Li, J.; Wu, H.; Gong, J.; Liu, Y.; Wu, Y. Dysfunctional immunoregulation in human liver allograft rejection associated with compromised galectin-1/CD7 pathway function. Cell Death Dis. 2018, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Ye, Y.; Jia, J.; He, Y.; Yang, Z.; Zhu, X.; Huang, H.; Wang, W.; Geng, L.; Yin, S.; et al. Galectin-1-induced tolerogenic dendritic cells combined with apoptotic lymphocytes prolong liver allograft survival. Int. Immunopharmacol. 2018, 65, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Banh, A.; Zhang, J.; Cao, H.; Bouley, D.M.; Kwok, S.; Kong, C.; Giaccia, A.J.; Koong, A.C.; Le, Q.T. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011, 71, 4423–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, Q.T.; Shi, G.; Cao, H.; Nelson, D.W.; Wang, Y.; Chen, E.Y.; Zhao, S.; Kong, C.; Richardson, D.; O’Byrne, K.J.; et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8932–8941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.Y.; Chen, T.T.; Xia, L.; Guo, M.; Xu, Y.; Yue, F.; Jiang, Y.; Chen, G.Q.; Zhao, K.W. Hypoxia inducible factor-1 mediates expression of galectin-1: The potential role in migration/invasion of colorectal cancer cells. Carcinogenesis 2010, 31, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Correa, S.G.; Sotomayor, C.E.; Aoki, M.P.; Maldonado, C.A.; Rabinovich, G.A. Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology 2003, 13, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Fulcher, J.A.; Hashimi, S.T.; Levroney, E.L.; Pang, M.; Gurney, K.B.; Baum, L.G.; Lee, B. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J. Immunol. 2006, 177, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Baker, G.J.; Chockley, P.; Yadav, V.N.; Doherty, R.; Ritt, M.; Sivaramakrishnan, S.; Castro, M.G.; Lowenstein, P.R. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer Res. 2014, 74, 5079–5090. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Stannard, K.; Gabutero, E.; Clark, A.M.; Neo, S.Y.; Onturk, S.; Blanchard, H.; Ralph, S.J. Galectin-1 as a potent target for cancer therapy: Role in the tumor microenvironment. Cancer Metastasis Rev. 2012, 31, 763–778. [Google Scholar] [CrossRef] [Green Version]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef]
- Chen, L.M.; Tseng, H.Y.; Chen, Y.A.; Al Haq, A.T.; Hwang, P.A.; Hsu, H.L. Oligo-Fucoidan Prevents M2 Macrophage Differentiation and HCT116 Tumor Progression. Cancers 2020, 12, 421. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Beigier-Bompadre, M.; Ilarregui, J.M.; Toscano, M.A.; Bianco, G.A.; Isturiz, M.A.; Rabinovich, G.A. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: Galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J. Immunol. 2007, 178, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.N.; Ludvigsen, M.; Abildgaard, N.; Petruskevicius, I.; Hjortebjerg, R.; Bjerre, M.; Honoré, B.; Møller, H.J.; Andersen, N.F. Serum galectin-1 in patients with multiple myeloma: Associations with survival, angiogenesis, and biomarkers of macrophage activation. OncoTargets Ther. 2017, 10, 1977–1982. [Google Scholar] [CrossRef] [Green Version]
- Van Woensel, M.; Mathivet, T.; Wauthoz, N.; Rosière, R.; Garg, A.D.; Agostinis, P.; Mathieu, V.; Kiss, R.; Lefranc, F.; Boon, L.; et al. Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci. Rep. 2017, 7, 1217. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Han, B.; Meng, X.; Duan, C.; Yang, C.; Wu, Z.; Magafurov, D.; Zhao, S.; Safin, S.; Jiang, C.; et al. Immunogenomic analysis reveals LGALS1 contributes to the immune heterogeneity and immunosuppression in glioma. Int. J. Cancer 2019, 145, 517–530. [Google Scholar] [CrossRef]
- Paclik, D.; Werner, L.; Guckelberger, O.; Wiedenmann, B.; Sturm, A. Galectins distinctively regulate central monocyte and macrophage function. Cell. Immunol. 2011, 271, 97–103. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Kambayashi, T.; Laufer, T.M. Atypical MHC class II-expressing antigen-presenting cells: Can anything replace a dendritic cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef]
- Thiemann, S.; Man, J.H.; Chang, M.H.; Lee, B.; Baum, L.G. Galectin-1 regulates tissue exit of specific dendritic cell populations. J. Biol. Chem. 2015, 290, 22662–22677. [Google Scholar] [CrossRef] [Green Version]
- Ilarregui, J.M.; Croci, D.O.; Bianco, G.A.; Toscano, M.A.; Salatino, M.; Vermeulen, M.E.; Geffner, J.R.; Rabinovich, G.A. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 2009, 10, 981–991. [Google Scholar] [CrossRef]
- Soldati, R.; Berger, E.; Zenclussen, A.C.; Jorch, G.; Lode, H.N.; Salatino, M.; Rabinovich, G.A.; Fest, S. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int. J. Cancer 2012, 131, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Hung, J.Y.; Huang, S.K.; Chou, S.H.; Cheng, D.E.; Jong, Y.J.; Hung, C.H.; Yang, C.J.; Tsai, Y.M.; Hsu, Y.L.; et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J. Immunol. 2011, 186, 1521–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ge, X.H.; Guo, X.J.; Guan, S.B.; Li, X.M.; Gu, W.; Xu, W.G. Bone Marrow Mesenchymal Stem Cells Inhibit the Function of Dendritic Cells by Secreting Galectin-1. BioMed Res. Int. 2017, 2017, 3248605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovich, G.A.; Ariel, A.; Hershkoviz, R.; Hirabayashi, J.; Kasai, K.I.; Lider, O. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology 1999, 97, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.V.; Sampaio, A.L.; Cooper, D.; Perretti, M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. Fed. Am. Soc. Exp. Biol. 2008, 22, 682–690. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Aguilera, T.; Cao, H.; Kwok, S.; Kong, C.; Bloomstein, J.; Wang, Z.; Rangan, V.S.; Jiang, D.; von Eyben, R.; et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Investig. 2019, 129, 5553–5567. [Google Scholar] [CrossRef] [Green Version]
- Van der Leij, J.; van den Berg, A.; Blokzijl, T.; Harms, G.; van Goor, H.; Zwiers, P.; van Weeghel, R.; Poppema, S.; Visser, L. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: An important tool in the regulation of the immune response. J. Pathol. 2004, 204, 511–518. [Google Scholar] [CrossRef]
- Stowell, S.R.; Qian, Y.; Karmakar, S.; Koyama, N.S.; Dias-Baruffi, M.; Leffler, H.; McEver, R.P.; Cummings, R.D. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008, 180, 3091–3102. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, H.; Cruz-Adalia, A.; Martinez Del Hoyo, G.; Cibrián-Vera, D.; Bonay, P.; Pérez-Hernández, D.; Vázquez, J.; Navarro, P.; Gutierrez-Gallego, R.; Ramirez-Huesca, M.; et al. The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with galectin-1. Mol. Cell. Biol. 2014, 34, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, J.T.; Evans, D.P.; Galvan, M.; Pace, K.E.; Leitenberg, D.; Bui, T.N.; Baum, L.G. CD45 modulates galectin-1-induced T cell death: Regulation by expression of core 2 O-glycans. J. Immunol. 2001, 167, 5697–5707. [Google Scholar] [CrossRef] [Green Version]
- Vespa, G.N.; Lewis, L.A.; Kozak, K.R.; Moran, M.; Nguyen, J.T.; Baum, L.G.; Miceli, M.C. Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J. Immunol. 1999, 162, 799–806. [Google Scholar] [CrossRef]
- Chung, C.D.; Patel, V.P.; Moran, M.; Lewis, L.A.; Miceli, M.C. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J. Immunol. 2000, 165, 3722–3729. [Google Scholar] [CrossRef] [Green Version]
- Pace, K.E.; Hahn, H.P.; Pang, M.; Nguyen, J.T.; Baum, L.G. CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J. Immunol. 2000, 165, 2331–2334. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.D.; Nguyen, J.T.; He, J.; Wang, W.; Ardman, B.; Green, J.M.; Fukuda, M.; Baum, L.G. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J. Immunol. 2006, 177, 5328–5336. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Baum, L.G. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Investig. 2006, 86, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Rabinovich, G.A.; Alonso, C.R.; Sotomayor, C.E.; Durand, S.; Bocco, J.L.; Riera, C.M. Molecular mechanisms implicated in galectin-1-induced apoptosis: Activation of the AP-1 transcription factor and downregulation of Bcl-2. Cell Death Differ. 2000, 7, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Brandt, B.; Abou-Eladab, E.F.; Tiedge, M.; Walzel, H. Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death Dis. 2010, 1, e23. [Google Scholar] [CrossRef] [Green Version]
- Brandt, B.; Büchse, T.; Abou-Eladab, E.F.; Tiedge, M.; Krause, E.; Jeschke, U.; Walzel, H. Galectin-1 induced activation of the apoptotic death-receptor pathway in human Jurkat T lymphocytes. Histochem. Cell Biol. 2008, 129, 599–609. [Google Scholar] [CrossRef]
- Matarrese, P.; Tinari, A.; Mormone, E.; Bianco, G.A.; Toscano, M.A.; Ascione, B.; Rabinovich, G.A.; Malorni, W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J. Biol. Chem. 2005, 280, 6969–6985. [Google Scholar] [CrossRef] [Green Version]
- Ion, G.; Fajka-Boja, R.; Kovács, F.; Szebeni, G.; Gombos, I.; Czibula, A.; Matkó, J.; Monostori, E. Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cell. Signal. 2006, 18, 1887–1896. [Google Scholar] [CrossRef]
- Kovács-Sólyom, F.; Blaskó, A.; Fajka-Boja, R.; Katona, R.L.; Végh, L.; Novák, J.; Szebeni, G.J.; Krenács, L.; Uher, F.; Tubak, V.; et al. Mechanism of tumor cell-induced T-cell apoptosis mediated by galectin-1. Immunol. Lett. 2010, 127, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Ion, G.; Fajka-Boja, R.; Tóth, G.K.; Caron, M.; Monostori, E. Role of p56lck and ZAP70-mediated tyrosine phosphorylation in galectin-1-induced cell death. Cell Death Differ. 2005, 12, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, H.P.; Pang, M.; He, J.; Hernandez, J.D.; Yang, R.Y.; Li, L.Y.; Wang, X.; Liu, F.T.; Baum, L.G. Galectin-1 induces nuclear translocation of endonuclease G in caspase- and cytochrome c-independent T cell death. Cell Death Differ. 2004, 11, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.K.; Moll, G.; Smith, C.; Dua, U.; Lambley, E.; Ramuz, O.; Gill, D.; Marlton, P.; Seymour, J.F.; Khanna, R. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood 2007, 110, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Maybruck, B.T.; Pfannenstiel, L.W.; Diaz-Montero, M.; Gastman, B.R. Tumor-derived exosomes induce CD8(+) T cell suppressors. J. Immunother. Cancer 2017, 5, 65. [Google Scholar] [CrossRef]
- Rubinstein, N.; Alvarez, M.; Zwirner, N.W.; Toscano, M.A.; Ilarregui, J.M.; Bravo, A.; Mordoh, J.; Fainboim, L.; Podhajcer, O.L.; Rabinovich, G.A. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004, 5, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Corapi, E.; Carrizo, G.; Compagno, D.; Laderach, D. Endogenous Galectin-1 in T Lymphocytes Regulates Anti-prostate Cancer Immunity. Front. Immunol. 2018, 9, 2190. [Google Scholar] [CrossRef]
- Liu, S.D.; Tomassian, T.; Bruhn, K.W.; Miller, J.F.; Poirier, F.; Miceli, M.C. Galectin-1 tunes TCR binding and signal transduction to regulate CD8 burst size. J. Immunol. 2009, 182, 5283–5295. [Google Scholar] [CrossRef] [Green Version]
- Juszczynski, P.; Ouyang, J.; Monti, S.; Rodig, S.J.; Takeyama, K.; Abramson, J.; Chen, W.; Kutok, J.L.; Rabinovich, G.A.; Shipp, M.A. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2007, 104, 13134–13139. [Google Scholar] [CrossRef] [Green Version]
- Cedeno-Laurent, F.; Watanabe, R.; Teague, J.E.; Kupper, T.S.; Clark, R.A.; Dimitroff, C.J. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood 2012, 119, 3534–3538. [Google Scholar] [CrossRef] [Green Version]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef]
- Cedeno-Laurent, F.; Opperman, M.; Barthel, S.R.; Kuchroo, V.K.; Dimitroff, C.J. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J. Immunol. 2012, 188, 3127–3137. [Google Scholar] [CrossRef] [Green Version]
- McHugh, R.S.; Whitters, M.J.; Piccirillo, C.A.; Young, D.A.; Shevach, E.M.; Collins, M.; Byrne, M.C. CD4(+)CD25(+) immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 2002, 16, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Garín, M.I.; Chu, C.C.; Golshayan, D.; Cernuda-Morollón, E.; Wait, R.; Lechler, R.I. Galectin-1: A key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007, 109, 2058–2065. [Google Scholar] [CrossRef] [Green Version]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Cagnoni, A.J.; Giribaldi, M.L.; Blidner, A.G.; Cutine, A.M.; Gatto, S.G.; Morales, R.M.; Salatino, M.; Abba, M.C.; Croci, D.O.; Mariño, K.V.; et al. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8(+) regulatory T cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2102950118. [Google Scholar] [CrossRef]
- Verschuere, T.; Toelen, J.; Maes, W.; Poirier, F.; Boon, L.; Tousseyn, T.; Mathivet, T.; Gerhardt, H.; Mathieu, V.; Kiss, R.; et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int. J. Cancer 2014, 134, 873–884. [Google Scholar] [CrossRef]
- Orozco, C.A.; Martinez-Bosch, N.; Guerrero, P.E.; Vinaixa, J.; Dalotto-Moreno, T.; Iglesias, M.; Moreno, M.; Djurec, M.; Poirier, F.; Gabius, H.J.; et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc. Natl. Acad. Sci. USA 2018, 115, E3769–E3778. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.; Comba, A.; Faisal, S.M.; Kadiyala, P.; Baker, G.J.; Alghamri, M.S.; Doherty, R.; Zamler, D.; Nuñez, G.; Castro, M.G.; et al. A novel miR1983-TLR7-IFNβ circuit licenses NK cells to kill glioma cells, and is under the control of galectin-1. Oncoimmunology 2021, 10, 1939601. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Wang, Y.; Han, F.; Cai, W.; Zhao, B.; Li, Y.; Han, S.; Wu, X.; Hu, D. Murine Sertoli cells promote the development of tolerogenic dendritic cells: A pivotal role of galectin-1. Immunology 2016, 148, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Dettin, L.; Rubinstein, N.; Aoki, A.; Rabinovich, G.A.; Maldonado, C.A. Regulated expression and ultrastructural localization of galectin-1, a proapoptotic beta-galactoside-binding lectin, during spermatogenesis in rat testis. Biol. Reprod. 2003, 68, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chui, K.; Trivedi, A.; Cheng, C.Y.; Cherbavaz, D.B.; Dazin, P.F.; Huynh, A.L.; Mitchell, J.B.; Rabinovich, G.A.; Noble-Haeusslein, L.J.; John, C.M. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011, 20, 619–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, C.V.; Gómez, L.G.; Gualdoni, G.S.; Lustig, L.; Rabinovich, G.A.; Guazzone, V.A. Dual roles of endogenous and exogenous galectin-1 in the control of testicular immunopathology. Sci. Rep. 2015, 5, 12259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, T.; Moos, S.; Klug, J.; Aslani, F.; Bhushan, S.; Wahle, E.; Fröhlich, S.; Meinhardt, A.; Fijak, M. Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling. Sci. Rep. 2018, 8, 3741. [Google Scholar] [CrossRef] [Green Version]
- Toegel, S.; Weinmann, D.; André, S.; Walzer, S.M.; Bilban, M.; Schmidt, S.; Chiari, C.; Windhager, R.; Krall, C.; Bennani-Baiti, I.M.; et al. Galectin-1 Couples Glycobiology to Inflammation in Osteoarthritis through the Activation of an NF-κB-Regulated Gene Network. J. Immunol. 2016, 196, 1910–1921. [Google Scholar] [CrossRef] [Green Version]
- Santucci, L.; Fiorucci, S.; Rubinstein, N.; Mencarelli, A.; Palazzetti, B.; Federici, B.; Rabinovich, G.A.; Morelli, A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology 2003, 124, 1381–1394. [Google Scholar] [CrossRef]
- Muglia, C.I.; Gobbi, R.P.; Smaldini, P.; Delgado, M.L.; Candia, M.; Zanuzzi, C.; Sambuelli, A.; Rocca, A.; Toscano, M.A.; Rabinovich, G.A.; et al. Inflammation Controls Sensitivity of Human and Mouse Intestinal Epithelial Cells to Galectin-1. J. Cell. Physiol. 2016, 231, 1575–1585. [Google Scholar] [CrossRef]
- Fernandez-Perez, R.; Lopez-Santalla, M.; Sánchez-Domínguez, R.; Alberquilla, O.; Gutiérrez-Cañas, I.; Juarranz, Y.; Bueren, J.A.; Garin, M.I. Enhanced Susceptibility of Galectin-1 Deficient Mice to Experimental Colitis. Front. Immunol. 2021, 12, 687443. [Google Scholar] [CrossRef]
- Iwatani, S.; Shinzaki, S.; Amano, T.; Otake, Y.; Tani, M.; Yoshihara, T.; Tsujii, Y.; Hayashi, Y.; Inoue, T.; Okuzaki, D.; et al. Oligosaccharide-dependent anti-inflammatory role of galectin-1 for macrophages in ulcerative colitis. J. Gastroenterol. Hepatol. 2020, 35, 2158–2169. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Daly, G.; Dreja, H.; Tailor, H.; Riera, C.M.; Hirabayashi, J.; Chernajovsky, Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J. Exp. Med. 1999, 190, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.R.; Shiau, A.L.; Chen, S.Y.; Cheng, Z.S.; Li, Y.T.; Lee, C.H.; Yo, Y.T.; Lo, C.W.; Lin, Y.S.; Juan, H.Y.; et al. Intra-articular lentivirus-mediated delivery of galectin-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene Ther. 2010, 17, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, A.J.; Cooper, D.; Vugler, A.; Gittens, B.R.; Moore, A.; Perretti, M. Endogenous galectin-1 exerts tonic inhibition on experimental arthritis. J. Immunol. 2013, 191, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Harjacek, M.; Diaz-Cano, S.; De Miguel, M.; Wolfe, H.; Maldonado, C.A.; Rabinovich, G.A. Expression of galectins-1 and -3 correlates with defective mononuclear cell apoptosis in patients with juvenile idiopathic arthritis. J. Rheumatol. 2001, 28, 1914–1922. [Google Scholar]
- Mendez-Huergo, S.P.; Maller, S.M.; Farez, M.F.; Mariño, K.; Correale, J.; Rabinovich, G.A. Integration of lectin-glycan recognition systems and immune cell networks in CNS inflammation. Cytokine Growth Factor Rev. 2014, 25, 247–255. [Google Scholar] [CrossRef]
- Starossom, S.C.; Mascanfroni, I.D.; Imitola, J.; Cao, L.; Raddassi, K.; Hernandez, S.F.; Bassil, R.; Croci, D.O.; Cerliani, J.P.; Delacour, D.; et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 2012, 37, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Offner, H.; Celnik, B.; Bringman, T.S.; Casentini-Borocz, D.; Nedwin, G.E.; Vandenbark, A.A. Recombinant human beta-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1990, 28, 177–184. [Google Scholar] [CrossRef]
- Mari, E.R.; Rasouli, J.; Ciric, B.; Moore, J.N.; Conejo-Garcia, J.R.; Rajasagi, N.; Zhang, G.X.; Rabinovich, G.A.; Rostami, A. Galectin-1 is essential for the induction of MOG35-55 -based intravenous tolerance in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2016, 46, 1783–1796. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, M.; Thomas, L.; Mathieu, P.; Carabias, P.; Troncoso, M.F.; Pasquini, J.M.; Rabinovich, G.A.; Pasquini, L.A. Galectin-1 circumvents lysolecithin-induced demyelination through the modulation of microglial polarization/phagocytosis and oligodendroglial differentiation. Neurobiol. Dis. 2016, 96, 127–143. [Google Scholar] [CrossRef]
- Santucci, L.; Fiorucci, S.; Cammilleri, F.; Servillo, G.; Federici, B.; Morelli, A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology 2000, 31, 399–406. [Google Scholar] [CrossRef]
- Perone, M.J.; Bertera, S.; Shufesky, W.J.; Divito, S.J.; Montecalvo, A.; Mathers, A.R.; Larregina, A.T.; Pang, M.; Seth, N.; Wucherpfennig, K.W.; et al. Suppression of autoimmune diabetes by soluble galectin-1. J. Immunol. 2009, 182, 2641–2653. [Google Scholar] [CrossRef] [Green Version]
- Toscano, M.A.; Commodaro, A.G.; Ilarregui, J.M.; Bianco, G.A.; Liberman, A.; Serra, H.M.; Hirabayashi, J.; Rizzo, L.V.; Rabinovich, G.A. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J. Immunol. 2006, 176, 6323–6332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez Allo, V.C.; Hauk, V.; Sarbia, N.; Pinto, N.A.; Croci, D.O.; Dalotto-Moreno, T.; Morales, R.M.; Gatto, S.G.; Manselle Cocco, M.N.; Stupirski, J.C.; et al. Suppression of age-related salivary gland autoimmunity by glycosylation-dependent galectin-1-driven immune inhibitory circuits. Proc. Natl. Acad. Sci. USA 2020, 117, 6630–6639. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Hornung, Á.; Monostori, É.; Bocskai, M.; Czibula, Á.; Kovács, L. Altered Cell Surface N-Glycosylation of Resting and Activated T Cells in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 4455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-González, R.; Cibrian, D.; Fernández-Gallego, N.; Ramírez-Huesca, M.; Saiz, M.L.; Navarro, M.N.; Fresno, M.; de la Fuente, H.; Sánchez-Madrid, F. Galectin-1 Expression in CD8(+) T Lymphocytes Controls Inflammation in Contact Hypersensitivity. J. Investig. Dermatol. 2021, 141, 1522–1532.E3. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Dai, M.; Wang, M.; Chen, F.; Liu, R. Anti-inflammatory Property of Galectin-1 in a Murine Model of Allergic Airway Inflammation. J. Immunol. Res. 2019, 2019, 9705327. [Google Scholar] [CrossRef]
- Ge, X.N.; Ha, S.G.; Greenberg, Y.G.; Rao, A.; Bastan, I.; Blidner, A.G.; Rao, S.P.; Rabinovich, G.A.; Sriramarao, P. Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1. Proc. Natl. Acad. Sci. USA 2016, 113, E4837–E4846. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Cuellar, S.; de la Fuente, H.; Cruz-Adalia, A.; Lamana, A.; Cibrian, D.; Giron, R.M.; Vara, A.; Sanchez-Madrid, F.; Ancochea, J. Reduced expression of galectin-1 and galectin-9 by leucocytes in asthma patients. Clin. Exp. Immunol. 2012, 170, 365–374. [Google Scholar] [CrossRef]
- Mello, C.B.; Ramos, L.; Gimenes, A.D.; Andrade, T.R.; Oliani, S.M.; Gil, C.D. Immunomodulatory effects of galectin-1 on an IgE-mediated allergic conjunctivitis model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.T.; Shu, Q.; Luo, X.Q.; Liu, Z.Q.; Qiu, S.Q.; Liu, J.Q.; Guo, H.J.; Li, L.J.; Li, M.G.; Liu, D.B.; et al. Long-term effects: Galectin-1 and specific immunotherapy for allergic responses in the intestine. Allergy 2018, 73, 106–114. [Google Scholar] [CrossRef]
- Ito, K.; Scott, S.A.; Cutler, S.; Dong, L.F.; Neuzil, J.; Blanchard, H.; Ralph, S.J. Thiodigalactoside inhibits murine cancers by concurrently blocking effects of galectin-1 on immune dysregulation, angiogenesis and protection against oxidative stress. Angiogenesis 2011, 14, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Cumpstey, I.; Sundin, A.; Leffler, H.; Nilsson, U.J. C2-symmetrical thiodigalactoside bis-benzamido derivatives as high-affinity inhibitors of galectin-3: Efficient lectin inhibition through double arginine-arene interactions. Angew. Chem. 2005, 44, 5110–5112, International ed. in English. [Google Scholar] [CrossRef]
- Bianchet, M.A.; Ahmed, H.; Vasta, G.R.; Amzel, L.M. Soluble beta-galactosyl-binding lectin (galectin) from toad ovary: Crystallographic studies of two protein-sugar complexes. Proteins 2000, 40, 378–388. [Google Scholar] [CrossRef]
- Stannard, K.A.; Collins, P.M.; Ito, K.; Sullivan, E.M.; Scott, S.A.; Gabutero, E.; Darren Grice, I.; Low, P.; Nilsson, U.J.; Leffler, H.; et al. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010, 299, 95–110. [Google Scholar] [CrossRef]
- Cumpstey, I.; Salomonsson, E.; Sundin, A.; Leffler, H.; Nilsson, U.J. Studies of arginine-arene interactions through synthesis and evaluation of a series of galectin-binding aromatic lactose esters. ChemBioChem 2007, 8, 1389–1398. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.C.; Lin, H.Y.; Tu, Z.; Kuo, Y.H.; Hsu, S.D.; Lin, C.H. Dissecting the Structure-Activity Relationship of Galectin-Ligand Interactions. Int. J. Mol. Sci. 2018, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Griffioen, A.W.; van der Schaft, D.W.; Barendsz-Janson, A.F.; Cox, A.; Struijker Boudier, H.A.; Hillen, H.F.; Mayo, K.H. Anginex, a designed peptide that inhibits angiogenesis. Biochem. J. 2001, 354, 233–242. [Google Scholar] [CrossRef]
- Dings, R.P.; van der Schaft, D.W.; Hargittai, B.; Haseman, J.; Griffioen, A.W.; Mayo, K.H. Anti-tumor activity of the novel angiogenesis inhibitor anginex. Cancer Lett. 2003, 194, 55–66. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [Green Version]
- Salomonsson, E.; Thijssen, V.L.; Griffioen, A.W.; Nilsson, U.J.; Leffler, H. The anti-angiogenic peptide anginex greatly enhances galectin-1 binding affinity for glycoproteins. J. Biol. Chem. 2011, 286, 13801–13804. [Google Scholar] [CrossRef] [Green Version]
- Dings, R.P.; Miller, M.C.; Nesmelova, I.; Astorgues-Xerri, L.; Kumar, N.; Serova, M.; Chen, X.; Raymond, E.; Hoye, T.R.; Mayo, K.H. Antitumor agent calixarene 0118 targets human galectin-1 as an allosteric inhibitor of carbohydrate binding. J. Med. Chem. 2012, 55, 5121–5129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dings, R.P.; Chen, X.; Hellebrekers, D.M.; van Eijk, L.I.; Zhang, Y.; Hoye, T.R.; Griffioen, A.W.; Mayo, K.H. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J. Natl. Cancer Inst. 2006, 98, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Koonce, N.A.; Griffin, R.J.; Dings, R.P.M. Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. Int. J. Mol. Sci. 2017, 18, 2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [CrossRef] [PubMed]
- Leung, Z.; Ko, F.C.F.; Tey, S.K.; Kwong, E.M.L.; Mao, X.; Liu, B.H.M.; Ma, A.P.Y.; Fung, Y.M.E.; Che, C.M.; Wong, D.K.H.; et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J. Exp. Clin. Cancer Res. CR 2019, 38, 423. [Google Scholar] [CrossRef] [Green Version]
- Shih, T.C.; Liu, R.; Wu, C.T.; Li, X.; Xiao, W.; Deng, X.; Kiss, S.; Wang, T.; Chen, X.J.; Carney, R.; et al. Targeting Galectin-1 Impairs Castration-Resistant Prostate Cancer Progression and Invasion. Clin. Cancer Res. 2018, 24, 4319–4331. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.T.; Li, C.Y.; Huang, Y.H.; Chang, T.S.; Lin, C.Y.; Chuang, C.H.; Wang, C.Y.; Anuraga, G.; Chang, T.H.; Shih, T.C.; et al. Galectin-1 orchestrates an inflammatory tumor-stroma crosstalk in hepatoma by enhancing TNFR1 protein stability and signaling in carcinoma-associated fibroblasts. Oncogene 2022, 41, 3011–3023. [Google Scholar] [CrossRef]
- Cedeno-Laurent, F.; Opperman, M.J.; Barthel, S.R.; Hays, D.; Schatton, T.; Zhan, Q.; He, X.; Matta, K.L.; Supko, J.G.; Frank, M.H.; et al. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. J. Investig. Dermatol. 2012, 132, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.T.; Liang, C.H.; Yu, J.H.; Huang, K.C.; Tung, C.H.; Wu, J.E.; Wu, Y.Y.; Chang, C.H.; Hong, T.M.; Chen, Y.L. A DNA Aptamer Targeting Galectin-1 as a Novel Immunotherapeutic Strategy for Lung Cancer. Mol. Therapy. Nucleic Acids 2019, 18, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Juszczynski, P.; Rodig, S.J.; Green, M.R.; O’Donnell, E.; Currie, T.; Armant, M.; Takeyama, K.; Monti, S.; Rabinovich, G.A.; et al. Viral induction and targeted inhibition of galectin-1 in EBV+ posttransplant lymphoproliferative disorders. Blood 2011, 117, 4315–4322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, D.O.; Salatino, M.; Rubinstein, N.; Cerliani, J.P.; Cavallin, L.E.; Leung, H.J.; Ouyang, J.; Ilarregui, J.M.; Toscano, M.A.; Domaica, C.I.; et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J. Exp. Med. 2012, 209, 1985–2000. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Gentilini, L.D.; Giribaldi, L.; Delgado, V.C.; Nugnes, L.; Croci, D.O.; Al Nakouzi, N.; Sacca, P.; Casas, G.; Mazza, O.; et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013, 73, 86–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez Sáez, J.M.; Hockl, P.F.; Cagnoni, A.J.; Méndez Huergo, S.P.; García, P.A.; Gatto, S.G.; Cerliani, J.P.; Croci, D.O.; Rabinovich, G.A. Characterization of a neutralizing anti-human galectin-1 monoclonal antibody with angioregulatory and immunomodulatory activities. Angiogenesis 2021, 24, 1–5. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, Y.; Zhang, Z.; Hao, J.; Zheng, Y.; Liu, Q.; Liu, Y.; Shi, L. An Antibody-like Polymeric Nanoparticle Removes Intratumoral Galectin-1 to Enhance Antitumor T-Cell Responses in Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 22159–22168. [Google Scholar] [CrossRef]
- Femel, J.; van Hooren, L.; Herre, M.; Cedervall, J.; Saupe, F.; Huijbers, E.J.M.; Verboogen, D.R.J.; Reichel, M.; Thijssen, V.L.; Griffioen, A.W.; et al. Vaccination against galectin-1 promotes cytotoxic T-cell infiltration in melanoma and reduces tumor burden. Cancer Immunol. Immunother. 2022, 71, 2029–2040. [Google Scholar] [CrossRef]
- Liebscher, L.; Weißenborn, C.; Langwisch, S.; Gohlke, B.O.; Preissner, R.; Rabinovich, G.A.; Christiansen, N.; Christiansen, H.; Zenclussen, A.C.; Fest, S. A minigene DNA vaccine encoding peptide epitopes derived from Galectin-1 has protective antitumoral effects in a model of neuroblastoma. Cancer Lett. 2021, 509, 105–114. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Tang, Z.; Li, X.; Wang, G. Reversal of galectin-1 gene silencing on resistance to cisplatin in human lung adenocarcinoma A549 cells. Biomed. Pharmacother. 2016, 83, 265–270. [Google Scholar] [CrossRef]
- Le Mercier, M.; Mathieu, V.; Haibe-Kains, B.; Bontempi, G.; Mijatovic, T.; Decaestecker, C.; Kiss, R.; Lefranc, F. Knocking down galectin 1 in human hs683 glioblastoma cells impairs both angiogenesis and endoplasmic reticulum stress responses. J. Neuropathol. Exp. Neurol. 2008, 67, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T.; et al. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef]
- Miller, M.C.; Klyosov, A.; Mayo, K.H. The alpha-galactomannan Davanat binds galectin-1 at a site different from the conventional galectin carbohydrate binding domain. Glycobiology 2009, 19, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Demotte, N.; Bigirimana, R.; Wieërs, G.; Stroobant, V.; Squifflet, J.L.; Carrasco, J.; Thielemans, K.; Baurain, J.F.; Van Der Smissen, P.; Courtoy, P.J.; et al. A short treatment with galactomannan GM-CT-01 corrects the functions of freshly isolated human tumor-infiltrating lymphocytes. Clin. Cancer Res. 2014, 20, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.I.; Friedman, S.L. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.C.; Ribeiro, J.P.; Roldós, V.; Martín-Santamaría, S.; Cañada, F.J.; Nesmelova, I.A.; André, S.; Pang, M.; Klyosov, A.A.; Baum, L.G.; et al. Structural aspects of binding of α-linked digalactosides to human galectin-1. Glycobiology 2011, 21, 1627–1641. [Google Scholar] [CrossRef] [Green Version]
- Traber, P.G.; Zomer, E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS ONE 2013, 8, e83481. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Dennis, A.; Fiore, M.M.; Kelly, M.D.; Kelly, C.J.; Paredes, A.H.; Whitehead, J.M.; Neubauer, S.; Traber, P.G.; Banerjee, R. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS ONE 2018, 13, e0203054. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Abdelmalek, M.F.; Garcia-Tsao, G.; Vuppalanchi, R.; Alkhouri, N.; Rinella, M.; Noureddin, M.; Pyko, M.; Shiffman, M.; Sanyal, A.; et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology 2020, 158, 1334–1345.E5. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Marri, S.R.; Chalasani, N.; Kohli, R.; Aronstein, W.; Thompson, G.A.; Irish, W.; Miles, M.V.; Xanthakos, S.A.; Lawitz, E.; et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment. Pharmacol. Ther. 2016, 44, 1183–1198. [Google Scholar] [CrossRef] [Green Version]
- Sturgill, E.R.; Rolig, A.S.; Linch, S.N.; Mick, C.; Kasiewicz, M.J.; Sun, Z.; Traber, P.G.; Shlevin, H.; Redmond, W.L. Galectin-3 inhibition with belapectin combined with anti-OX40 therapy reprograms the tumor microenvironment to favor anti-tumor immunity. Oncoimmunology 2021, 10, 1892265. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Qian, J.; Ding, L.; Yin, S.; Zhou, L.; Zheng, S. Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities. Int. J. Mol. Sci. 2023, 24, 6501. https://doi.org/10.3390/ijms24076501
Yu X, Qian J, Ding L, Yin S, Zhou L, Zheng S. Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities. International Journal of Molecular Sciences. 2023; 24(7):6501. https://doi.org/10.3390/ijms24076501
Chicago/Turabian StyleYu, Xizhi, Junjie Qian, Limin Ding, Shengyong Yin, Lin Zhou, and Shusen Zheng. 2023. "Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities" International Journal of Molecular Sciences 24, no. 7: 6501. https://doi.org/10.3390/ijms24076501






